Abstract
Context:
Bryophyllum pinnatum is used as traditional medicine in India, Africa, Tropical America and China for treatment of various diseases. B. pinnatum contains different groups of phytoconstituents viz., flavonoid, terpenoids, alkaloid, phenolic compounds.
Aim:
The present study was carried out to evaluate the gastroprotective activity of B. pinnatum whole plant aqueous extract, and mucilage (MUC) isolated from the whole plant against ethanol induced gastric ulcer.
Materials and Methods:
Pretreatment of rats with aqueous extract at dose level of 500 and 750 mg/kg b.w., MUC at 500 mg/kg dose level and standard drug Rabeprazole at dose level of 20 mg/kg b.w. where given for 7 days.
Results:
The aqueous whole plant extract of B. pinnatum at dose of 750 mg/kg p.o. and MUC at dose of 500 mg/kg p.o. markedly decrease the incidence of ulcers in ethanol induced ulcer rats. In ethanol induced ulcer rats, there was a decrease in the gastric volume, free and total acidity and ulcerative index as compared to the control group. Total carbohydrate content was found to be an increase as compare to control the group. The aqueous whole plant extract of B. pinnatum at dose of 750 mg/kg showed a significant reduction in the above parameters which was comparable to the standard drug rabeprazole (20 mg/kg). B. pinnatum extract and MUC showed protection index 72.69 and 69.65% respectively, whereas standard drug rabeprazole showed protection index 75.49%.
Conclusions:
Whole plant extracts of B. pinnatum and MUC has potent gastroprotective effect which can be further clinically studied for new drug development.
KEY WORDS: Bryophyllum pinnatum, gastroprotective, mucilage
INTRODUCTION
Peptic ulcer is one of the most common gastrointestinal diseases. The exact causes of peptic ulcer disease are not known, but it may be the result of an imbalance between acid-pepsin secretion and mucosal defense factors.[1] In Ayurveda, peptic ulcer mostly refers to amlapitta or pariṇāmaśūla. Amlapitta is a disease of the gastrointestinal tract, especially of the stomach. Amlapitta literally means pitta leading to the sour taste.[2] A number of antiulcer drugs such as H2 receptor antagonists, proton pump inhibitors and cytoprotectants are available for curing ulceration, but due to their several adverse reaction and toxicities and may alter the biochemical mechanisms of the body upon chronic usage.[3] Herbal medicines are considered safer because of the natural ingredients with little or no side effects.[4]
Bryophyllum pinnatum (Lam.) Kurz. is a medicinally important plant having different group of phytoconstituents with a wide range of pharmacological activities. Leaves of B. pinnatum has been reported to have flavonoid, phenolic compounds, alkaloids, terpenoids, carbohydrates, minerals and glycosides.[5] Lightly roasted leaves are used against cancer, inflammations and a leaf infusion for fevers by Creoles. Mixture of leaf juice with coconut oil is used as a remedy for migraines and headaches in Palikur. The Siona indigenous people heat the leaves and apply them topically to boils and skin ulcers. Rio Pastaza natives in Ecuador, use a leaf infusion to treat broken bones and internal bruises. Infusion of leaves and stem in cold water are used by indigenous tribes of Peru for heartburn, urethritis, fevers, and for all sorts of respiratory conditions. The root infusion is also used in epilepsy. In Kerala, tribals use plant for treating cancer symptoms.[5] Leaves of B. pinnatum are known for their antinociceptive, anti-inflammatory and antidiabetic activity,[6] antihypertensive activity,[7] immunosuppressive effect,[8] anti-tumor activity.[9] Antiulcer potentials of herbal drug have been reported in various scientific studies. There is a need to evaluate the potential of Ayurvedic remedies as adjuvant to counteract side effects of modern therapy. In the present study, isolation of mucilage (MUC) from the whole plant and gastroprotective effect of aqueous extract of whole plant has been evaluated on ethanol induced ulcer model (rats).
MATERIALS AND METHODS
Plant materials
Whole plant of B. pinnatum was collected from Kalyan, Maharashtra in the month of March 2010. Plant was authenticated from Blatter Herbarium, St. Xavier's college, Mumbai by Dr. Rajendra Shinde. The plant material was washed, and excess water was drained off and dried on filter paper. Shade dried plant material was crushed in electrical mix grinder to a fine powder, and it was further used for the studies.
Extraction condition
Five-hundred gram of whole plant was defatted with petroleum ether and exhaustively extracted with distilled water for 7 days (changing distilled water every day). The content was filtered through Whatman filter paper No. 1 and evaporated to syrupy content at 95 ± 2°C and labelled as an aqueous extract of B. pinnatum (AEBP). This extract was used for gastroprotective study.
Isolation of mucilage
Two hundred and fifty gram of whole plant was extracted in distilled water by boiling it in the water bath at 100 ± 2°C for 2 h. and kept for extraction for 4 days in mechanical shaking. The content was filtered and concentrated. Equal amount of ethanol was poured in the concentrated aqueous extract to obtain faint red precipitate; precipitate was washed with acetone and oven dried at 45 ± 2°C. Isolated MUC was further hydrolyzed in 1 M H2SO4 to separate the sugar subunit present in it and was characterized by gas chromatography-mass spectroscopy (GC-MS), Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR).
Animals
Thirty-Six healthy female Wistar rats weighing between 180 and 250 g were used in this study. Animals were maintained at 25 ± 2°C and kept in well-ventilated animal house under natural photoperiodic condition in polypropylene cages with paddy husk as bedding with free access to food and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC NO: MVC/IEAC/04/12) and the study was conducted in accordance with CPCSEA guidelines.
Acute toxicity studies (fixed dose method)
The animals in group 1 served as control and received 1.0 ml of phosphate buffer saline (PBS). The animals in groups 2 and 3 received 2000 mg/kg b. w. of AEBP and MUC respectively through oral administration with a cannula attached to a graduated syringe. They were all placed under observation for 30 min, 4, 24 h and post 14 days and cage side observations were recorded.
Gastroprotective study
Six groups (n = 6) of female Wistar rats were used to study the Gastroprotective activity of AEBP. PBS, aqueous extract (AEBP), rabeprazole (20 mg), MUC and ethanol were administered to the animals per orally (p.o.).
Group 1: Received PBS (10 ml/kg) through the experimental period (7 days) and served as control.
Group 2: Received PBS (10 ml/kg) for 7 days and served as ulcer control.
Group 3: Received rabeprazole (20 mg/kg) for 7 days.
Groups 4 and 5: Were respectively administered with 500 and 750 mg/kg AEBP for 7 days respectively.
Group 6: Was administered with 500 mg/kg of MUC for 7 days.
All the groups were fasted for 24 h and administered with the extract or drug at respective dose. After 30 min of this treatment, animals of groups 2–6 were administered with 2 ml/200 g b.w. ethanol to induce an ulcer. After 30 min of ethanol administration, all the animals were sacrificed using anesthetic ether. Stomach of each rat was opened along the greater curvature and examined macroscopically for gastric erosions under a dissecting microscope (×20). The length and width (mm) of the ulcer on the gastric mucosa were measured by plane glass square (10 mm × 10 mm). The ulcer area (UA) was calculated. The % of protection (P%) provided to the animals through the action of various treatments was calculated using the formula:
P % = (UA ulcer control − UA treatment)/UA ulcer control × 100
Histopathology
After collecting the gastric contents and measuring the UA, small pieces of stomachs from each group were embedded in paraffin wax. Sections of 5 μm thick were cut in a microtome and mounted on glass slides using standard techniques. After staining the tissues with H and E stain, the slides were viewed under a light microscope equipped for photography.
Ulcer index
Ulcer healing was observed via measurement severity of the lesion. Arbitrary scoring system described by Nie et al. was used to grade the incidence and severity of the lesion.[10]
The stomach was then examined under microscope (×100) to observe erosions and scored as 1–5: 1 – Small round hemorrhagic erosion, 2 – Hemorrhagic erosion <1 mm, 3 – Hemorrhagic erosion = 1–2 mm, 4 – Hemorrhagic erosion = 2–3 mm, 5 – Hemorrhagic erosion >4 mm. The score was multiplied by 2 when the width of the erosion is larger than 1 mm.
Collection of gastric juice: Antisecretory potential
After postoperative period, animals were sacrificed by cervical dislocation and the stomach was dissected out as a whole by passing a ligature at the esophageal end. Gastric content was evacuated into a graduated tube by cutting along the greater curvature of the stomach, and was centrifuged at 3000 rpm for 10 min.
pH, gastric juice volume, free acidity, total acidity determined by titrating with 0.01 N NaOH, using phenolphthalein as indicator and was expressed as mEq. Dissolved mucosal substances were estimated in 90% alcoholic precipitate of the gastric juice. The precipitate thus obtained was either dissolved in 1 ml of 0.1 N NaOH or 1 ml of 0.1 N H2SO4. The former was used for the estimation of protein,[11] while the latter was used for the estimation of carbohydrate.[12]
Estimation of free radical generation
The fundic part of the stomach was homogenized (5%) in ice cold 0.9% saline with a glass homogenizer for 30 s. The homogenate was then centrifuged at 1000 rpm for 10 min followed by centrifugation of the supernatant at 12,000 g for 15 min and the obtained fraction was used for the estimation of lipid peroxidase, superoxide dismutase (SOD)[13] activity and catalase (CAT)[14] activity.
RESULTS
Acute oral toxicity study
Acute oral toxicity was carried out fixed dose method. It is found that AEBP and MUC were found to be safe at dose 2000 mg/kg with no mortality in studied subjects. Cage side observation was found to be normal in AEBP and MUC groups. Body weight, food and water intake of AEBP and MUC were found to be normal.
Effect of the aqueous extract of Bryophyllum pinnatum and mucilage on ethanol induced gastric ulcers in rats
Ethanol administration in rats (1 ml/200 g b.w.) induced ulceration of the gastric mucosa of the control group, characterized by hemorrhagic gastric lesions. AEBP and MUC caused a reduction in the severity of these lesions induced by ethanol which was evident by a significant (P < 0.05) reduction in the ulcer index and an increase in the percentage protection of ulcers when compared with the control group [Figure 1].
Figure 1.
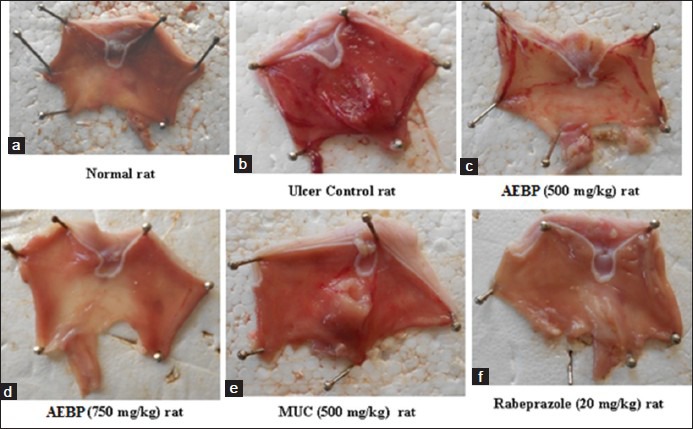
(a) Ulcer lesion-normal rat; (b) ethanol-induced rats; (c) Aqueous extract of Bryophyllum pinnatum (AEBP) (500 mg/kg) pretreated + ethanol induced rats; (d) AEBP (750 mg/kg) pretreated + ethanol induced rats; (e) mucilage + ethanol induced rats; (f) rabeprazole (20 mg/kg) pretreated + ethanol induced rats
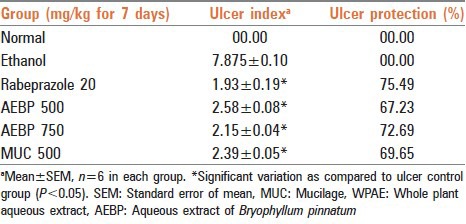
At 500 and 750 mg/kg b.w. AEBP showed a protection index of 67.23% and 72.69% respectively and MUC at 500 mg/kg b.w. dose level showed protection index of 69.65% [Table 1]. The results were comparable to rabeprazole (20 mg/kg) which reduced the ulcer index significantly [Table 1].
Table 1.
Effect of Bryophyllum pinnatum extract (AEBP) and MUC on gastric lesions in ethanol induced ulcer-genesis rats
Histopathological studies
The control group of rats treated with absolute alcohol showed histopathological changes of the gastric mucosa characterized by loss of glandular architecture, edema and erosions of the epithelial layer, evident edema, congestion and infiltration by inflammatory cells. The rats treated with the AEBP at 500 mg/kg, b.w. Showed minimum ulceration and edema but gastric epithelium was not intact. However, at a dose of 750 mg/kg the rats showed significant regenerative changes indicating healing [Figure 2].
Figure 2.
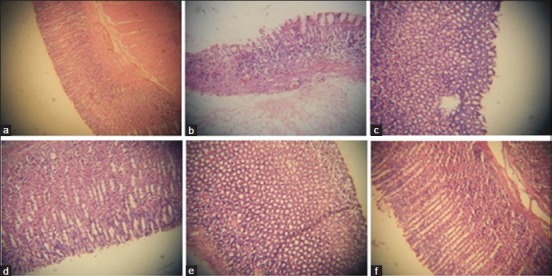
Histological examinations of gastric mucosal tissue sections of control and experimental rats (H and E, ×20). (a) Ulcer lesion-normal rat; (b) ethanol-induced rat; (c) Aqueous extract of Bryophyllum pinnatum (AEBP) (500 mg/kg) pretreated + ethanol induced rat; (d) AEBP (750 mg/kg) pretreated + ethanol induced rat; (e) Mucilage + ethanol induced rats; (f) Rabeprazole (20 mg/kg) pretreated + ethanol induced rat
Effect on biochemical parameters
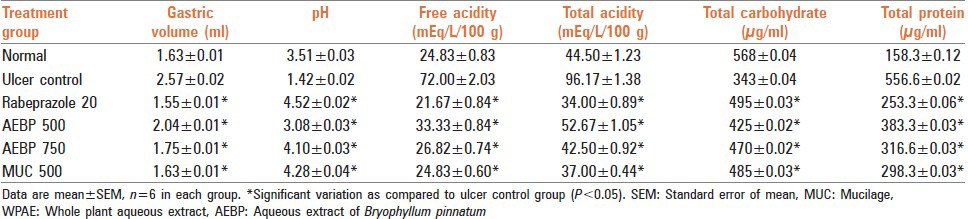
Gastric volume, pH, free and total acidity and total protein content of gastric juices in the AEBP treated groups indicated that there was a significant decrease at 500 and 750 mg/kg respectively. Similarly, MUC at dose level of 500 mg/kg b.w. Also showed a significant decrease in comparison to the control group (P < 0.05). Rabeprazole at 20 mg/kg also caused a significant decrease [Table 2]. Total carbohydrate content in rat treated with AEBP at dose level 500 and 750 mg/kg b.w. And MUC (500 mg/kg) showed significant increase in comparison to control group (P < 0.05). The results are tabulated in Table 2.
Table 2.
Effect of AEBP on gastric mucosal factors in ethanol induced gastric ulcer model
Effect of aqueous extract of Bryophyllum pinnatum and mucilage on in vivo antioxidant parameters
The results revealed that at dose levels of 500 and 750 mg/kg, AEBP decreased the CAT activity, and lipid peroxidation (LPO) level significantly in the glandular tissue, when compared to the control group. The activity of the group treated with 500 mg/kg of MUC was comparable as that of animals treated with Rabeprazole at 20 mg/kg (P < 0.05) [Table 3].
Table 3.
Effect of Bryophyllum pinnatum (AEBP) on ethanol-induced gastric ulcers in rats
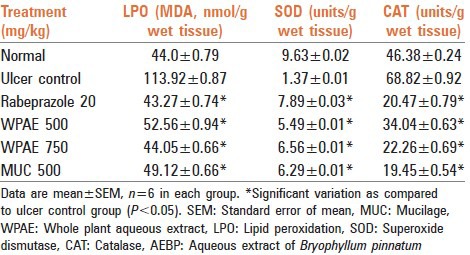
Superoxide dismutase activity in the glandular tissue was found to be significantly increased at dose level of 500 and 750 mg/kg of AEBP and MUC at 500 mg/kg b.w. As compared to control group (P < 0.05). The results are tabulated in Table 3.
Characterization of mucilage
1H NMR spectrum at 70°C of the partially depolymerized polysaccharide [Figure 3], containing uronic acid, showed signals at 4.87, 4.52, 4.37, 4.06 and 3.88 ppm which were assigned according to the literature to H1, H5, H4, H3 and H2, respectively of α-D galactopyranuronic acid units. The signal at 5.25 and 1.28 ppm was assigned to the anomeric and methyl protons at position-6, respectively, of a-L-rhamnopyranosyl residues. In the 13C NMR spectrum [Figure 4] a signal at 183.46 ppm was assigned to the carbonyl carbon of the carboxyl group.
Figure 3.
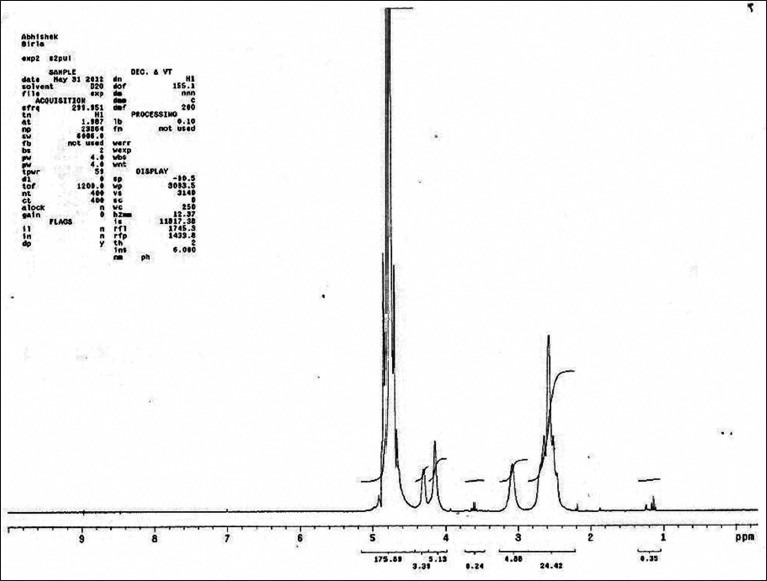
1H nuclear magnetic resonance spectra of mucilage isolated from Bryophyllum pinnatum
Figure 4.
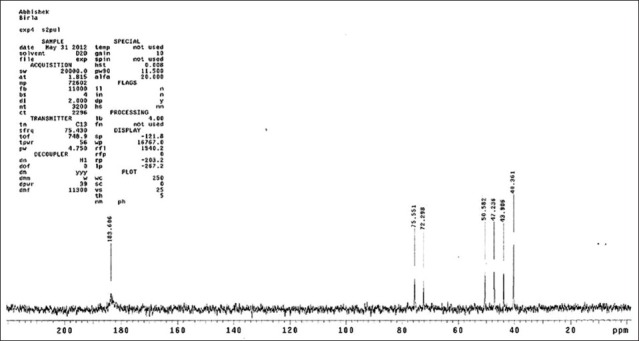
13C nuclear magnetic resonance spectra of mucilage isolated from Bryophyllum pinnatum
DISCUSSION
The gastroprotective effect AEBP and MUC were evaluated by ethanol induced gastric mucosal damage. AEBP and MUC reduced the ethanol induced lesions in the gastric mucosa as was evident from the significant (P < 0.05) reduction in the ulcer index in comparison to the ulcerated control group. The results were comparable to Rabeprazole, which reduced the ulcer index significantly. Histopathological study of the aqueous alcoholic extractives confirmed the antiulcer activity.
Ethanol induced gastric mucosal damage is mediated by active oxygen and free radicals generated both exogenously and endogenously. Hydroxyl radicals interact with the cell membrane to produce conjugated dienes and lipid hydroperoxides, which are lipid derived free radicals. Thus, LPO induced oxidative damage takes place. Ethanol treatment causes induction of oxidative stress intracellularly and leads to transition of mitochondrial permeability and depolarization, which further leads to cell death in the gastric mucosa.[15]
An important parameter like ulcer inhibition is generally used for antiulcer evaluation since this parameter has a direct relation to gastrointestinal parameters like gastric volume reduction and acidity changes. With the increase in pH, the ulcer score appeared less in the experimental animals. The reduction in free acid might have contributed to the ulcer preventive effect of the plant extractives.[16]
The gastric mucosal epithelial cells are impermeable to hydrogen ions and therefore form a physical barrier. This may have contributed to the mucosal defense mechanism as was evident from increased carbohydrate/protein ratio and an increase in carbohydrates like hexose, hexosamine, fucose and sialic acid.
Reactive oxygen species plays a role in the etiology and pathophysiology of inflammation and gastroduodenal ulcers.[14] From the data of ethanol induced ulcer model, it was found that the AEBP and MUC effectively increased the enzymatic antioxidants such as SOD and CAT. Conversely, it decreased the LPO level which suggests an antioxidant effect of the extractives.
From the results of antisecretory model, it was found that AEBP and MUC decreased the ulcer index in the doses used. AEBP and MUC decreased total carbohydrate, in the gastric juice compared with the ulcerated control group. On the other hand, the AEBP and MUC decreased the aggravating parameters such as gastric volume, total protein, total and free acidity in the gastric juice and increased the gastric pH. These findings suggest that AEBP and MUC show good gastroprotective effect in the ethanol induced ulcer model.
CONCLUSION
Bryophyllum pinnatum extract could significantly protect the gastric mucosa against ethanol-induced injury. Such protection was shown to be dose dependent as ascertained by the reduction of UAs in the gastric wall, as well as the reduction or inhibition of edema and leucocytes infiltration of sub-mucosal layers. Protection of gastric mucosa was most prominent at a dose of 750 mg/kg extract. Further, MUC isolated from B. pinnatum showed potent gastroprotective effect which when analyzed using NMR revealed the presence of galactopyranuronic acid units. In future AEBP and MUC can further be clinically checked for new drug development for the treatment of gastric ulcers.
ACKNOWLEDGMENTS
The authors express gratitude to the staff of the SAIF, IIT-Bombay for providing GC-MS, NMR and FT-IR instrumentation facilities which was helpful in interpreting the results.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Patidar DK. Anti-ulcer activity of aqueous extract of Murraya koenigii in albino Rats. Int J Pharm Biol Sci. 2011;2:524–9. [Google Scholar]
- 2.Dashputre NL, Naikwade NS. Evaluation of anti-ulcer activity of methanolic extract of Abutilon indicum Linn leaves in experimental rats. Int J Pharm Sci Drug Res. 2011;3:97–100. [Google Scholar]
- 3.Thirunavukkarasu P, Ramkumar L, Ramanathan T. Anti-ulcer activity of Excoecaria agallocha bark on NSAID-induced gastric ulcer in albino rats. Glob J Pharmacol. 2009;3:123–6. [Google Scholar]
- 4.Clouatre D, Rosenbaum M. New York: Keats Publishing; 1994. The Diet and Benefits of HCA; pp. 23–32. [Google Scholar]
- 5.Kamboj A, Saluja AK. Bryophyllum pinnatum (Lam.) Kurz: Phytochemical and pharmacological profile: A review. Pharmacogn Rev. 2009;3:364–74. [Google Scholar]
- 6.Ojewole JA. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J Ethnopharmacol. 2005;99:13–9. doi: 10.1016/j.jep.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Ghasi S, Egwuib C, Achukwu PU, Onyeanusi JC. Assessment of the medical benefit in the folkloric use of Bryophyllum pinnatum leaf among the Igbos of Nigeria for the treatment of hypertension. Afr J Pharm Pharmacol. 2011;5:83–92. [Google Scholar]
- 8.Bergmann BR, Costa SS, Borges MB, Silva SA, Noleto GR, Souza ML, et al. Immunosuppressive effect of the aqueous extract of Kalanchoe pinnata in mice. Phytother Res. 2006;8:399–402. [Google Scholar]
- 9.Supratman U, Fujita T, Akiyama K, Hayashi H, Murakami A, Sakai H, et al. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontianaxtubiflora. Biosci Biotechnol Biochem. 2001;65:947–9. doi: 10.1271/bbb.65.947. [DOI] [PubMed] [Google Scholar]
- 10.Nie SN, Qian XM, Wu XH, Yang SY, Tang WJ, Xu BH, et al. Role of TFF in healing of stress-induced gastric lesions. World J Gastroenterol. 2003;9:1772–6. doi: 10.3748/wjg.v9.i8.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosenborough NI, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 12.Goel RK, Chakrabarti A, Sanyal AK. The effect of biological variables on the anti-ulcerogenic effect of vegetable plantain banana. Planta Med. 1985:85–8. doi: 10.1055/s-2007-969412. [DOI] [PubMed] [Google Scholar]
- 13.Panneerselvam S, Arumugam G. A biochemical study on the gastroprotective effect of hydroalcoholic extract of Andrographis paniculata in rats. Indian J Pharmacol. 2011;43:402–8. doi: 10.4103/0253-7613.83110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repetto MG, Llesuy SF. Antioxidant properties of natural medicine for gastric ulcers. Braz J Med Biol. 2002;5:523–34. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 15.Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35:523–34. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 16.Malairajan P, Gopalakrishnan G, Narasimhan S, Veni KJ, Kavimani S. Anti-ulcer activity of crude alcoholic extract of Toona ciliata Roemer (heart wood) J Ethnopharmacol. 2007;110:348–51. doi: 10.1016/j.jep.2006.10.018. [DOI] [PubMed] [Google Scholar]


