Abstract
Activity of acetate kinase in cell-free extracts and individual fractions and the kinetic properties of the enzyme obtained from the Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9 intestinal bacterial strains were presented at the first time. The highest activity of the enzyme was measured in the cell-free extracts (1.52 ± 0.163 and 0.46 ± 0.044 U × mg-1 protein for D. piger Vib-7 and Desulfomicrobium sp. Rod-9, respectively) compared to other fractions. The specific activity of acetate kinase in the extracts of both bacterial strains was determined at different temperature and pH. Analysis of the kinetic properties of the purified acetate kinase was carried out. The acetate kinase activity, initial (instantaneous) reaction rate (V0) and maximum rate of the acetate kinase reaction (Vmax) in D. piger Vib-7 and Desulfomicrobium sp. Rod-9 intestinal bacterial strains were defined. Michaelis constants (KmAcetyl phosphate and KmADP) of the enzyme reaction (2.54 ± 0.26 and 2.39 ± 0.24 mM for D. piger Vib-7 as well as 2.68 ± 0.25 and 2.47 ± 0.27 mM for Desulfomicrobium sp. Rod-9, respectively) were calculated. The described results of acetate kinase, an important enzyme in the process of organic compounds oxidation and dissimilatory sulfate reduction would be perspective and useful for clarification of the etiological role of these bacteria in the development of inflammatory bowel diseases in humans and animals.
Keywords: Acetate kinase, inflammatory bowel diseases, kinetic analysis, sulfate-reducing bacteria.
INTRODUCTION
Intestinal sulfate-reducing bacteria consume the organic compounds as a carbon source and electron donor in the process of dissimilatory sulfate reduction [1]. The species of Desulfovibrio and Desulfomicrobium genera oxidize these compounds incompletely to acetate via pyruvate and other intermediates [2]. The process of organic compounds oxidation is a complex and multistage that provides the bacterial cells with energy. The lactate is the most common substrate used by the species belonging to the intestinal sulfate-reducing bacteria [3].
In previous researches, it was demonstrated that the lactate consumption by the intestinal sulfate-reducing bacteria D. piger Vib-7 and Desulfomicrobium sp. Rod-9 and acetate accumulation in cultivation medium [4]. Lactate oxidation to acetate occurs with the intermediate compounds formation: pyruvate, acetyl-CoA and acetyl phosphate [1, 2, 5].
Acetate kinase (ATP:acetate phosphotransferase, EC 2.7.2.1) plays a significant role in energy production and catalyzes the formation of acetate from acetyl phosphate. This enzyme is involved in the synthesis of most of the ATP formed catabolically [1, 6, 7]:
Lactate oxidation to acetate occurs together with the concurrent reduction of sulfate to sulfide [1, 2]. In the presence of lactate and sulfate in the human intestine contributes to the intensive bacteria growth and the accumulation of their final metabolism products, acetate and hydrogen sulfide, which are toxic and mutagenic to epithelial intestinal cells [3, 8]. The increased number of the sulfate-reducing bacteria and intensity of dissimilatory sulfate reduction in the gut can cause inflammatory bowel diseases of humans and animals [3, 8-10].
Acetate kinase from intestinal sulfate-reducing bacteria D. piger and Desulfomicrobium has never been well-characterized. In literature, there are some data on acetate kinase in various organisms as well as in the sulfate-reducing bacteria isolated from environment [6, 11-18]. However, the data on the activity and the kinetic properties of this enzyme from intestinal sulfate-reducing bacteria Desulfovibrio piger and Desulfomicrobium sp. has not been reported yet.
The aim of this work was to study acetate kinase activity in cell-free extracts of intestinal sulfate-reducing bacteria D. piger Vib-7 and Desulfomicrobium sp. Rod-9 and to carry out the kinetic analysis of enzymatic reaction.
MATERIALS AND METHODS
Objects of the study were sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9 isolated from the human large intestine and identified by the sequence analysis of the 16S rRNA gene [4, 19].
Bacterial Growth and Cultivation
Bacteria were grown in a nutrition-modified Kravtsov-Sorokin's liquid medium [4]. Before seeding bacteria in the medium, 0.05 ml/l of sterile solution of Na2S×9H2O (1%) was added. A sterile 10N solution of NaOH (0.9 ml/l) in the medium was used to provide the final pH 7.2. The medium was heated in boiling water for 30 min in order to obtain an oxygen-free medium, and then cooled to +30°C. The bacteria were grown for 72 hours at +37°C under anaerobic conditions. The tubes were brim-filled with medium and closed to provide anaerobic conditions.
Obtaining Cell-free Extracts
Cells were harvested at the beginning of the stationary phase, centrifuged and suspended in 100 ml of 50 mM Tris(hydroxymethyl)aminomethane (Tris)-hydrochloride, pH 7.0 (henceforth referred to as Tris buffer), containing 1 mM ethylenediaminetetraacetate (EDTA). A suspension of cells (150–200 mg/ml) was obtained and homogenized using the ultrasonic disintegrator at 22 kHz for 5 minutes at 0 °C to obtain cell-free extracts. The homogenate was centrifuged for 20 min at 16,000 g to remove the cell debris. The pellet was then used as sedimentary fraction, and the supernatant obtained was termed the soluble fraction. The supernatant fluid and a Tris buffer wash of the pellet were subjected to a second centrifugation at 16,000 g for 40 min [20]. The soluble extract constituted by the supernatant was used as the source of the enzyme. A pure supernatant, containing the soluble fraction, was then used as cell-free extract. Protein concentration in the cell-free extracts was determined by the Lowry method [21].
Assays for Acetate Kinase Activity
The acetate kinase activity was assayed colorimetrically as described previously in paper [17]. The reaction mixture of 1 ml contained 50 µmol imidazole buffer of pH 7.3, 800 µmol potassium acetate, 10 µmol ATP, 20 µmol MgCl2, 200 µmol neutralized hydroxylamine and 0.5–1.0 U of acetate kinase dissolved in 0.1 M phosphate buffer of pH 7.4 containing 0.005 M cysteine. After incubation for l0 min at 29°C the reaction was stopped by the addition of 1.0 ml 10% trichloroacetic acid. For the immobilization screening and storage stability tests, 1.0 ml of the same reaction mixture was pipetted onto the enzyme-loaded glass beads. After reaction for 10 min at 29°C the glass beads were allowed to settle and 800 µl of the supernatant was added to 1.0 ml 10% trichloroacetic acid. For both assays colour was developed by adding 4.0 ml of 1.25% FeCl3 in 1.0 M HCl and absorbance was measured at 510 nm. The enzyme was also purified as described previously in paper [6]. One unit of acetate kinase is defined as that amount of acetate kinase which forms one micromole of acethydroxamic acid per minute under the conditions described. Specific enzyme activity was expressed as U × mg-1 protein. The specific activity of the studied enzyme in the cell-free extracts of both bacterial strains under the effect of different temperature (+20, +25, +30, +35, +40, +45°C) and pH (5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, 10.0) in the incubation medium was measured.
Kinetic Analysis
Kinetic analysis of the enzyme reaction was performed in a standard incubation medium (as it was described above) with modified physical and chemical characteristics of the respective parameters (the incubation time, substrate concentration, temperature and pH). The kinetic parameters characterizing the acetate kinase reaction are the initial (instantaneous) reaction velocity (V0), maximum velocity of the reaction (Vmax), maximum amount of the reaction product (Pmax) and characteristic reaction time (time half saturation) τ were determined. The amount of the reaction product was calculated stoichiometrically. The kinetic parameters characterizing acetate kinase reactions such as Michaelis constant (Km) and maximum reaction velocity of substrate decomposition were determined by Lineweaver-Burk plot [22]. For analysis of the substrate kinetic mechanism of acetate kinase, initial velocities were measured under standard assay conditions with different substrate concentrations. The resulting data were also analyzed by global curve fitting in SigmaPlot to model the kinetic data for rapid equilibrium rate equations describing ordered sequential, V=(Vmax [A] [B])/(KA KB+KB [A]+[A] [B]), and random sequential, V=(Vmax [A] [B])/(α KA KB+KB [A]+KA [B]+[A] [B]), kinetic mechanisms, where V is the initial velocity, Vmax is the maximum velocity, KA and KB are the Km values for substrates A and B, respectively, and α is the interaction factor if the binding of one substrate changes the dissociation constant for the other [23].
Statistical Analysis
Kinetic and statistical calculations of the results were carried out using the software MS Office and Origin computer programs. The research results were treated by the methods of variation statistics using Student t-test. The equation of the straight line that the best approximates the experimental data was calculated by the method of least squares. The absolute value of the correlation coefficient r was from 0.90 to 0.98. The statistical significance of the calculated parameters of line was tested by the Fisher’s F-test. The accurate approximation was when P ≤ 0.05 [24].
RESULTS AND DISCUSSION
Specific activity of acetate kinase, an important enzyme in the process of organic compounds oxidation in intestinal sulfate-reducing bacteria, was measured in different fractions obtained from D. piger Vib-7 and Desulfomicrobium sp. Rod-9 cells (Table 1).
Table 1.
Acetate kinase activity in different fractions obtained from the bacterial cells.
| Sulfate-reducing bacteria | Specific activity of the enzyme (U × mg-1 protein) | ||
|---|---|---|---|
| Cell-free extract | Individual fractions | ||
| Soluble | Sedimentary | ||
| Desulfovibrio piger Vib-7 | 1.52 ± 0.163 | 1.14 ± 0.118 | 0 |
| Desulfomicrobium sp. Rod-9 | 0.46 ± 0.044*** | 0.27 ± 0.029*** | 0 |
Comment: The assays were carried out at a protein concentration of 41.17 mg/ml (for D. piger Vib-7) and 38.12 mg/ml (for Desulfomicrobium sp. Rod-9). Enzyme activity was determined after 10 min incubation. Statistical significance of the values M ± m, n = 5; ***P<0.001, compared to D. piger Vib-7 strain.
Results of the study showed that the highest activity of the enzyme was measured in cell-free extracts (1.52 ± 0.163 and 0.46 ± 0.044 U × mg-1 protein for D. piger Vib-7 and Desulfomicrobium sp. Rod-9, respectively). The slightly lower values of activity of acetate kinase were determined in the soluble fraction compared to cell-free extracts. Its values designated 1.14 ± 0.118 U × mg-1 protein for D. piger Vib-7 and 0.27 ± 0.029 U × mg-1 protein for Desulfomicrobium sp. Rod-9. The enzyme activity was not observed in sedimentary fraction.
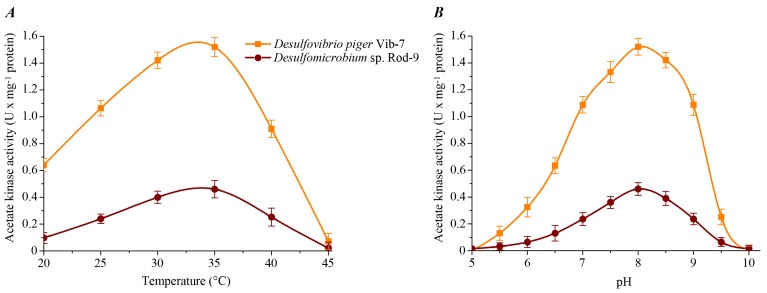
The effect of temperature and pH of the reaction mixture on acetate kinase activity in the cell-free extracts of the sulfate-reducing bacteria was studied (Fig. 1). The enzyme activity exhibited typical bell-shaped curves as a function of temperature and pH. The maximum specific activity for both bacterial strains was determined at +35ºC. The highest enzyme activity of acetate kinase for D. piger Vib-7 and Desulfomicrobium sp. Rod-9 was measured at pH 8.0.
Fig. (1).
The effect of temperature (A) and pH (B) on the acetate kinase activity in the cell-free extracts of the intestinal sulfate-reducing bacteria.
Thus, temperature and pH optimum of this enzyme was +35ºC and pH 8.0, respectively. An increasing or decreasing of temperature and pH led to a decrease of the activity of studied enzyme in the cell-free bacterial extracts of the sulfate-reducing bacteria.
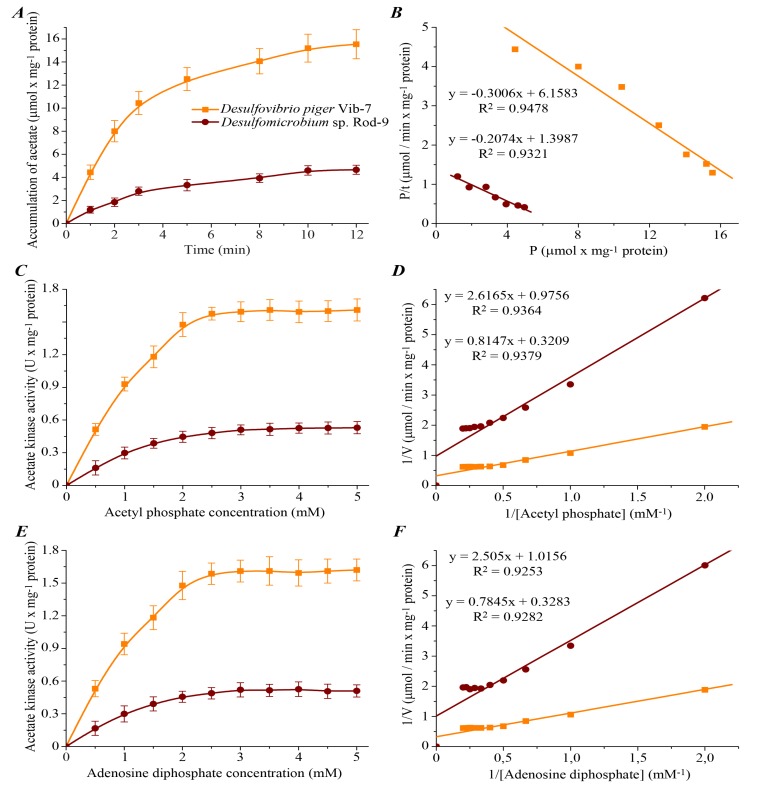
To study the characteristics and mechanism of acetate kinase reaction, the initial (instantaneous) reaction velocity (V0), maximum velocity of the reaction (Vmax), maximum amount of reaction product (Pmax) and reaction time (τ) were defined. Dynamics of reaction product accumulation was studied for investigation of the kinetic parameters of acetate kinase (Fig. 2).
Fig. (2).
Kinetic parameters of acetate kinase activity in D. piger Vib-7 and Desulfomicrobium sp. Rod-9: A – dynamics of product accumulation (M ± m, n = 5); B – linearization of curves of product accumulation in {P/t; P} coordinates (n = 5; R2 > 0.93; F <0.02); C, E – the effect of different concentrations of substrate (acetyl phosphate and adenosine diphosphate) on the enzyme activity (M ± m, n = 5); D, F – linearization of concentration curves, which are shown in fig. 2C, E, in the Lineweaver-Burk plot, where V is velocity of the enzyme reaction and [Acetyl phosphate] or [Adenosine diphosphate] is substrate concentration (n = 5; R2 > 0.92; F < 0.005).
Experimental data showed that the kinetic curves of acetate kinase activity have tendency to saturation (Fig. 2A). Analysis of the results allows to reach the conclusion that the kinetics of acetate kinase activity in the sulfate-reducing bacteria was consistent to the zero-order reaction in the range of 0–3 min (the graph of the dependence of product formation on the incubation time was almost linear in this interval of time). Therefore the duration of the incubation of bacterial cells extracts was 3 min in subsequent experiments.
Amount of product of acetate kinase reaction in the D. piger Vib-7 was the higher (20.48 ± 2.51 µmol × mg-1 protein) compared to the Desulfomicrobium sp. Rod-9 (6.74 ± 0.68 µmol × mg-1 protein) in the entire range of time factor. The basic kinetic properties of the reaction in the sulfate-reducing bacteria were calculated by linearization of the data in the {P/t; P} coordinates (Fig. 2B, Table 2).
Table 2.
Kinetic parameters of the acetate kinase from intestinal sulfate-reducing bacteria.
| Kinetic parameters | Sulfate-reducing bacteria | |
|---|---|---|
| Desulfovibrio piger Vib-7 | Desulfomicrobium sp. Rod-9 | |
| V0 (µmol × min-1 × mg-1 protein) | 6.16 ± 0.63 | 1.39 ± 0.14*** |
| Pmax (µmol × mg-1 protein) | 20.48 ± 2.51 | 6.74 ± 0.68*** |
| τ (min) | 3.33 ± 0.34 | 4.82 ± 0.49 |
Comment: V0 is initial (instantaneous) reaction velocity; Pmax is maximum amount (plateau) of the product of reaction; is the reaction time (half saturation period). Statistical significance of the values M ± m, n = 5; ***P<0.001, compared to the D. piger Vib-7 strain.
The kinetic parameters of acetate kinase from both D. piger Vib-7 and Desulfomicrobium sp. Rod-9 were significantly different. Values of initial (instantaneous) reaction velocity (V0) for the enzyme was calculated by the maximal amount of the product reaction (Pmax). As shown in Table 2, V0 for acetate kinase reaction was slightly higher (6.16 ± 0.63 µmol × min-1 × mg-1 protein) in D. piger Vib-7 compared to Desulfomicrobium sp. Rod-9 (1.39 ± 0.14 µmol × min-1 × mg-1 protein). In this case, the values of the reaction time (τ) were 3.33 ± 0.34 and 4.82 ± 0.49 minutes for D. piger Vib-7 and Desulfomicrobium sp. Rod-9, respectively. Based on these data, we may assume that the D. piger Vib-7 can consume lactate ion much faster in their cells than a Desulfomicrobium sp. Rod-9. Moreover, this hypothetical assumption can be also confirmed by obtained data on maximal velocities of accumulation of the final reaction products, where Vmax for enzyme reaction in D. piger Vib-7 were also more intensively compared to Desulfomicrobium sp. Rod-9 (Table 3).
Table 3.
Kinetic parameters of acetate kinase reaction.
| Kinetic parameters | Sulfate-reducing bacteria | |
|---|---|---|
| Desulfovibrio piger Vib-7 | Desulfomicrobium sp. Rod-9 | |
| VmaxAcetyl phosphate (µmol × min-1 × mg-1 protein) | 3.12 ± 0.32 | 1.03 ± 0.098*** |
| KmAcetyl phosphate (mM) | 2.54 ± 0.26 | 2.68 ± 0.25 |
| VmaxADP (µmol × min-1 × mg-1 protein) | 3.05 ± 0.31 | 0.98 ± 0.095*** |
| KmADP (mM) | 2.39 ± 0.24 | 2.47 ± 0.27 |
Comment: Vmax is maximum velocity of the enzyme reaction; Km is Michaelis constant which was determined by substrate (acetyl phosphate and ADP). Statistical significance of the values M ± m, n = 5; ***P<0.001, compared to the D. piger Vib-7 strain.
The kinetic analysis of acetate kinase activity dependence on concentration of substrate (acetyl phosphate and ADP) was carried out. The increasing of acetyl phosphate and ADP concentrations from 0.5 to 5.0 mM caused a monotonic rise of the studied enzyme activity and the activity was maintained on unchanged level (plateau) under substrate concentrations over 2.5 mM (Fig. 2C, E). Curves of the dependence {1/V; 1/[S]} were distinguished by the tangent slope and intersect the vertical axis in one point (Fig. 2D,F). The basic kinetic parameters of acetate kinase activity in D. piger Vib-7 and Desulfomicrobium sp. Rod-9 were identified by linearization of the data in the Lineweaver-Burk plot (Table 3).
Calculation of the kinetic parameters of enzyme activity indicates that the maximum velocities (Vmax) of acetylphosphate and ADP in the D. piger Vib-7 and Desulfomicrobium sp. Rod-9 were significantly different from each other. It was observed a correlative relationship between VmaxAcetyl phosphate and VmaxADP as well as KmAcetyl phosphate and KmADP in both intestinal bacterial strains. Michaelis constants (KmAcetyl phosphate and KmADP) of the enzyme reaction (2.54 ± 0.26 and 2.39 ± 0.24 mM for D. piger Vib-7 as well as 2.68 ± 0.25 and 2.47 ± 0.27 mM for Desulfomicrobium sp. Rod-9, respectively) were calculated.
Acetate kinase, discovered in 1944 by Lipmann (1944), is one of the earliest phosphoryl transfer enzymes recognized [25]. Following the first purification in 1954 from E. coli [17], the enzyme was the subject of investigations leading to two proposals for the catalytic mechanism: a direct in-line transfer of the γ-phosphoryl group of ATP to acetate [11, 18] or a triple-displacement mechanism involving two covalent phosphoenzyme intermediates [26]. The acetate kinase was also assayed and purified from cell extract of Methanosarcina thermophila by Ferry (2011). Cell extract from acetate-grown the cells was prepared for purification of acetate kinase [12]. This enzyme from M. thermophila was evaluated for detection of acetate in various biological fluids using the hydroxamate assay [14]. Acetate kinase had nearly an eightfold lower Km for acetate (<3 mM) than the wild type which was lower than those reported for commercially available acetate kinase from Bacillus stearothermophilus (120 mM) [16] or E. coli (7–300 mM) [13, 16] increasing the sensitivity [12].
The enzyme acetate kinase was also purified from Desulfovibrio vulgaris by a combination of ammonium sulfate precipitation, hydroxylapatite and dye-affinity chromatography. The specific activity in crude extract supernatant from D. vulgaris was 1.6 U/mg of protein [6, 15]. These data are consistent to the obtained results for the specific activity in cell-free extract from D. piger Vib-7 where the activity was 1.52 ± 0.163 U × mg-1 protein.
Yu and coauthors (2001) have described two distinct forms of acetate kinase which were purified to homogeneity from a sulfate-reducing bacterium Desulfbvibrio vulgaris Miyazaki F [7]. The enzymes were separated from the soluble fraction of the cells. Total activity in the crude extract from D. vulgaris was 1.252 µmol/min. The dependence of initial velocity of AKI as measured by the rate of ATP formation on the ADP concentration under the fixed acetyl phosphate concentration of 2.5 mM. In the presence of fixed acetyl phosphate concentrations of 0.5, 1.0, 2.5, and 5.0 mM, the dependence of initial velocity of AK-I and AK-II on the ADP concentration was examined [7].
Acetate kinase from an thermophile, B. stearothermophilus, was purified and crystallized by Nakajima et al. (1978). This enzyme shared many common enzymatic properties with the counterpart from mesophiles, i.e. pH optimum, substrate specificity, requirement of metal ions and essential amino acid residues necessary for the catalytic activity. However, this enzyme was remarkably thermostable [16].
Thus, the acetate kinase, an important enzyme in process of dissimilatory sulfate reduction and lactate oxidation in sulfate-reducing bacteria, carries out the central step in the formation of acetate from acetyl phosphate.
CONCLUSION
The acetate kinase activity, initial (instantaneous) reaction velocity and maximum velocity of the enzyme reaction were significantly higher in the D. piger Vib-7 cells than Desulfomicrobium sp. Rod-9. The maximum enzyme activity for both strains was determined at +35ºC and at pH 8.0. The kinetic parameters of enzyme reaction depended on substrate concentration. Michaelis constants for acetyl phosphate and ADP were quite similar (2.54 ± 0.26 and 2.39 ± 0.24 mM for D. piger Vib-7 as well as 2.68 ± 0.25 and 2.47 ± 0.27 mM for Desulfomicrobium sp. Rod-9, respectively) in both intestinal bacterial strains. The studies of the acetate kinase in the process of dissimilatory sulfate reduction and kinetic properties of this enzyme in the D. piger Vib-7 and Desulfomicrobium sp. Rod-9, their production of acetate in detail may be perspective for clarification of their etiological role in the development in pathogenesis of the humans and animals bowel diseases. These studies might help in predicting the development of diseases of the gastrointestinal tract, by providing further details on the etiology of bowel diseases which are very important for the clinical diagnosis of these disease types.
ACKNOWLEDGEMENTS
The author expresses his gratitude to Dr. Roman Fafula, MD, Ph.D. from Biophysics Department of Faculty of Pharmacy, Danylo Halytsky Lviv National Medical University (Ukraine) for his assistance in performing the kinetic analysis and critical reading of the manuscript.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Barton LL, Hamilton WA, editors. Environmental and Engineered Systems. Cambridge Cambridge University Press. 2010. Sulphate-Reducing Bacteria. [Google Scholar]
- 2.Kushkevych IV. Sulfate-reducing bacteria of the human intestine. I.; Dissimilatory sulfate reduction. Studia Biologica. 2012;6(1):149–80. [Google Scholar]
- 3.Kushkevych IV. Sulfate-reducing bacteria of the human intestine. II.; The role in the diseases development. Studia Biologica. 2012;6(2):221–50. [Google Scholar]
- 4.Kushkevych IV. Identification of sulfate-reducing bacteria strains of human large intestine. Studia Biologica. 2013;7(3):115–24. [Google Scholar]
- 5.Sadana JC. Pyruvate oxidation in Desulphovibrio desulphuricans. J Bacteriol. 1954;67(5):547–53. doi: 10.1128/jb.67.5.547-553.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannens G, Slegers G, Claeys A. Purification and immobilization of acetate kinase from Desulfovibrio vulgaris. Biotech Lett. 1988;10(8):563–8. [Google Scholar]
- 7.Yu L, Ishida T, Ozawa K , et al. Purification and characterization of homo- and hetero-dimeric acetate kinases from the sulfate-reducing bacterium desulfbvibrio vulgaris. J Biochem. 2001;129:411–21. doi: 10.1093/oxfordjournals.jbchem.a002872. [DOI] [PubMed] [Google Scholar]
- 8.Pitcher MC, Cummings JH. Hydrogen sulphide a bacterial toxin in ulcerative colitisκ. Gut. 1996;39:1 – 4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings JH, Macfarlane GT, Macfarlane S. Intestinal bacteria and ulcerative colitis. Curr Issues Intest Microbiol. 2003;4:9–20. [PubMed] [Google Scholar]
- 10.Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate-reducing bacteria in gut contents of health subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991;86:103–12. [Google Scholar]
- 11.Blattler WA, Knowles Jr. Stereochemical course of phosphokinases.The use of adenosine [gamma-(S)-16O 17O 18O] triphosphate and the mechanistic consequences for the reactions catalyzed by glycerol knasehexokinase, pyruvate kinase, and acetate kinase. Biochem. 1979;18:3927–33. doi: 10.1021/bi00585a013. [DOI] [PubMed] [Google Scholar]
- 12.Ferry JG. Acetate kinase and phosphotransacetylase. Methods Enzymol. 2011;494:219–31. doi: 10.1016/B978-0-12-385112-3.00011-1. [DOI] [PubMed] [Google Scholar]
- 13.Fox DK, Roseman S. Isolation and characterization of homogeneous acetate kinase from Salmonella typhimurium and Escherichia coli. J Biol Chem. 1986;261:13487–97. [PubMed] [Google Scholar]
- 14.Iyer P, Ferry JG. Acetate kinase from Methanosarcina thermophila, a key enzyme for methanogenesis. Methods Biotech. 2005;17:239–46. [Google Scholar]
- 15.Mannens G, Slegers G, Lambrecht R. Immobilization of acetate kinase and phosphotransacetylase on derivatized glass beads. Enzyme Microb Technol. 1987;9:285–90. [Google Scholar]
- 16.Nakajima H, Suzuki K, Imahori K. Purification and properties of acetate kinase from Bacillus stearothermophilus. J Biochem. 1978;84:193–203. doi: 10.1093/oxfordjournals.jbchem.a132108. [DOI] [PubMed] [Google Scholar]
- 17.Rose IA, Grunberg-Manago M, Korey SR, Ochoa S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954;211(2):737–56. [PubMed] [Google Scholar]
- 18.Skarstedt MT, Silverstein E. Escherichia coli acetate kinase mechanism studied by net initial rate, equilibrium, and independent isotopic exchange kinetics. J Biol Chem. 1976;251:6775–83. [PubMed] [Google Scholar]
- 19.Kushkevych IV, Bartos M, Bartosova L. Sequence analysis of the 16S rRNA gene of sulfate-reducing bacteria isolated from human intestine. Int J Curr Microbiol Appl Sci. 2014;3(2):239–48. [Google Scholar]
- 20.Robinson Jr, Sagers RD. Phosphotransacetylase from Clostridium acidiurici. J Bacteriol. 1972;112(1):465–73. doi: 10.1128/jb.112.1.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein determination with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Keleti T, Kramer M. Basic Enzyme Kinetics. Akademiai Kiado. 1988 [Google Scholar]
- 23.Segal IH, editor. John Wiley & Sons New York. 1975. Enzyme kinetics behavior and analysis of rapid equilibrium and steady-state enzyme systems. [Google Scholar]
- 24.Bailey NTJ, editor. Cambridge Cambridge University Press. 1995. Statistical methods in biology. [Google Scholar]
- 25.Lipmann F. Enzymatic synthesis of acetyl phosphate. J Biol Chem. 1944;155:55–70. [Google Scholar]
- 26.Spector LB. Acetate kinase A triple-displacement enzyme. Proc Natl Acad Sci USA. 1980;77:2626–30. doi: 10.1073/pnas.77.5.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]