Abstract
High Density Lipoprotein (HDL) has been witnessed to possess a range of different functions that contribute to its atheroprotective effects. These functions are: the promotion of macrophage cholesterol efflux, reverse cholesterol transport, anti-inflammatory, anti-thrombotic, anti-apoptotic, pro-fibrinolytic and anti-oxidative functions. Paraoxonase 1 (PON1) is an HDL associated enzyme esterase/homocysteinethiolactonase that contributes to the anti-oxidant and anti-atherosclerotic capabilities of HDL. PON1 is directly involved in the etiopathogenesis of atherosclerosis through the modulation of nitric oxide (NO) bioavailability. The aim of this review is to summarize the role of HDL on endothelial homeostasis, and also to describe the recently characterized molecular pathways involved.
Keywords: Endothelial function, high-density lipoprotein, homocysteine, nitric oxide, oxLDL, oxidative stress, paraoxonase.
INTRODUCTION
Atherosclerosis is the leading cause of death in old adults. The vascular endothelial cell is the master goal for pathological or mechanical injuries caused by the putative risk factors of the atherosclerotic process, which include smoking, increased systolic blood pressure and total cholesterol, and decreased high density lipoprotein (HDL) cholesterol [1].
The recent academic contributions to the existing literature present the highlights of the extensive shift in the understanding of atherosclerotic disease. The old concept was quite simple: HDL is good, and therefore increase the amount of HDL. Unfortunately, the concept is not this simple. Despite having high levels of HDL, subjects that participated in a study experienced unexpected complications. After an in-depth investigation, we have reached a conclusion: “HDL-functionality”. The term “HDL-functionality” was introduced in the mid-90s [1-3].
At the presence of causative factors, the putative protector HDL becomes potentially pro-atherogenic. “Dysfunctional-HDL” is a profile where endothelial-protective properties of the particle are markedly impaired [1]. Various mechanisms may lead to these non-functional endothelial effects of HDL in patients with atherosclerotic process, including the oxidative modification of HDL-associated proteins, such as PON-1, lipids or HDL-bound sphingosine-1-phosphate (S1P), and alterations of HDL-proteome [4-9]. “Normal–HDL” from healthy subjects have been observed to exert potential direct anti-atherogenic effects by modulating these vascular endothelial functions [4-6]. The revision of vascular endothelial functions, including reduced eNOS coupling and loss of NO bioavailability, increased endothelial cell apoptosis, and pro-thrombotic activation is thought to add to the pathophysiology of atherosclerosis [6].
The aim of this study is to summarize the recent data on nitric oxide (NO), and also to make connections between different findings to understand the concept as a whole. We focus on HDL-associated antioxidant enzyme PON1 and oxLDL, and search for clues for the impact of “HDL- functionality “on the endothelial dysfunction.
Multifaceted High-density Lipoprotein
Lipoproteins play a pivotal role in the pathogenesis of atherosclerosis. The lipid rich “α-globulin” from serum was introduced in 1929 [1, 2, 10]. The particle has later become the popular HDL [1, 2]. HDL has been observed to have a range of different functions that contribute to its atheroprotective effects. These effects are: the promotion of macrophage cholesterol efflux, reverse cholesterol transport [RCT], anti-inflammatory, anti-thrombotic, anti-apoptotic, pro-fibrinolytic and anti-oxidative functions [1, 3, 4].
The first HDL-associated protein fraction was defined in the late 1960’s. By the early 1990’s, HDL was generally thought to contain approximately 15 proteins. Currently, up to more than 200 individual proteins have been detected in human HDL samples [1, 5-11].
The enormous functional heterogeneity innate to HDL is determined in large part by its compositional heterogeneity [9]. Recently, academic studies indicate that the HDL proteome can change in a variety of disease states, and these modifications are often related to the proteomic analyses of HDL- function [9-11].
Serum HDL-cholesterol concentration measurements fall short to suggest the functions and composition of HDL, and this is considered to be the key point that creates contradictions in previous studies. Commonly used definitions of HDL are listed in Table 1 [1, 6-10].
Table 1.
New definitions of HDL.
| “normal-HDL” |
| “dsyfunctional –HDL” |
| “HDL- dysfunction” |
| “HDL- malformed” |
| “Healthy -HDL” |
| “coronary artey disease-HDL” |
| “chronic kidney disease-HDL” |
Anti-oxidative Function of High Density Lipoprotein
The classical function of HDL is reverse cholesterol transport (RCT). The major HDL apolipoprotein A-I (apoA-I) binds to the high affinity HDL soluble receptor –B1 (SR-BI) of the target tissue [12].
HDL has well reported anti-oxidative properties. HDL has been observed to anticipate oxidative modification of LDL, thus reducing macrophage foam cell generation in a vessel’s wall [13]. Oxidatively damaged proteins and lipid peroxidation products have been shown to accumulate in the vascular endothelium of atherosclerotic diseases, such as acute coronary sydrome and stroke, and oxidized lipoprotein is considered to be toxic and endothelial–degenerative. Hence, oxLDL is the main cause in endothelial dysfunction. oxLDL induces endothelial damage, monocyte adhesion, platelet agregation and inhibits apoptosis and eNOS expression/ activity, all of which contribute to atherosclerotic process [14]. HDL can counter-attack LDL induction of platelet aggregation, serotonin release, thromboxane B2 production and can inhibit oxLDL inhibition of eNOS [11-15].
The exact anti-oxidant mechanism and endogen substrate of the PON1 enzyme is still unknown [16]. The incubation of purified PON1 with hydrogen peroxide or lipid peroxides partly decomposes them. PON1 may interact with apolipoprotein A-I and lecithin cholesterol acly –transferases (LCAT) to decrease LDL oxidation, with the combination preventing LCAT inactivation. In addition, purified PON1 protects HDL and LDL from oxidation catalyzed by copper ions [16-22].
Endothelial Dsyfunction and oxLDL
NO plays a role in a number of different significant biological processes, which are the avoiding of vascular thrombosis, interception of inflammatory cell injury, and arrangement of endothelial harmony and cell proliferation. NO is generated by incubating endothelial cells with L-arginine by means of eNOS. However, to determine NO as the marker of endothelial dysfunction may be insufficient. Therefore, endothelial dysfunction should evaluate NO along with HDL dysfunction and oxLDL [23, 24].
OxLDL are powerfull inducers of endothelial dysfunction. Protective effects of HDL on endothelial function are quite likely due to their capacity to counteract the effects of oxLDL [1, 12]. A decreased serum HDL level is an independent predictor of endothelial dysfunction in atherosclerosis [25].
A reduced NO bioavailability is a pronounced hallmark of endothelial dysfunction. Injury to vascular endothelium induces the expression of cell adhesion molecules (CAMs), such as vascular cell adhesion molecule-1, inter-cellular adhesion molecule-1, E-selectin, and P-selectin [25-33], whereas HDL down regulate TNF-α-induced CAMs expression in vascular endothelial cells [34-36]. Recently, studies have shown that dysfunctional-HDL reduced endothelial NO availability via toll-like receptor-2 [TLR-R-2], leading to impaired endothelial repair in patients with kidney disease [37, 38].
HDL-associated PON1 enzyme activity is directly involved in the pathogenesis of endothelial dsyfunction by the modulation of NO bioavailability [25]. Several basic mechanisms have been advanced for HDL-associated enzyme PON1’s anti-atherogenic effects, incorporating the capability of HDL to inhibit inflammation and regulate NO production by endothelial cells [25].
The findings of a recent study are of concern: normal-HDL from healthy subjects caused an increase in bioavailable eNO, while HDL from patients with atherosclerotic diseases caused no increase or an actual decrease in eNO [25-28].
Normal-HDL from healthy subjects activates the production of the anti-atherosclerotic and anti-thrombotic signaling molecule NO by eNOS [25-27].
Normal-HDL includes the anti-oxidant enzyme PON1, which suppresses the formation of oxidized lipids and lipoproteins, such as MDA [1-6]. In contrast, “Dsyfunctional-HDL” has a decreased PON1 enzyme activity that potentially causes a greater production of MDA, which activates the lectin-like oxidized LDL receptor-1 (LOX-1), and thereby stimulates PKCß [6, 38]. The LOX-1 is an oxLDL receptor expressed in vascular endothelium, and a multiligand receptor implicated in endothelial dysfunction and atherosclerosis [38-41]. It is also unknown whether the loss in PON1 enzyme activity leads to alterations in other HDL constituents besides MDA that activate LOX-1 [6, 38].
HDL-Associated Sphingosine 1-Phosphate
The vascular endothelial cell is a major target for injuries caused by putative risk factors of atherosclerosis, such as cholesterol crystaline material [42]. It was previously demonstrated that circulating cholesterol crystals could injure the endothelial lining of the arterial wall, consequently diminishing normal vasoreactivity [42]. HDL-associated sphingosine 1-Phosphate (S1P) is a plasma-borne lysosphingolipid that has been shown to regulate endothelial barrier integrity [3, 43-46]. It has traditionally been considered an inert component of the vascular endothelial wall, but quiet endothelium produces NO, which acts to slow down cellular pathways of inflammation, proliferation, and thrombosis [25, 30-33]. An increasing body of evidence suggests that several anti-inflammatory effects exerted by HDL can be attributed to the presence of lysosphingolipids in this lipoprotein fraction. The majority of the lipoprotein particle-associated S1P (>50%) is bound to HDL. For example, HDL-associated S1P and related molecules may activate the lysophospholipid receptor S1P3 in order to stimulate eNOS [3, 47]. A number of recent studies point to the S1P cargo of HDL as being a mediator of many of the cardiovascular effects of HDL, including the ability to inhibit/reverse atherosclerosis [3, 47].
Endothelial Nitric Oxide Synthase and HDL
Numerous previous studies reported an impaired eNOS-activating capacity of HDL in atherosclerosis. More precisely, those who developed an important inflammatory response have found to have circulating HDL ineffective in stimulating endothelial eNOS and NO production [25].
eNOS is a key signaling protein that promotes vascular smooth muscle cell (VSMC) relaxation, and also reduces platelet aggregation and provides atheroprotection through the production of NO [48, 49]. The concentration of eNOS is therefore a key marker of endothelial dysfunction [49]. Indeed, part of HDL’s anti-atherogenic effect is by stimulating endothelial NO production and inhibiting oxidant stress and inflammation [10, 39]. In VSCM, HDL natures pro-inflammatory, pro-migratory, and degradative actions on endothelium and platelets [12]. Thus, by adjusting the production of a number of diverse endothelium-derived factors, such as NO, prostaglandin I2 (PGI2), platelet-activating factors (PAFs), and von willebrand factors (vWFs), HDL may affect both vascular tone and thrombogenicity [23, 50]. vWF is another protein released by vascular endothelial cells that plays a fundamental role in platelet adhesion and aggregation. The blood vWF expressions are counter correlated with plasma HDL, recommending that HDL may block vWF production [31].
Previous studies have shown the ability of HDL added to endothelial cells in an in-vitro medium to significantly enhance eNOS activity in a manner that is dependent on SR-BI.
It was also demonstrated that HDL interaction with SR-BI modifies endothelial cell membrane lipid distribution and morphology, thus potentially influencing eNOS activity [51-54].
Scavenger Receptor Class B Member 1(SR-BI), ApoA-1 and Platelet-activating Factor Acetylhydrolase (PAF-AH)
SR-BI appears to be a major player in HDL-induced vasodilation, mediating the production of PGI2, another potent vasodilator. HDL can also regulate the expression of cyclooxygenase 2 (COX-2) and PGI-2 release in endothelial cells to exert anti-atherogenic functions [54, 55]. It was hypothesized that HDLs stimulate Cox-2 expression through NF-κB activation. It is common that S1P, binding to S1P receptors, can increase COX-2 expression and PGI-2 release through p38MAPK/CREB pathway [23, 24, 55].
Recently, it was reported that the HDL-associated protein apoA-I induces COX-2 expression and PGI-2 release through ATP-binding cassette transporter1 (ABCA1) and the actuation of intra-cellular p38 mitogen activated protein kinases (MAPK), extracellular signal regulated kinases (ERK1/2), and janus kinase 2 (JAK2) pathways. ApoA-I can reinforce these effects with S1P in vascular endothelial cells [54]. Similarly, HDL inhibits the secretion of endothelin (ET-1), and therefore may prevent the vasoconstrictor effects of ET-1. Additionally, HDL inhibits vascular endothelial inflammation by increasing 3β-hydroxysteroid-Δ24 reductase expression and inducing heme oxygenase-1 [45]. In vascular endothelial cells and their progenitors, HDL slows down apoptosis and encourages proliferation and migration [32]. It was suggested that the HDL-induced proliferation occurs through a protein kinase C-mediated pathway. HDL-related-lipoproteins (apoA-1) were required for this effect [32]. Pro-inflammatory cytokines (IL-1 beta and Il-18), products of lipid peroxidation (oxLDL), and growth factors are potent apoptotic stimuli for endothelial cells [50-56]. In fact, HDL protects vascular endothelial cells from TNF-α-induced apoptosis [57, 58]. The apolipoprotein composition of HDL affects its anti-apoptotic activity, with apoA-I-containing particles being the most effective [20-25].
HDL also has various anti-inflammatory actions in vascular endothelial cells. Therefore, HDL-associated - PAF-AH is an antioxidant enzyme avoiding LDL oxidation by hydrolysis of oxidized phospholipids [12, 46]. Consequently, by limiting PAF production by endothelial cells and increasing its degradation by circulating enzymes, HDL may avoid PAF-induced adhesion of leukocytes to the activated endothelium, which may contribute to the anti-adhesive effects of HDL [33]. As previously mentioned, HDL raises NO and PGI2 production, and limits PAF activity for reduced atherosclerotic disease [47].
Homocysteine and Nitric Oxide
Moderately elevated plasma homocysteine (Hcy) levels are highly prevalent in the general population in developing countries [59]. Oxidative radicals, generated by Hcy, are capable of oxidizing LDL particles in plasma [60]. Patients with Hiperhomocsyteinemia (HHcy) show end products of lipid peroxidation (MDA), mimicking the decreased HDL-associated PON1 enzyme activity, which is associated with increased MDA, both leading to endothelial dysfunction [61]. The association of HHcy and endothelial dysfunction mainly depends on the molecules exact damaging effect on eNOS coupling. Loss of NO bioavailability has a pivotal role on endothelial dysfunction, preceding the appearance of atherosclerosis [61-63].
The increase in extracellular Hcy is toxic to cells and tissues, and has the potential to initiate a broad array of vascular and endothelial complications [64, 65]. Oxidative radicals generated by Hcy are capable of oxidizing LDL in the plasma. Patients with hiperhomocysteinemia have an increase in MDA, very similar to decreased HDL-associated PON1 enzyme activity that is associated with increased MDA, to act on endothelial dsyfunction [26].
The association of Hcy and endothelial dysfunction greatly depends on molecules’ damaging effect on eNOS coupling, and loss of NO bioavailability [65, 66].
Tetrahydrobiopterin (BH4) is an essential cofactor for eNOS. Hcy was shown to promote the oxidation of the essential eNOS cofactor tetrahydrobiopterin, resulting in the uncoupling of the enzyme, timely spontaneous oxygen radical synthesis, and decreased NO production. The reduction in BH4 availability, followed by the uncoupling of eNOS, is the significant hallmark in Hcy-mediated oxidative stress [26, 61, 64, 65].
Hcy also induces NADPH oxidase activity, which contributes to increased ROS production [61-64]. Hcy is also known to decrease NO production by increasing asymmetrical dimethylarginine (ADMA). Superoxide generated by Hcy indirectly decreases NO bioavailability by rapid consumption of NO, resulting in the generation of ONOO- [67]. Finally, a reduction in NO synthesis and release by injured ECs causes the release of multiple growth factors to provoke proliferation of VSMC [67-69].
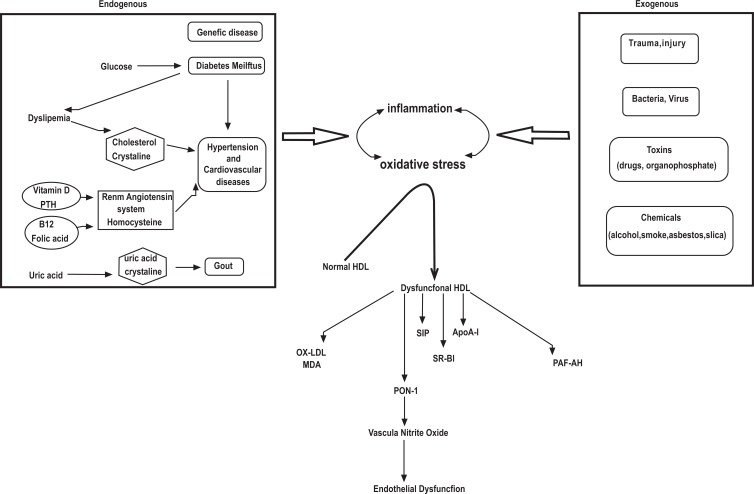
Oxidative radicals generated by Hcy inevitably initiate the oxidative degradation of cell membrane lipids of the endothelium, leading to loss of membrane function [61-64]. Hcy has also been shown to activate the Fas cell-death pathway, the p53/Noxa pathway, and the cytochrome-c activated caspase 3 and 9 pathway, in endothelial cells [70]. Elucidating the redox dependent mechanisms involved in the physiological and pathophysiological regulation of endothelial cells by ROS will provide critical new insights on cardio-vascular diseases, and may lead to the identification of novel treatment targets related to HDL, HDL-associated PON 1 enzyme and Hcy modulated vascular responses (Fig. 1).
Fig. (1).
Generation of oxidative stress and dysfunctional HDL, and effect on endothelial dysfunction. Oxidative stress and inflammation are increased via endogenous and exogenous factors. These effects transform from Normal HDL to Dysfunctional HDL. oxLDL is increased and HDL's protective effect is destroyed. Finally, NO decreases and endothelial dysfunction occurs.
CONCLUSION
HDL possesses numerous features that contribute to its role in protecting against atherosclerosis: “Normal - HDL”, specifically the particle that contains the active anti-oxidant PON1enzyme, has the power to suppress the formation of lipids and lipoproteins, such as oxLDL and malondialdehyde. Impaired HDL function and increased oxLDL are a leading factors for the atherosclerotic process by triggering platelet aggregation, release of serotonin, production of thromboxane B2 and the inhibition of eNOS. Analyses of HDL-function, PON1 enzyme activity and eNOS expression would be more important to diagnose endothelial dysfunction. Additionally, the treatment strategy of atherosclerosis to improve endothelial function and a basis for assessing the effects of HDL-targeted therapies would be worthy to investigate in the near future.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Eren E, Yilmaz N, Aydin O. High Density Lipoprotein and it's Dysfunction. Open Biochem. J. 2012;6:78–93. doi: 10.2174/1874091X01206010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein it's not just about lipid transport anymore. Trends Endocrinol. Metab. 2011;22(1):9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan LR, Wang DX, Liu H, Zhang XX, Zhao H, Hua L, Xu P, Li YS. A pro-atherogenic HDL profile in coronary heart disease patients an iTRAQ labelling-based proteomic approach. PLoS One. 2014;9(5):e98368. doi: 10.1371/journal.pone.0098368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J. Am. Coll. Cardiol. 2008;51(23):2199–2211 . doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Movva IR, Rader D. Laboratory Assessment of HDL Heterogeneity and Function. Clin. Chem. 2008;54:88–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 6.Mineo C, Shaul PW. PON-dering differences in HDL function in coronary artery disease. J. Clin. Invest. 2011;121(7):2545–2548. doi: 10.1172/JCI57671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kon V, Ikizler TA, Fazio S. Importance of high-density lipoprotein quality evidence from chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013;22(3):259–265. doi: 10.1097/MNH.0b013e32835fe47f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine1-phosphate promotes endothelial barrier function. J. Biol. Chem. 2008;283(36):25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AS, Tan L, Lu LJ, Davidson WS. The proteomic diversity of high density lipoproteins Our emerging understanding of its importance in lipid transport and beyond. J. Lipid. Res. 2013;54(10):2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell S, Genest J. HDL-C clinical equipoise and vascular endothelial function. Expert Rev. Cardiovasc. Ther. 2013;11(3):343–53. doi: 10.1586/erc.13.17. [DOI] [PubMed] [Google Scholar]
- 11.Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, vonEckardstein A, Lüscher TF, Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease role of high-density lipoprotein-proteome remodeling. Circultion. 2013; 127(8):891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 12.Mineo C, Deguchi H, Griffi JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006;98(11):1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 13.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ. Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Mehta JL. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect against oxidized low-density lipoprotein-induced endothelial dysfunction. Endothlium. 2003; 10(1):17–21. doi: 10.1080/10623320303355. [DOI] [PubMed] [Google Scholar]
- 15.Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat. Rev. Cardiol. 2011;8(5):266–277. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellidag HY, Eren E, Aydin O, Yκldκrκm M, Sezer C, Yilmaz N. Multiple myeloma relationship to antioxidant esterases. Med Princ Pract. 2014;3(1):18–23. doi: 10.1159/000355826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackness B, Mackness M. The antioxidant properties of high-density lipoproteins in atherosclerosis. Panminerva Med. 2012;54(2):83–90. [PubMed] [Google Scholar]
- 18.Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006;26(7):1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 19.Aviram M. Introduction to paraoxonases. J. Lipids. 2012;2012:687273. doi: 10.1155/2012/687273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvinov D, Mahini H, Garelnabi M. Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N. Am. J. Med. Sci. 2012;4(11):523–532. doi: 10.4103/1947-2714.103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farid AS, Horii Y. Modulation of paraoxonases during infectious diseases and its potential impact on atherosclerosis. Lipids Health Dis. 2012;11:92. doi: 10.1186/1476-511X-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borowczy kK, Shih DM, Jakubowski H. Metabolism and neurotoxicity of homocysteine thiolactone in mice evidence for a protective role of paraoxonase 1. J. Alzheimers. Dis. 2012;30(2):225–231. doi: 10.3233/JAD-2012-111940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J. Am. Coll. Cardiol. 2008;51(23):2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Loscalzo J. Regulation of tissue factor expression in human microvascular endothelial cells by nitric oxide. Circultion. 2000;101(18):2144–2148. doi: 10.1161/01.cir.101.18.2144. [DOI] [PubMed] [Google Scholar]
- 25.Eren E, Yilmaz N, Aydin O. Functionally defective high-density lipoprotein and paraoxonase a couple for endothelial dysfunction in atherosclerosis. Cholesterol. 2013;2013:792090. doi: 10.1155/2013/792090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, vonEckardstein A, Fogelman AM, Lüscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2003;23(10):1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 28.Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of High-density lipoprotein alterations in cardiovascular disease. EMBO Mol. Med. 2012;4(4):251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stancu CS, Toma L, Sima AV. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012;349(2):433–446. doi: 10.1007/s00441-012-1437-1. [DOI] [PubMed] [Google Scholar]
- 30.Kotosai M, Shimada S, Kanda M, Matsuda N, Sekido K, Shimizu Y, Tokumura A, Nakamur T, Murota K, Kawai Y, Terao J. Plasma HDL reduces nonesterified fatty acid hydroperoxides originating from oxidized LDL a mechanism for its antioxidant ability. Lpids. 2013;48(6):569–578. doi: 10.1007/s11745-013-3779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norata GD, Catapano AL. Molecular mechanisms responsible for the antiinflammatory and prot ective effect of HDL on the endothelium. Vasc. Health Risk Manag. 2005;1(2):119–129. doi: 10.2147/vhrm.1.2.119.64083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mineo C, Shaul PW. Novel biological functions of high-density lipoprotein cholesterol. Circ. Res. 2012;111(8):1079–90. doi: 10.1161/CIRCRESAHA.111.258673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugano M, Tsuchida K, Makino N. High-density lipoproteins protect endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Biochem. Biophys. Res. Commun. 2000;272:872–876. doi: 10.1006/bbrc.2000.2877. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-González J, Badimon L. Mechanisms underlying the cardiovascular effects of COX-inhibition benefits and risks. Curr. Pharm. Des. 2007;13(22):2215–2227 . doi: 10.2174/138161207781368774. [DOI] [PubMed] [Google Scholar]
- 35.Shao B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochem. Biophys.Acta. 2012;1821(3):490–501. doi: 10.1016/j.bbalip.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eren E, Ellidag HY, Aydin O, Yκlmaz N. Homocysteine, Paraoxonase-1 and vascular endothelial dysfunction Omnibus viis romam pervenitur. J. Clin. Diagn. Res. 2014;8(9) doi: 10.7860/JCDR/2014/7827.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Müller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, vonEckardstein A, Lüscher TF, Fliser D, Bahlmann FH, Landmesser U. Abnormal High-Density Lipoprotein Induces Endothelial Dysfunction via Activation of Toll-Like Receptor-2. Immunity. 2013;18, 38(4):754–768. doi: 10.1016/j.immuni.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc. Drugs Ther. 2011;25(5):379–391. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawamura T, Kakino A, Fujita Y. LOX-1: a multiligand receptor at the crossroads of response to danger signals. Curr. Opin. Lipidol. 2012;23(5):439–445. doi: 10.1097/MOL.0b013e32835688e4. [DOI] [PubMed] [Google Scholar]
- 40.Huang CX, Zhang YL, Wang JF, Jiang JY, Bao JL. MCP-1 impacts reverse cholesterol transport by repressing ABCA1, ABCG1 and SR-BI through PI3K /Akt post-translational regulation in HepG2 cells. J. Lipid Res. 2013;54(5):1231–1240. doi: 10.1194/jlr.M032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong X, Peng H, Liu D, Ji L, Niu C, Ren J, Pan B, Hu J, Zheng L, Huang Y. High-density lipoprotein of patients with Type 2 Diabetes Mellitus upregulates cyclooxgenase-2 expression and prostacyclin I-2 release in endothelial cells relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc. Diabetol. 2013;30, 12(1):27. doi: 10.1186/1475-2840-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadeela N, Rubinstein J, Tamhane U, Huang R, Pathak DR, Hosein HA, Rich M, Dhar G, Abela GS. The impact of circulating cholesterol crystals on vasomotor function implications for no-reflow phenomenon. JACC Cardiovasc. Interv. 2011;4 (5):521–9. doi: 10.1016/j.jcin.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem. 2012;287(53):44645–44653. doi: 10.1074/jbc.M112.423426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yokoi K, Adachi H, Hirai Y, Enomoto M, Fukami A, Ogata K, Tsukagawa E, Kasahara A, Imaizumi T. Plasma endothelin-1 level is a predictor of 10-year mortality in a general population the Tanushimaru study. Circ. J. 2012;76(12):2779–2784. doi: 10.1253/circj.cj-12-0469. [DOI] [PubMed] [Google Scholar]
- 45.Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ, Rye KA. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3κ-hydroxysteroid-κ24 reductase expression and inducing heme oxygenase-1. Circ. Res. 2013;18, 112(2):278–288. doi: 10.1161/CIRCRESAHA.111.300104. [DOI] [PubMed] [Google Scholar]
- 46.Bayrak T, Dursun P, Bayrak A, Gültekin M, Kolusarκ A, Cakκr E, Ozyurt M, Zeyneloκlu HB. Paraoxonase lactonase activity (PON-HTLase): asymmetric dimethylarginine (ADMA) and platelet activating factor-acetylhydrolase (PAF-AH) activity in non-obese women with PCOS. Gynecol. Endocrinol. 2012;28 (11):874–878. doi: 10.3109/09513590.2012.683068. [DOI] [PubMed] [Google Scholar]
- 47.Arya D, Chang S, DiMuzio P, Carpenter J, Tulenko TN. Sphingosine-1-phosphate promotes the differentiation of adipose-derived stem cells into endothelial nitric oxide synthase (eNOS) expressing endothelial-like cells. J. Biomed. Sci. 2014;4:21–55. doi: 10.1186/1423-0127-21-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 49.Mudau M, Genis A, Lochner A, Strijdom H. Endothelial dysfunction the early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012;23(4):222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Y, Liu G, Yang J, Zheng R, Jiang L, Bao P. The association between metabolic syndrome and vascular endothelial dysfunction in adolescents. Exp. Ther. Med. 2013;5(6):1663–1666. doi: 10.3892/etm.2013.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunet R, How M, Trigatti BL. Modulators of Protein Kinase C Affect SR-BI-Dependent HDL Lipid Uptake in Transfected HepG2 Cells. Cholesterol. 2011;2011:687939. doi: 10.1155/2011/687939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saddar S, Mineo C, Shaul PW. Signaling by the high-affinity HDL receptor scavenger receptor B type, I. Arterioscler. Thromb. Vasc. Biol. 2010;30 (2):144–150. doi: 10.1161/ATVBAHA.109.196170. [DOI] [PubMed] [Google Scholar]
- 53.Mineo C, Shaul PW. Role of high-density lipoprotein and scavenger receptor B type I in the promotion of endothelial repair. Trends Cardiovasc. Med. 2007;17(5):156–161. doi: 10.1016/j.tcm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Liu D, Ji L, Tong X, Pan B, Han JY, Huang Y, Chen YE, Pennathur S, Zhang Y, Zheng L. Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. Am. J. Physiol. Cell Physiol. 2011;301 (3):C739–748. doi: 10.1152/ajpcell.00055.2011. [DOI] [PubMed] [Google Scholar]
- 55.Liu D, Ji L, Wang Y, Zheng L. Cyclooxygenase-2 expression, prostacyclin production and endothelial protection of high-density lipoprotein. Cardiovasc. Hematol. Disord. Drug Tagets. 2012;12 (2):98–105. doi: 10.2174/1871529x11202020098. [DOI] [PubMed] [Google Scholar]
- 56.Yamagata K, Tanaka N, Matsufuji H, Chino M. κ-carotene reverses the IL-1κ-mediated reduction in paraoxonase-1 expression via induction of the CaMKKII pathway in human endothelial cells. Microvasc. Res. 2012;84(3):297–305. doi: 10.1016/j.mvr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 57.Eren E, Ellidag HY, Yκlmaz A, Aydκn O, Yκlmaz N. Acute Phase Response Implication in ST-segment Elevation Myocardial Infarction. Open Biochem. J. 2014;8:44–51. doi: 10.2174/1874091X01408010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mascarenhas-Melo F, Sereno J, Teixeira-Lemos E, Marado D, Palavra F, Pinto R, Rocha-Pereira P, Teixeira F, Reis F. Implication of low HDL-c levels in patients with average LDL-c levels a focus on oxidized LDL, large HDL subpopulation, and adiponectin. Mediators Inflamm. 2013;2013:612038. doi: 10.1155/2013/612038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yilmaz N. Relationship between paraoxonase and homocysteine crossroads of oxidative diseases. Arch. Med. Sci. 2012;29, 8(1):138–153. doi: 10.5114/aoms.2012.27294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Domagaκa TB, κacinski M, Trzeciak WH, Mackness B, Mackness MI, Jakubowski H. The correlation of homocysteine-thiolactonase activity of the paraoxonase (PON1):protein with coronary heart disease status. Cell Mol. Biol. (Noisy-le-grand): 2006;31, 52(5):4–10. [PubMed] [Google Scholar]
- 61.Perκa-Kaján J, Jakubowski H. Paraoxonase 1 and homocysteine metabolism. Amino cids. 2012; 43 (4):1405–1417. doi: 10.1007/s00726-012-1321-z. [DOI] [PubMed] [Google Scholar]
- 62.Kundu S, Kumar M, Sen U, Mishra PK, Tyagi N, Metreveli N, Lominadze D, Rodriguez W, Tyagi SC. Nitroty-rosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/ CBS double knockout mice. J. Cell Biochem. 2009;106(1):119–126. doi: 10.1002/jcb.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antoniades C, Shirodaria C, Warrick N, Cai S, deBono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels effectws on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circultion. 2006;114 (11):1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 64.Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J. Physiol. Pharmacol. 2008;59(Suppl 9 ):155–167. [PubMed] [Google Scholar]
- 65.He L, Zeng H, Li F, Feng J, Liu S, Liu J, Yu J, Mao J, Hong T, Chen AF, Wang X, Wang G. Homocysteine impairs coronary artery endothelial function by inhibiting tetrahydrobiopterin in patients with hyperhomocysteinemia. Am. J. Physiol. Endocrinol. Metab. 2010;299(6):E1061–E1065. doi: 10.1152/ajpendo.00367.2010. [DOI] [PubMed] [Google Scholar]
- 66.Maron BA, Michel T. Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase. Circ. J. 2012;76(11):2497–2512. doi: 10.1253/circj.cj-12-1207. [DOI] [PubMed] [Google Scholar]
- 67.Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;(Suppl 1 ):S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 68.Sharma P, Senthilkumar RD., Brahmahari V., Sundaramoorthy E., Mahajan A., Sharma A., Sengupta S. Mining literature for a comprehensive pathway analysis a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis. 2006;5:1. doi: 10.1186/1476-511X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss N, Papatheodorou L, Morihara N, Hilge R, Ide N. Aged garlic extract restores nitric oxide bioavailability in cultured human endothelial cells even under conditions of homocysteine elevation. J. Ethnopharmacol. 2013;145(1):162–167. doi: 10.1016/j.jep.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 70.Ma S, Zhang H, Sun W, Gong H, Wang Y, Ma C, Wang J, Cao C, Yang X, Tian J, Jiang Y. Hyperhomocysteinemia induces cardiac injury by up-regulation of p53-dependent Noxa and Bax expression through the p53 DNA methylation in ApoE(-/-) mice. Acta Biochim. Biophys. Sin (Shanghai): 2013;45(5):391–400. doi: 10.1093/abbs/gmt030. [DOI] [PubMed] [Google Scholar]