Abstract
Although the reported percentage of bone-implant contact is far lower than 100%, the cause of such low levels of bone formation has rarely been investigated. This study tested the negative biological effect of hydrocarbon deposition onto titanium surfaces, which has been reported to be inevitable. Osteogenic MC3T3-E1 cells were cultured on titanium disks on which the carbon concentration was experimentally regulated to achieve carbon/titanium (C/Ti) ratios of 0.3, 0.7, and 1.0. Initial cellular activities such as cell attachment and cell spreading were concentration-dependently suppressed by the amount of carbon on the titanium surface. The osteoblastic functions of alkaline phosphatase activity and calcium mineralization were also reduced by more than 40% on the C/Ti (1.0) surface. These results indicate that osteoblast activity is influenced by the degree of hydrocarbon contamination on titanium implants and suggest that hydrocarbon decomposition before implant placement may increase the biocompatibility of titanium.
Keywords: implant dentistry, biomaterial(s), biocompatibility, surface chemistry, wettability, osseointegration
Introduction
One of the current problems in titanium implant therapy is incomplete integration. The reported bone-titanium contact percentage is 45% ± 16% (Weinlaender et al., 1992) or 50% to 65% (Ogawa and Nishimura, 2003) at maximum, which is quite less than the ideal value of 100%. Most implants fail because of incomplete fixation or early/late destructive changes at the bone-implant interface (Chuang et al., 2002). Until recently, a limited supply of stem cells had been the only hypothetical explanation for such incomplete bone formation (Tu et al., 2007; Li et al., 2008). It is necessary to investigate the cause of low levels of bone formation to reduce the need for revision surgery and expand the indications for clinical implant dentistry.
Biocompatibility, which is a critical factor in bone-implant integration, depends on surface physicochemical properties such as surface topography, wettability, and chemical composition (Zhao et al., 2005; Liu et al., 2007; Zareidoost et al., 2012). In this study, we focused on the effect of carbon adsorption on the bioactivity of titanium because carbon is the most prominent contaminant of titanium surfaces (Morra et al., 2003). Previous studies have reported that progressive deposition of hydrocarbons onto titanium surfaces is inevitable. A study analyzing the chemical composition of several types of implant surfaces showed 17.9% to 76.5% carbon deposition on 34 different implants (Morra et al., 2003). Such adsorption of hydrocarbon was found on titanium surfaces regardless of topography, suggesting that hydrocarbon deposition may occur on titanium-based materials with any surface texture. Differences in osteoconductivity arising from differences in the amount of carbon deposition on implants could create a critical problem in clinical implant dentistry related to discrepancy in the quality of the implants. However, the effect of carbon on biocompatibility with osteoblastic cells has not been sufficiently evaluated. In the present study, we tested the hypothesis that organic contaminants adsorbed on titanium surfaces decrease the bioactivity and osteoconductivity of titanium. The objective of this study was to examine the effects of carbon deposition on titanium in terms of various in vitro behaviors and functions of osteoblastic cells on a titanium substrate.
Materials & Methods
The surfaces of commercially pure grade-2 titanium disks (8 mm diameter × 1.5 mm thickness; Rare Metallic Co. Ltd., Tokyo, Japan) were mirror-polished. The carbon concentration on the titanium surface was experimentally regulated by soaking each disk in acetone for 3 different durations (60, 15, and 5 min) after the titanium surface was varnished with machine oil composed of various types of hydrocarbons (CxHy). Therefore, to evaluate whether the cytotoxic molecules were contained in the machine oil, we tested cellular viability for the osteoblasts on the oil-varnished glass plates compared with the cells on the polystyrene plates. Both the attached cells on the substrates and the floating cells in the culture medium were collected for measurement after 24 and 72 hr of culture. The quantification of the viable cells was performed with the use of a cell-counting reagent and water-soluble tetrazolium salt, WST-8 (Dojindo Molecular Technologies, Inc., Rockville, MD, USA). The ratio of the integrated intensity of the C1s electron peak to that of the Ti2p electron peak was defined as C/Ti. The final C/Ti ratios were 0.3, 0.7, and 1.0, which were confirmed by chemical analysis by x-ray photoelectron spectroscopy (XPS) (JEOL, Ltd., Tokyo, Japan). The hydrophilicity of the titanium surface was measured by means of an automated contact angle measuring device (DM-301; Kyowa Interface Science, Saitama, Japan) to determine the contact angle of 10 µL of H2O. Protein adsorption onto the titanium disks was measured with bovine serum albumin, fraction V (Pierce Biotechnology, Inc., Rockford, IL, Inc.), as a model protein. Osteogenic MC3T3-E1 cells were cultured on titanium disks. Initial cell attachment was evaluated by measurement of the quantity of the cells attached to the titanium substrate after 6 and 24 hr of incubation. The quantification was performed by colorimetry with water-soluble tetrazolium salt, WST-8, which is reduced by dehydrogenase activities in cells to give a formazan dye. Furthermore, after 6 hr of incubation, the cells were stained with calcein for observation under a fluorescent microscope to confirm the cell density results. Vinculin expression per cell after 6 hr of incubation was quantified as pixel-based density in an image analyzer. Cell morphological features and cytoskeletal arrangement after 12 hr of culture were also analyzed by microscopic image-based quantification. Alkaline phosphatase (ALP) activity at day 10 and mineralization capability at day 20 were examined in the cultured osteoblastic cells by colorimetry-based quantification. Details of the cell culture and assay protocols can be found in the Appendix.
Five samples were used in each analysis. We used one-way analysis of variance (ANOVA) with significance defined as p < .05 to examine differences in the variables among the different C/Ti ratios. When necessary, Tukey’s multiple-comparison test was used for post hoc evaluation.
Results
Surface Characteristics of Titanium Samples
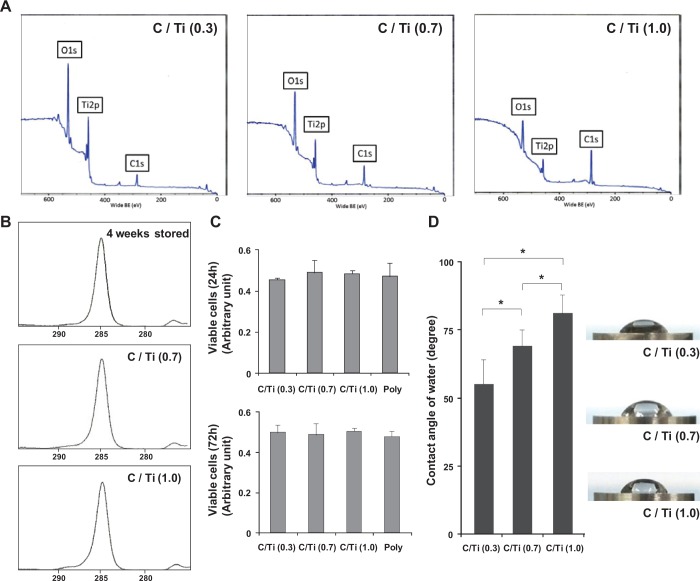
X-ray photoelectron spectroscopy (XPS) spectra showed only peaks of Ti2p, O1s, and C1s for each titanium surface, indicating the absence of contamination by impurities other than these elements (Fig. 1A). The XPS spectra also showed that the C1s peak increased with carbon contamination, whereas the Ti2p peaks decreased. The O1s peak originating from titanium oxides also decreased as a result of carbon contamination (Fig. 1A). The magnified C1s profiles of a disk stored for 4 wk (untreated disk), a C/Ti (0.7) disk, and a C/Ti (1.0) disk showed similarities in shape and typical hydrocarbon waveforms, indicating that natural hydrocarbon contamination was successfully simulated (Fig. 1B). The viability of the osteoblastic cells was not decreased on the oil-varnished glass plates, even compared with the cells cultured on the polystyrene plates (Fig. 1C), indicating that the cytotoxic molecules were not contained in the machine oil or were virtually eliminated in this experimental condition. The contact angle increased as the carbon amount increased; thus, the wettability changed with the increased hydrophobicity of the surface (Fig. 1D). The C/Ti (0.7) and (1.0) surfaces were hydrophobic, as shown in the images, and exhibited a contact angle of > 70° for a 10 µL H2O drop (Fig. 1E).
Figure 1.
Surface characterization of the carbon-contaminated titanium surfaces used in this study. (A) C/Ti changes in the XPS profile for the C1s, Ti2p, and O1s elements. (B) Magnified C1s profile for a disk stored for 4 wk (untreated), C/Ti (0.7) disk, and C/Ti (1.0) disk. (C) Cellular viability of MC3T3E1 cells on the carbon-varnished glass plates and the polystyrene plates evaluated by WST-8 colorimetry after 24 and 72 hr of culture. (D) Hydrophilicity changes after carbon contamination were evaluated as the contact angle of 10 µL of H2O. Data have been expressed in terms of mean ± SD values (n = 5). *p < .05, indicating a statistically significant difference among three different C/Ti titanium surfaces. (E) Side-view images of 10 µL of H2O dropped onto titanium disks have also been presented.
Decreased Cell Attachment Because of Carbon Contamination
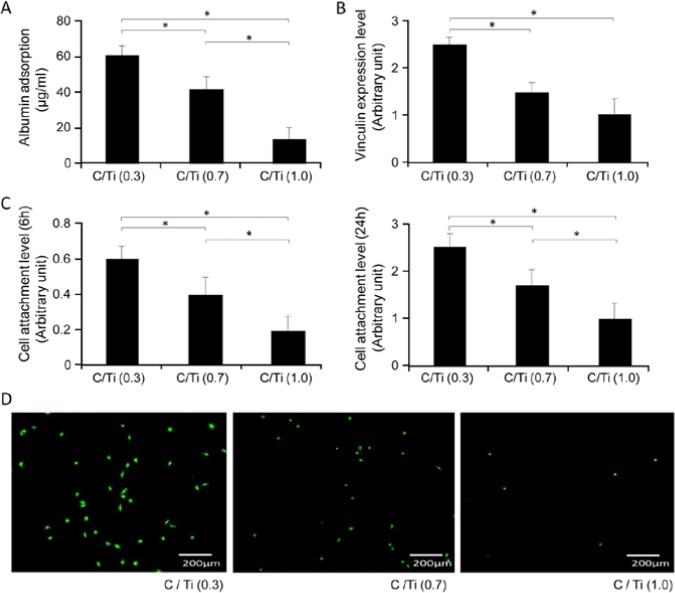
The adsorption rate of albumin, a protein that is important for the attachment of cells to biomaterials, was 70% lower on the C/Ti (1.0) surface than on the C/Ti (0.3) surface after 3 hr of incubation (Fig. 2A). After 6 hr of incubation, the expression of vinculin, a focal adhesion protein, had also decreased according to the amount of carbon, with a decrease of 50% observed for the C/Ti (1.0) surface relative to the C/Ti (0.3) surface. Quantitative analysis of cell attachment in the WST-8 assay showed a 60% reduction in the amount of cells on the C/Ti (1.0) surface relative to that on the C/Ti (0.3) surface after 6 hr of culture (Fig. 2C). The negative effect of carbon contamination on cell attachment was evident even after 24 hr, with a 50% decrease observed in cell density (Fig. 2C). Fluorescent microscopic images of osteoblastic cells stained with calcein confirmed this effect (Fig. 2D).
Figure 2.
Protein and cell affinity to titanium surfaces. (A) Albumin adsorption to titanium surfaces during 3 hr of incubation. (B) Vinculin expression in cells on titanium surfaces after 6 hr of incubation. (C) Initial attachment of osteoblastic cells evaluated by WST-8 colorimetry after 6 and 24 hr of culture. Data have been expressed in terms of mean ± SD values (n = 5). *p < .05, indicating a statistically significant difference among three different C/Ti titanium surfaces. (D) Fluorescent microscopic images of osteoblastic cells stained with calcein after 6 hr of culture on titanium samples.
Suppression of Spreading of Osteoblastic Cells by Carbon Contamination
Fluorescent microscopic images of the osteoblastic cells after being stained with rhodamine-phalloidin showed that the cells on the C/Ti (0.3) disk were larger, with cytoskeletal stretching and actin fiber formation, whereas most cells on C/Ti (1.0) surfaces were rounded and exhibited neither cytoskeletal stretching nor actin fiber formation after 12 hr of incubation (Fig. 3A). The 3 different C/Ti conditions were significantly different with regard to cytomorphometric parameters such as cell area, cell perimeter, and Feret’s diameter, the results of which supported the qualitative observations (Fig. 3B).
Figure 3.
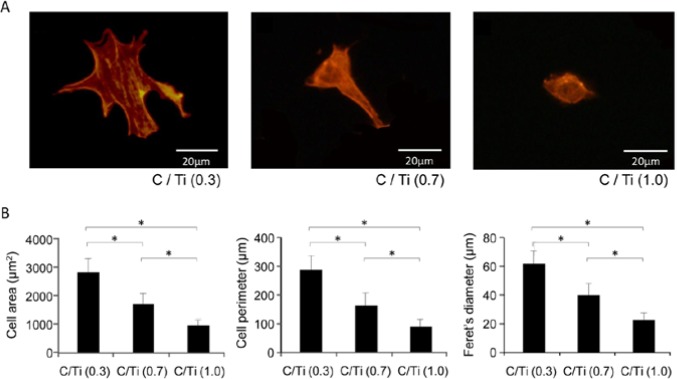
Initial spreading and cytoskeletal arrangement of osteoblastic cells 12 hr after being seeded onto 3 different C/Ti titanium surfaces. (A) Representative fluorescent microscopic images of cells stained with rhodamine phalloidin for actin filaments. (B) Cytomorphometric evaluations of cell area, cell perimeter, and Feret’s diameter were performed with an image analyzer. Data have been expressed in terms of mean ± SD values (n = 5). *p < .05, indicating a statistically significant difference among the 3 different C/Ti titanium surfaces.
Decreased Osteoblastic Differentiation
The osteoblastic phenotype, as represented by ALP activity, decreased in a concentration-dependent manner according to carbon accumulation. The total ALP activity on the C/Ti (1.0) surface decreased by 50% relative to that on the C/Ti (0.3) surface at day 10 (Fig. 4A). The mineralization capability of cultured osteoblastic cells was examined by colorimetry-based quantification of calcium deposition at day 20 (Fig. 4B), and a 40% reduction was observed for C/Ti (1.0) relative to C/Ti (0.3) culture.
Figure 4.
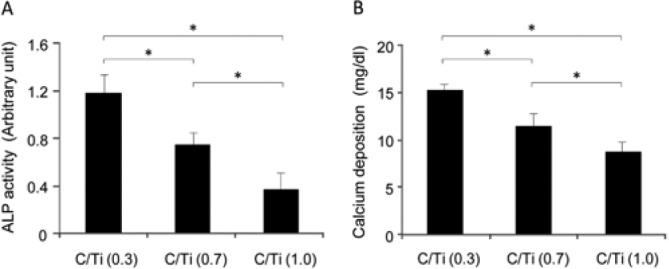
Suppression of osteoblastic function on carbon-contaminated titanium surfaces. (A) Total alkaline phosphatase (ALP) activity measured by a colorimetry-based assay on culture day 10. (B) Mineralizing capability of osteoblasts evaluated by total calcium deposition at culture day 20. Data have been expressed in terms of mean ± SD (n = 5). *p < .05, indicating a statistically significant difference among the 3 different C/Ti titanium surfaces.
Discussion
To our knowledge, this is the first study to evaluate the adverse biological effects of carbon deposition on the osteoconductivity of titanium. Recent studies have reported that ultraviolet treatment (Att et al., 2009) or gamma-ray irradiation (Ueno et al., 2012) could increase the bioactivity of the titanium surface and simultaneously remove the hydrocarbon. Although the negative effect of carbon deposition on cellular response seems to be suggested, it might possibly be only presumptive evidence, but may not infer a mechanism of the relationship between these 2 phenomena. In addition, some researchers have reported that carbon was highly biocompatible because of the presence of this element in the human body (Couvrat et al., 1995) and carbon has been widely applied to biomaterials (Martins-Junior et al., 2013). Further, surface treatment changes not only chemical composition but also other properties such as surface energy or electrostatic potential (Hori et al., 2010), making the interpretation of the mechanism of biological enhancement complicated. Therefore, there is a need to clarify the cause-and-effect relationship between cellular activity and experimentally regulated hydrocarbons.
Calcium deposition, which is representative of osteoconductivity, decreased by 40% on C/Ti (1.0). ALP activity, a marker of differentiation, also decreased by 50% on C/Ti (1.0). Therefore, the bone-titanium integration of a carbon-coated surface may be reduced by half compared with that for a freshly prepared surface. The reduction seen in vitro in osteoconductivity resulted from a decrease in protein adsorption, a decrease in osteoblastic cell attachment, and suppression of cell spreading, which are closely inter-related processes. For instance, decreased protein adsorption may have decreased cell attachment via decreased interactions between proteins and cellular integrins. Decreased cell attachment may have decreased osteoblastic differentiation through reduced cell-to-cell interactions. The cytoskeleton, which consists of actin fibers/filaments, maintains the cellular shape and is responsible for resisting tension (Endlich and Endlich, 2006; Mierke, 2009). As seen in the microscopic images at 24 hr of incubation, actin fibers emerged earlier and were arranged in a more advanced manner in osteoblastic cells on surfaces with a low C/Ti ratio than on C/Ti (1.0) surfaces. The reduction in osteoblast adhesion to carbon-contaminated surfaces was associated with decreased expression of vinculin, which is involved in linkage among cell adhesion molecules, integrins, and actin filaments and plays a key role in initiating and establishing cell adhesion, cell shape formation, and cytoskeletal development (Humphries et al., 2007; Wen et al., 2009). The observed down-regulation of vinculin provides a molecular explanation for how the adhesion of osteoblasts to carbon-contaminated titanium surfaces is suppressed. There may also have been an indirect effect of inhibited cytoskeletal development. In addition, cell attachment on C/Ti (1.0) titanium disks did not reach or even approach the level of cell attachment observed on C/Ti (0.3) surfaces after a longer cultivation time of 24 hr, which implies that the initial difference in biological potential may determine the subsequent bioactivity of titanium, potentially influencing osseointegration.
Some previous studies reported the biocompatibility of carbon deposited onto titanium substrates (Ianno et al., 1995; Mitura et al., 1996). Those authors suggested the better biocompatibility of carbon-coated titanium due to its high corrosion resistance, low friction, decreased wear metal particles, smoothness, or high mechanical strength. In those studies, crystalline diamond-like carbon adhered strongly to titanium substrates via sputtering and dense plasma methods. Although the method of carbon coating is quite different from ours, our present study showed the inconsistent results of low biocompatibility with osteoblasts by carbon deposition. One important thing was that these past studies did not perform biological experiments such as in vitro cellular response, though the targets of the studies were orthopedic prostheses or cardiovascular surgery. Therefore, issues such as the attachment and initial behavior of other cell types need to be addressed in future studies; the biological responses of mesenchymal stem cells and fibroblasts to carbon-coated titanium will be of particular interest.
Titanium constantly absorbs organic impurities, such as polycarbonyls and hydrocarbons, from the atmosphere, water, and cleaning solutions (Kilpadi et al., 2000; Serro and Saramago, 2003). The frequent detection of high levels of carbon on titanium surfaces indicates that such contamination may be unavoidable (Massaro et al., 2002; Buser et al., 2004). Previous reports have suggested a link between surface hydrocarbons and the hydrophilicity of titanium; the contact angle of H2O has been found to increase with increased absorption of hydrocarbons (Takeuchi et al., 2005). Oxygen species derived from O2 under air, which effectively increase hydrophilicity, are covered by hydrocarbon adsorption. It is thereby likely that the wettability declines by adsorption of organic molecules onto titanium dioxide surfaces. This phenomenon is consistent with the results of the present study, as indicated by elevation of the C1s peak accompanied by a reduction in the Ti2p and O1s peaks, as confirmed by XPS analysis. Generally, wettability is governed by the number of surface hydroxyl (OH) groups (Takeda et al., 1999). One study demonstrated that increased OH groups increased wettability and both cell adhesion protein adsorption and cell attachment (Arima and Iwata, 2007). A recent study also reported that protein immobilization was enhanced with an increased number of OH groups on TiO2 surfaces (Kim et al., 2009). Further, active surface OH groups dissociate in aqueous solution and form electric charges, which are governed by the pH of the surrounding solution (Boehm, 1971; Westall and Hohl, 1980). The electric charges play an important role in the immobilization of molecules such as cell adhesion protein (Gongadze et al., 2011). Thus, for the present study, functional OH groups may be inhibited by hydrocarbon masking, resulting in the reduction of the initial biological capacity of titanium, accompanied by the increase of contact angle.
In this study, untreated titanium disks were not used as a control because the C/Ti values for untreated disks varied from 0.2 to 0.7. This finding is consistent with the previous result measuring chemical composition from different implant surfaces, as mentioned in the Introduction (Morra et al., 2003). The results of the present study suggest that the amount of hydrocarbons adsorbed onto titanium surfaces at the time of implantation is crucial for determining the initial affinity for osteoblasts and consequently increasing the amount of bone-titanium integration. The titanium implants currently used clinically and experimentally have been found to be contaminated by hydrocarbons (Massaro et al., 2002; Morra et al., 2003; Takeuchi et al., 2005). The progressive accumulation of organic molecules on titanium surfaces cannot be avoided under ambient conditions and may explain the relatively low values of bone-titanium contact mentioned previously (Weinlaender et al., 1992; Berglundh et al., 2007). Detailed analyses of the surface energy, electric charge, and other physicochemical properties are also required to identify the mechanism underlying osseointegration; however, the results of this study suggest that removal of hydrocarbons may be an important step in promoting the bioactivity and osseointegration of titanium. Some studies have reported that ultraviolet treatment (Att et al., 2009), gamma-ray irradiation (Ueno et al., 2012), or storage in physiological saline solution (Buser et al., 2004; Zhao et al., 2005) could promote osteoblastic differentiation and growth factor production, thereby enhancing bone-implant integration while maintaining low carbon contamination of the implant surface. These methods can be used to decompose carbon or to prevent hydrocarbon contamination on titanium surfaces. An optimal method for surface enhancement needs to be developed to achieve better osseointegration.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24592903).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arima Y, Iwata H. (2007). Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 28:3074-3082. [DOI] [PubMed] [Google Scholar]
- Att W, Hori N, Iwasa F, Yamada M, Ueno T, Ogawa T. (2009). The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 30:4268-4276. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Abrahamsson I, Albouy JP, Lindhe J. (2007). Bone healing at implants with a fluoride-modified surface: an experimental study in dogs. Clin Oral Implants Res 18:147-152. [DOI] [PubMed] [Google Scholar]
- Boehm HP. (1971). Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss Faraday Soc 52:264-275. [Google Scholar]
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. (2004). Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 83:529-533. [DOI] [PubMed] [Google Scholar]
- Chuang SK, Wei LJ, Douglass CW, Dodson TB. (2002). Risk factors for dental implant failure: a strategy for the analysis of clustered failure-time observations. J Dent Res 81:572-577. [DOI] [PubMed] [Google Scholar]
- Couvrat P, Denis M, Langer M, Mitura S, Niedzielski P, Marciniak J. (1995). The corrosion tests of amorphous carbon coatings deposited by r.f. dense plasma onto steel with different chromium contents. Diamond and Related Materials 4:1251-1254. [Google Scholar]
- Endlich N, Endlich K. (2006). Stretch, tension and adhesion – adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol 85:229-234. [DOI] [PubMed] [Google Scholar]
- Gongadze E, Kabaso D, Bauer S, Slivnik T, Schmuki P, van Rienen U, et al. (2011). Adhesion of osteoblasts to a nanorough titanium implant surface. Int J Nanomedicine 6:1801-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori N, Ueno T, Minamikawa H, Iwasa F, Yoshino F, Kimoto K, et al. (2010). Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater 6:4175-4180. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. (2007). Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 179:1043-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianno NJ, Dillon RO, Ali A, Ahmad A. (1995). Deposition of diamond-like carbon on a titanium biomedical alloy. Thin Solid Films 270:275-278. [Google Scholar]
- Kilpadi DV, Lemons JE, Liu J, Raikar GN, Weimer JJ, Vohra Y. (2000). Cleaning and heat-treatment effects on unalloyed titanium implant surfaces. Int J Oral Maxillofac Implants 15:219-230. [PubMed] [Google Scholar]
- Kim WJ, Kim S, Lee BS, Kim A, Ah CS, Huh C, et al. (2009). Enhanced protein immobilization efficiency on a TiO2 surface modified with a hydroxyl functional group. Langmuir 25:11692-11697. [DOI] [PubMed] [Google Scholar]
- Li S, Tu Q, Zhang J, Stein G, Lian J, Yang PS, et al. (2008). Systemically transplanted bone marrow stromal cells contributing to bone tissue regeneration. J Cell Physiol 215:204-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lim JY, Donahue HJ, Dhurjati R, Mastro AM, Vogler EA. (2007). Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: phenotypic and genotypic responses observed in vitro. Biomaterials 28:4535-4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Junior PA, Alcantara CE, Resende RR, Ferreira AJ. (2013). Carbon nanotubes: directions and perspectives in oral regenerative medicine. J Dent Res 92:575-583. [DOI] [PubMed] [Google Scholar]
- Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, et al. (2002). Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J Mater Sci Mater Med 13:535-548. [DOI] [PubMed] [Google Scholar]
- Mierke CT. (2009). The role of vinculin in the regulation of the mechanical properties of cells. Cell Biochem Biophys 53:115-126. [DOI] [PubMed] [Google Scholar]
- Mitura E, Niedzielska A, Niedzielski P, Klimek L, Rylski A, Mitura S, et al. (1996). The properties of carbon layers deposited onto titanium substrates. Diamond and Related Materials 5:998-1001. [Google Scholar]
- Morra M, Cassinelli C, Bruzzone G, Carpi A, Di Santi G, Giardino R, et al. (2003). Surface chemistry effects of topographic modification of titanium dental implant surfaces: 1. Surface analysis. Int J Oral Maxillofac Implants 18:40-45. [PubMed] [Google Scholar]
- Ogawa T, Nishimura I. (2003). Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int J Oral Maxillofac Implants 18:200-210. [PubMed] [Google Scholar]
- Serro AP, Saramago B. (2003). Influence of sterilization on the mineralization of titanium implants induced by incubation in various biological model fluids. Biomaterials 24:4749-4760. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamamoto K, Hayasaka Y, Matsumoto K. (1999). Surface OH group governing wettability of commercial glasses. J Non-Crystalline Solids 249:41-46. [Google Scholar]
- Takeuchi M, Sakamoto K, Martra G, Coluccia S, Anpo M. (2005). Mechanism of photoinduced superhydrophilicity on the TiO2 photocatalyst surface. J Phys Chem B 109:15422-15428. [DOI] [PubMed] [Google Scholar]
- Tu Q, Valverde P, Li S, Zhang J, Yang P, Chen J. (2007). Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng 13:2431-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Takeuchi M, Hori N, Iwasa F, Minamikawa H, Igarashi Y, et al. (2012). Gamma ray treatment enhances bioactivity and osseointegration capability of titanium. J Biomed Mater Res B Appl Biomater 100:2279-2287. [DOI] [PubMed] [Google Scholar]
- Weinlaender M, Kenney EB, Lekovic V, Beumer J, 3rd, Moy PK, Lewis S. (1992). Histomorphometry of bone apposition around three types of endosseous dental implants. Int J Oral Maxillofac Implants 7:491-496. [PubMed] [Google Scholar]
- Wen KK, Rubenstein PA, DeMali KA. (2009). Vinculin nucleates actin polymerization and modifies actin filament structure. J Biol Chem 284:30463-30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall J, Hohl H. (1980). A comparison of electrostatic models for the oxide/solution interface. Adv Colloid Interf Sci 12:265-294. [Google Scholar]
- Zareidoost A, Yousefpour M, Ghaseme B, Amanzadeh A. (2012). The relationship of surface roughness and cell response of chemical surface modification of titanium. J Mater Sci Mater Med 23:1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. (2005). High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A 74:49-58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.