Abstract
Recently, involvement of the sympathetic nervous system in bone metabolism has attracted attention. β2-Adrenergic receptor (β2-AR) is presented on osteoblastic and osteoclastic cells. We previously demonstrated that β-AR blockers at low dose improve osteoporosis with hyperactivity of the sympathetic nervous system via β2-AR blocking, while they may have a somewhat inhibitory effect on osteoblastic activity at high doses. In this study, the effects of butoxamine (BUT), a specific β2-AR antagonist, on tooth movement were examined in spontaneously hypertensive rats (SHR) showing osteoporosis with hyperactivity of the sympathetic nervous system. We administered BUT (1 mg/kg) orally, and closed-coil springs were inserted into the upper-left first molar. After sacrifice, we calculated the amount of tooth movement and analyzed the trabecular microarchitecture and histomorphometry. The distance in the SHR control was greater than that in the Wistar-Kyoto rat group, but no significant difference was found in the SHR treated with BUT compared with the Wistar-Kyoto rat control. Analysis of bone volume per tissue volume, trabecular number, and osteoclast surface per bone surface in the alveolar bone showed clear bone loss by an increase of bone resorption in SHR. In addition, BUT treatment resulted in a recovery of alveolar bone loss. Furthermore, TH-immunoreactive nerves in the periodontal ligament were increased by tooth movement, and BUT administration decreased TH-immunoreactive nerves. These results suggest that BUT prevents alveolar bone loss and orthodontic tooth movement via β2-AR blocking.
Keywords: bone remodeling, sympathetic nervous system, orthodontics, osteoporosis, periodontal disease/periodontitis, osteoclast
Introduction
Previous studies have shown that the sympathetic nervous system is involved in bone metabolism (Togari et al., 2005; Bonnet et al., 2008a; Bonnet et al., 2008b; Togari and Arai, 2008). Because immunohistochemical studies have also shown that mammalian bones are widely innervated by sympathetic nerves (Bjurholm, 1991; Hill and Elde, 1991), the possibility of sympathetic nervous regulation of bone metabolism has been demonstrated. In addition, the size, shape, microarchitecture, and mineral content of bone are affected by bone modeling, and this remodeling is changed by mechanical stress (Seeman, 2002). It is considered that alveolar bone is governed by the sympathetic nervous system and affected by mechanical stress associated with tooth movement.
Spontaneously hypertensive rats (SHR) are characterized by increased blood pressure, heart rate, plasma catecholamine levels, and dopamine β-hydroxylase and adrenal tyrosine hydroxylase (TH) levels, which are markers of sympathetic nervous activity (Nagatsu et al., 1974; Zhang and Thorén, 1998; Klemola et al., 1999). In comparison with Wistar-Kyoto rats (WKY), considered normotensive genetic controls, SHR have an overactive sympathetic nervous system. SHR also show decreased cortical and cancellous bone mass and increased bone turnover (Izawa et al., 1985; Wang et al., 1993). In addition, we recently demonstrated that SHR exhibited bone loss and increased fragility in cancellous bone associated with decreased bone formation and increased bone resorption (Sato et al., 2010).
Epidemiologic studies have demonstrated that high blood pressure is associated with increased bone loss at the femoral neck and low bone mineral density and that β-blockers are potential candidates of therapeutic drugs for osteoporosis and fracture healing (Pasco et al., 2004; Schlienger et al., 2004; Bonnet et al., 2007; Graham et al., 2008). In an animal study, lower doses of propranolol, a nonselective β-blocker, increased bone mass without affecting blood pressure (Bonnet et al., 2008a; Sato et al., 2010). In addition, butoxamine (BUT)—a specific β2-AR antagonist not approved by the U.S. Food and Drug Administration—prevented bone loss and biomechanical fragility (Arai et al., 2013). These in vivo studies clearly indicate that β-blocker at low doses influences the remodeling of bone and can be effective against osteoporosis.
However, the effect of β-blocker on tooth movement that occurs because of the acceleration of bone remodeling is still unclear. Kondo et al. (2013) reported that propranolol, a nonselective β-blocker, decreased initial tooth movement without influencing bone mass. In a study using genetically engineered mice, β1-adrenergic signaling was suggested to promote bone formation indirectly (Pierroz et al., 2012). One possible reason propranolol did not influence bone mass in the study of Kondo et al. (2013), in addition to the fact that long-term bone mass was not observed, could be that the bone formation–depressing effect exerted by β1-AR blocking of propranolol may have been indirect, an effect that could also have been involved in tooth movement. Therefore, we removed the indirect β1-AR effect by examining the effect of BUT, a specific β2-AR antagonist, in SHR and found that this showed hyperactivity of the sympathetic nervous system.
Materials & Methods
Animals and Reagents
We purchased eight-week-old male SHR/Izm: a genetic model of essential hypertension with elevated blood pressure, increased heart rate, raised plasma catecholamine level and dopamine β-hydroxylase activity, and adrenal TH and dopamine β-hydroxylase activities. In addition, we purchased WKY/Izm, a normotensive genetic control of SHR, from Japan SLC Inc. (Hamamatsu, Japan). Rats were housed in cages (3 rats/cage) under automatically controlled conditions of temperature (23°C ± 1°C), humidity (50% ± 10%), and a 12:12-hour light-dark cycle. They were given tap water and standard laboratory chow ad libitum. All procedures complied with the guidelines for animal experiments at the School of Dentistry, Aichi-Gakuin University. BUT hydrochloride—α-(1-[t-butylamino]ethyl)-2,5-dimethoxybenzyl alcohol—was purchased from Sigma (St. Louis, MO, USA).
Groups of SHR and WKY (6 rats/group) were administered BUT at a dose of 1 mg/kg (per os) with a gastric tube once daily for 6 wk. SHR and WKY control were administered saline (1 mL/kg) in the same way. Each rat received a calcein injection (15 mg/kg, intraperitoneal) at 10 and 3 days before sacrifice. All rats were sacrificed by drawing whole blood from the abdominal aorta via a heparinized syringe under ether anesthesia.
Experimental Tooth Movement
Two weeks after the initiation of administration, the rats were anesthetized with diethylether during the setting and adjustment of an orthodontic appliance as detailed previously (Dunn et al., 2007). A closed-coil nickel-titanium spring (Sentalloy, Tomy, Japan) providing a force of 50 g at 4-mm activation was connected between the maxillary first molar and maxillary central incisor teeth with 0.010-in. steel ligatures. After the ligatures were tied and cut, composite resin (Transbond; 3M Unitek, Japan) was placed over the wire to prevent slipping as well as pulpal irritation due to exposed dentin. According to the method of Dunn et al. (2007), the amount of tooth movement was also measured. Before and after treatment, polyvinylsiloxane (FUSION 2, GC, Japan) impressions of the maxillary arches were taken. Following fabrication of precise stone models (NEW FUJIROCK, GC, Japan), the occlusal tooth surfaces were scanned (ES-10000G, Epson, Japan) adjacent to a 100-mm ruler and then magnified 100× with calibrated imaging software (Adobe Photoshop CS5, Adobe Systems, Inc., San Jose, CA, USA). Distance from the distobuccal groove of the maxillary first molar to the most distal surface of the maxillary third molar was measured before and after treatment with Adobe Photoshop’s measuring tool. The amount of tooth movement was calculated as the difference between the 2 measurements.
Histomorphometry Analysis
Half the alveolar bone sample was embedded in resin, a mixture of methyl methacrylate, monomer, benzoyl peroxide, nonylphenyl-polyethyleneglycol acetate, and N,N-dimethyl-p-toluidine. Serial undecalcified 7-µm-thick sagittal sections were made. The bone formation rate per bone surface (BFR/BS; µm3/µm2/d) was calculated via the following formula: The mineral apposition rate was calculated as the mean distance between 2 labels of calcein divided by the labeling interval: (7 d) × (single-labeled surface / 2BS + double-labeled surface / BS). In addition, half the alveolar bone sample was decalcified in 20% EDTA (pH 7.4) for 3 wk. The specimens were embedded in paraffin, sectioned longitudinally at a thickness of 4 µm and stained for tartrate-resistant acid phosphatase (TRAP). TRAP staining–positive multinucleated cells attached to bone were scored as osteoclasts. Measurements were made within a randomly selected area of 0.8 mm2 (1.0 mm × 0.8 mm). Histomorphometry was conducted to quantify the osteoclast surface per bone surface (Oc.S/BS), as defined by Parfitt et al. (1987). For immunohistochemistry, TH-immunoreactive nerve fibers were visualized by the avidin–biotin–horseradish peroxidase complex method. ImageJ software (National Institutes of Health) was used to quantify immunohistochemical data.
Trabecular Microarchitecture
Alveolar bone was subjected to 3-dimensional micro–computed tomography (µCT) analysis via an R_mCT µCT scanner (Rigaku, Tokyo, Japan). TRI/3D-BON software (Ratoc, Tokyo, Japan) was used to analyze the cancellous parameters: alveolar bone volume / tissue volume (BV/TV) and trabecular number (Tb.N). BV/TV and Tb.N were defined in the region surrounded by 3 roots of the maxillary first molar, as detailed previously (Kondo et al., 2013). The coronal extent of the root was demarcated by the adjacent alveolar crest.
Plasma Biochemical Parameters
Blood samples obtained from the abdominal aorta were centrifuged. The concentration of osteocalcin and activity of TRAP 5b, the enzymatically active form of TRAP secreted from osteoclasts in the plasma, were tested via the rat osteocalcin EIA kit (Biomedical Technologies, Inc., MA, USA) and rat TRAP assay kit (SBA Sciences, Immunodiagnostic Systems Ltd., UK), respectively.
Statistical Analysis
All data are presented as the means ± SEM, and statistical analysis was carried out by one-way analysis of variance (Tukey multiple comparison test). All statistical analyses were performed with GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA); p < .05 was considered to be significant.
Results
Body Weight
The initial body weights of the WKY and SHR were 221 ± 4.5 g and 214 ± 3.1 g, respectively. All rats gained weight in the same way during the experiment. The final body weights were as follows: WKY, 391 ± 9.2 g; SHR control, 380 ± 7.7 g; WKY treated with BUT at a dose of 1 mg/kg, 395 ± 8.7 g; SHR treated with BUT at a dose of 1 mg/kg, 383 ± 6.4 g. No significant difference in body weight was observed between WKY and SHR groups at the start of the experiment or among all groups treated with or without BUT at the end of the experiment.
Tooth Movement
SHR control showed an increase in mesial movement of the first molar tooth compared with WKY control, and SHR treated with BUT showed a decrease (Fig. 1A, 1B). In addition, WKY treated with BUT showed a decrease in mesial movement of the first molar tooth when compared with the WKY control (Fig. 1A, 1B). There was no significant difference between WKY control and SHR treated with BUT (Fig. 1C).
Figure 1.
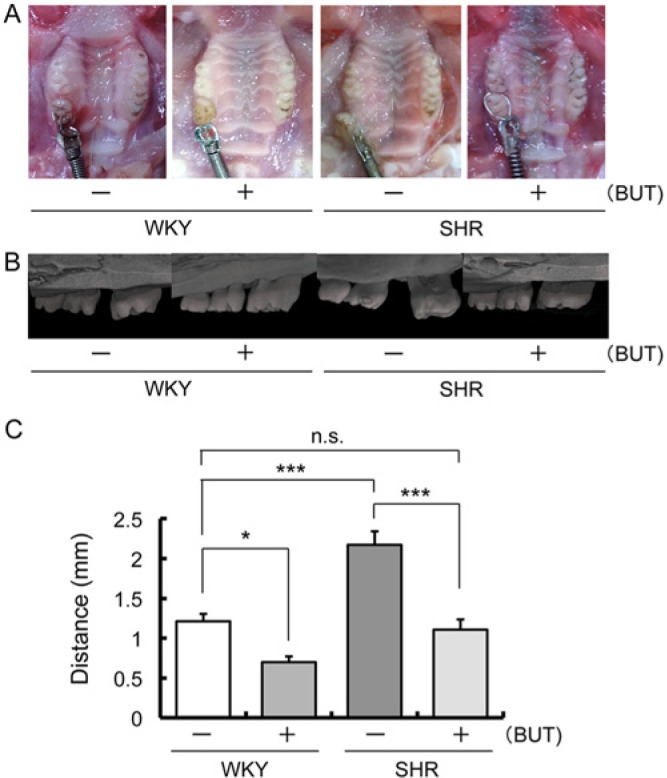
Tooth movement in spontaneously hypertensive rats (SHR) and Wistar-Kyoto rats (WKY) with 1 mg/kg of butoxamine (BUT) or vehicle administration. (A) Intraoral photographs 1 mo after spring insertion. (B) Representative microfocus X-ray computed tomography images of the maxillary first molar and second molar obtained 1 mo after spring insertion. (C) Mean distance between the maxillary first molar and second molar 1 mo after spring insertion (* p < .05; *** p < .001).
Histomorphometry Parameters of Alveolar Bone
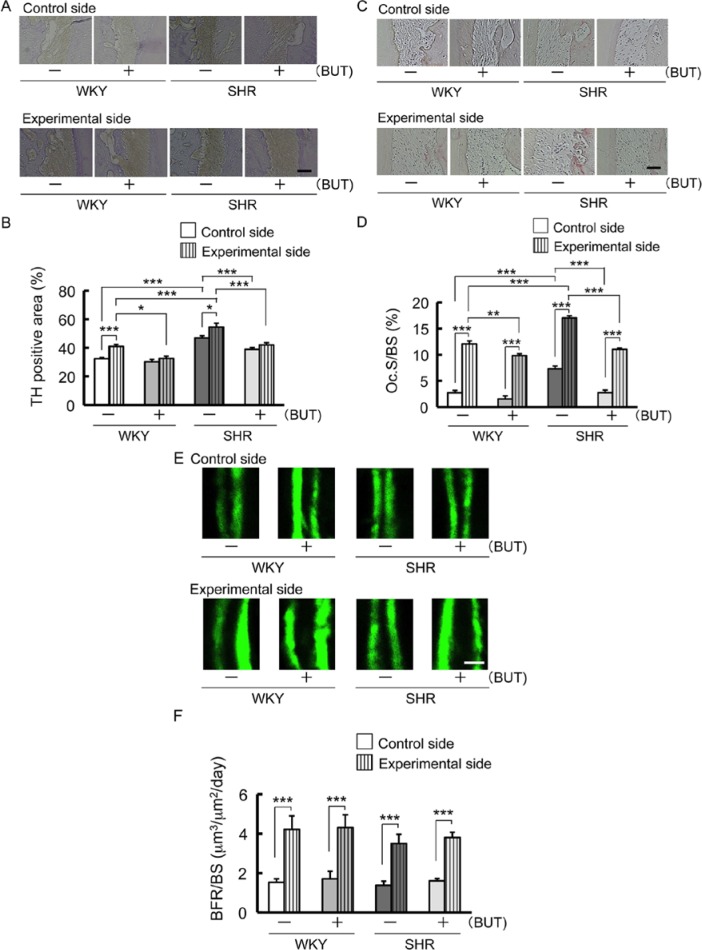
TH-immunoreactive nerves on the compression side were increased by tooth movement in WKY and SHR control (Fig. 2A, 2B). TH-positive area was larger in SHR control than in WKY control and decreased in SHR treated with BUT (Fig. 2B). Furthermore, BUT inhibited an increase of TH-positive area caused by tooth movement (Fig. 2B).
Figure 2.
Changes of tyrosine hydroxylase (TH) immunoreactivity in the periodontal ligament area on the compression side adjacent to the mesial root of the maxillary first molar; also, osteoclast activity on the compression side and bone formation parameter on the tension side along the alveolar bone 1 mo after spring insertion. Rats received an injection of calcein (15 mg/kg, intraperitoneal) 10 and 3 d before the end of the experiment. (A) Representative pictures of immunohistochemical photomicrographs in periodontal ligament area. Bar = 50 µm. (B) Comparison of changes in TH-positive area (* p < .05; *** p < .001). (C) Representative microscopic photographs of tartrate-resistant acid phosphatase (TRAP)–stained histologic sections of the alveolar bone. Bar = 50 µm. (D) Comparison of changes in osteoclast surface/bone surface (Oc.S/BS) (** p < .01; *** p < .001). (E) Representative fluorescent micrographs of undecalcified sections of the alveolar bone. Double labeling lines of calcein. Bar = 20 µm. (F) Comparison of changes in bone formation rate per bone surface (BFR/BS) (*** p < .001).
In the micrographs of TRAP-stained histologic sections on the compression side, red-stained osteoclasts were observed on the surface of alveolar bone (Fig. 2C). Oc.S/BS was increased by tooth movement (Fig. 2D). In addition, Oc.S/BS was higher in SHR control than in WKY control and decreased in SHR treated with BUT (Fig. 2D).
The distance between the 2 labeling lines of calcein on the fluorescent micrographs on the tension side was clearly increased by tooth movement (Fig. 2E). The value of BFR/BS was also increased by tooth movement (Fig. 2F).
µCT Analysis
In terms of tooth movement, bone mass measured by BV/TV of the alveolar bone was significantly decreased in SHR control in comparison with that in WKY control and increased in WKY and SHR treated with BUT in comparison with that in WKY and SHR control (Fig. 3B, 3C). In addition, BUT inhibited decreases of BV/TV and Tb.N caused by the tooth movement (Fig. 3C, 3D).
Figure 3.
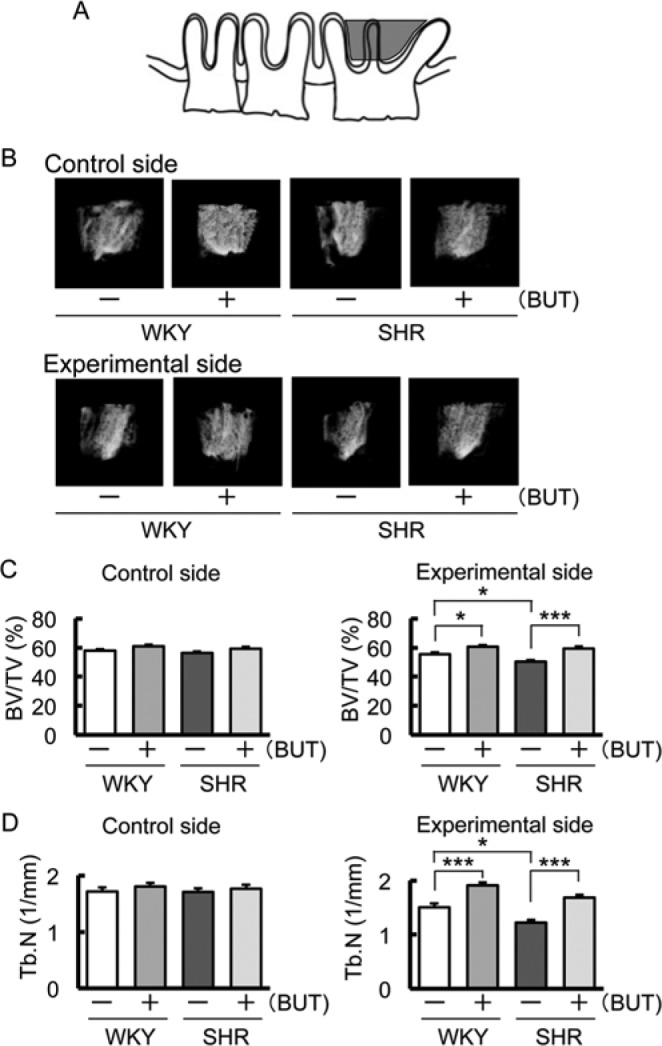
Comparison of bone volume/tissue volume (BV/TV) and trabecular number (Tb.N) measured from the furcation area to the tooth root apex of the maxillary first molar by micro–computed tomography 1 mo after spring insertion. (A) The region of the alveolar BV/TV and Tb.N is defined in the coronal region surrounded by the 3 roots of the first maxillary molar, as described in Materials & Methods (gray area). (B) Representative microfocus X-ray computed tomography images of alveolar BV/TV. (C) Comparison of changes in BV/TV (* p < .05; *** p < .001). (D) Comparison of changes in Tb.N (* p < .05; *** p < .001).
Plasma Biochemical Parameters in SHR
As shown in Figure 4, the osteocalcin concentration was increased in SHR treated with BUT compared with that in SHR control (Fig. 4A). In contrast, the plasma TRAP 5b level was increased in SHR control compared with that in WKY and decreased in SHR treated with BUT compared with that in SHR control (Fig. 4B).
Figure 4.
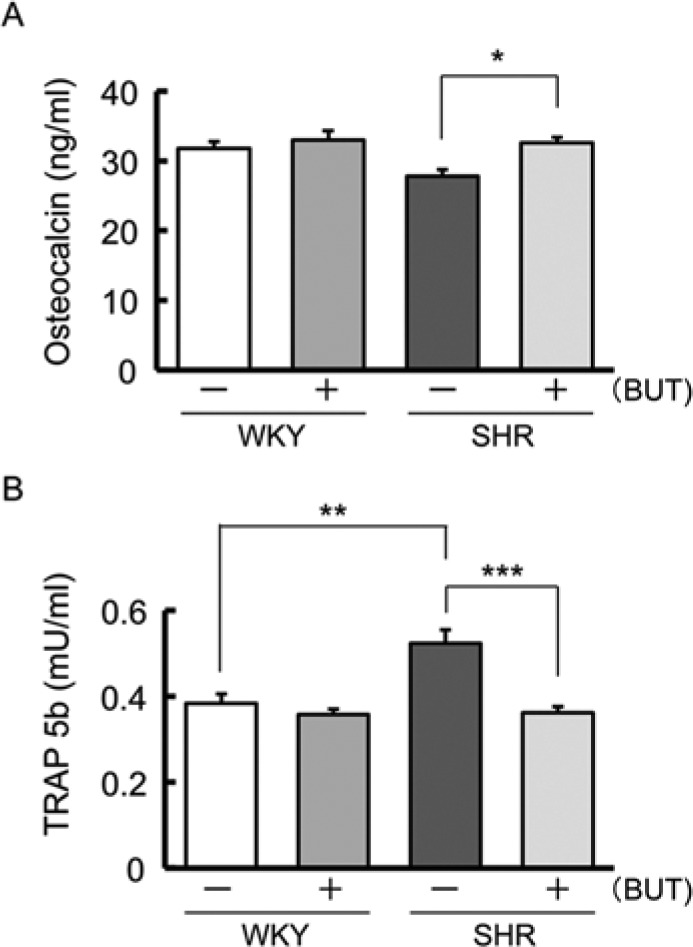
Mean plasma biochemical parameters of bone metabolism 1 mo after spring insertion. (A) Mean serum osteocalcin concentration in spontaneously hypertensive rats (SHR) and Wistar-Kyoto rats (WKY) 1 mo after spring insertion (** p < .01; *** p < .001). (B) Mean serum tartrate-resistant acid phosphatase (TRAP) activity in SHR and WKY 1 mo after spring insertion (* p < .05).
Discussion
In the present study, we examined the β2-AR blocking effect of BUT on tooth movement and alveolar bone mass. Increased tooth movement caused by an accelerated sympathetic nervous system was decreased by BUT. Our analysis of the histomorphometry suggested that bone resorption and formation were both accelerated by tooth movement. In addition, osteoclast appearance in alveolar bone and TH-immunoreactive nerves in the periodontal ligament on the compression side were increased, and BUT administration decreased osteoclast appearance and TH-immunoreactive nerves. On the compression side, the increases of bone resorption and TH-immunoreactive nerves and their decrease by BUT suggest a relationship between sympathetic nerve and bone resorption. BUT was used here at 1 mg/kg, but the results were similar at 10 mg/kg (data not shown). This supports the hypothesis that nerve fibers could act as mechanoreceptors in bone (Serre et al., 1999; Wada et al., 2001). However, in this study, the tension side and the sympathetic relationships were not clarified.
Orthodontic tooth movement causes alveolar bone loss (Ogaard, 1988; Bondemark, 1998; Janson et al., 2003). In our results, a decrease of alveolar bone mass by tooth movement was found in SHR. In addition, no difference was found in the alveolar bone mass in WKY and SHR on the control side, but the decrease in bone mass was greater in SHR than that in WKY when we induced tooth movement. Kondo et al. (2013) reported that propranolol did not influence bone mass. However, in this study, BUT surprisingly prevented bone loss associated with tooth movement, in addition to decreasing the amount of movement. To explain these different results, we considered that we observed the effect of the β2-AR alone without the indirect effect of β1-AR (Appendix Figure), as well as observing long-term bone mass. Plasma biochemical examination revealed increased osteocalcin and decreased TRAP 5b following BUT administration in SHR. However, although a tendency was found in WKY, this was not significant. No change in plasma TRAP 5b levels was observed in WKY and could explain why bone mass increased with BUT administration. We considered that the influence of BUT was easy to detect because sympathetic nervous activity is locally enhanced by tooth movement. These results show the possibility that BUT is useful as a drug to suppress the decrease of alveolar bone in orthodontic treatment. More than 50% of adults develop periodontal disease (Petersen et al., 2005), and in patients receiving orthodontic treatment with periodontal disease, BUT may decrease or prevent alveolar bone loss. Among healthy orthodontic patients, those with a thin width of alveolar bone may experience resorption of the alveolar bone and gingival recession due to tooth movement. BUT can prevent alveolar bone loss so that orthodontic treatment may be performed more safely.
In addition, the finding of an increase in tooth movement of the SHR, which is a model of hypertension, deserves attention. Because > 25% of adults have hypertension (Kearney et al., 2005), some patients who receive orthodontic treatment may be hypertensive. During orthodontic treatment of patients with hypertension, accelerated tooth movement and bone loss may occur. It has been suggested that tooth movement and bone loss due to orthodontic treatment in patients with hypertension can be controlled by use of BUT. We regard this as an important finding in orthodontic treatment.
However, the possibility that the α-adrenergic receptors affect bone mass was shown recently (Fonseca et al., 2011). It will be necessary to examine the effects that α-blockers have on tooth movement and to examine which blocker is most beneficial.
Conclusively, when quantitative load is measured via dental traction with a spring over a relatively long period in the clinical setting, BUT prevents alveolar bone loss and orthodontic tooth movement via β2-AR blocking. We suggest that tooth movement can be controlled by BUT, which will be a useful drug to suppress alveolar bone loss in orthodontic treatment.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (25870861).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arai M, Sato T, Takeuchi S, Goto S, Togari A. (2013). Dose effects of butoxamine, a selective b2-adrenoceptor antagonist, on bone metabolism in spontaneously hypertensive rat. Eur J Pharmacol 701:7-13. [DOI] [PubMed] [Google Scholar]
- Bjurholm A. (1991). Neuroendocrine peptides in bone. Int Orthop 15:325-329. [DOI] [PubMed] [Google Scholar]
- Bondemark L. (1998). Interdental bone changes after orthodontic treatment: a 5-year longitudinal study. Am J Orthod Dentofacial Orthop 114:25-31. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, et al. (2007). Protective effect of β blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40:1209-1216. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, et al. (2008a). Low dose β-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol 217:819-827. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Pierroz DD, Ferrari SL. (2008b). Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact 8:94-104. [PubMed] [Google Scholar]
- Dunn MD, Park CH, Kostenuik PJ, Kapila S, Giannobile WV. (2007). Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone 41:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca TL, Jorgetti V, Costa CC, Capelo LP, Covarrubias AE, Moulatlet AC, et al. (2011). Double disruption of α2A- and α2C-adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J Bone Miner Res 26:591-603. [DOI] [PubMed] [Google Scholar]
- Graham S, Hammond-Jones D, Gamie Z, Polyzois I, Tsiridis E, Tsiridis E. (2008). The effect of β-blockers on bone metabolism as potential drugs under investigation for osteoporosis and fracture healing. Expert Opin Investig Drugs 17:1281-1299. [DOI] [PubMed] [Google Scholar]
- Hill EL, Elde R. (1991). Distribution of CGRP-, VIP-, DβH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 264:469-480. [DOI] [PubMed] [Google Scholar]
- Izawa Y, Sagara K, Kadota T, Makita T. (1985). Bone disorders in spontaneously hypertensive rat. Calcif Tissue Int 37:605-607. [DOI] [PubMed] [Google Scholar]
- Janson G, Bombonatti R, Brandão AG, Henriques JF, de Freitas MR. (2003). Comparative radiographic evaluation of the alveolar bone crest after orthodontic treatment. Am J Orthod Dentofacial Orthop 124:157-164. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. (2005). Global burden of hypertension: analysis of worldwide data. Lancet 365:217-223. [DOI] [PubMed] [Google Scholar]
- Klemola R, Huttunen P, Laine M, Weckström M, Hirvonen J. (1999). Catecholamines in pericardial fluid of normotensive, spontaneously hypertensive and reserpine-treated rats. Acta Physiol Scand 165:293-297. [DOI] [PubMed] [Google Scholar]
- Kondo M, Kondo H, Miyazawa K, Goto S, Togari A. (2013). Experimental tooth movement-induced osteoclast activation is regulated by sympathetic signaling. Bone 52:39-47. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Kato T, Numata Y, Keiko I, Umezawa H. (1974). Serum dopamine β-hydroxylase activity in developing hypertensive rats. Nature 251:630-631. [DOI] [PubMed] [Google Scholar]
- Ogaard B. (1988). Marginal bone support and tooth lengths in 19-yearolds following orthodontic treatment. Eur J Orthod 10:180-186. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. (1987). Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595-610. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC; Geelong Osteoporosis Study (2004). β-Adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res 19:19-24. [DOI] [PubMed] [Google Scholar]
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. (2005). The global burden of oral diseases and risks to oral health. Bull World Health Organ 83:661-669. [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, et al. (2012). Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res 27:1252-1262. [DOI] [PubMed] [Google Scholar]
- Sato T, Arai M, Goto S, Togari A. (2010). Effects of propranolol on bone metabolism in spontaneously hypertensive rats. J Pharmacol Exp Ther 334:99-105. [DOI] [PubMed] [Google Scholar]
- Schlienger RG1, Kraenzlin ME, Jick SS, Meier CR. (2004). Use of β-blockers and risk of fractures. JAMA 292:1326-1332. [DOI] [PubMed] [Google Scholar]
- Seeman E. (2002). Pathogenesis of bone fragility in women and men. Lancet 359:1841-1850. [DOI] [PubMed] [Google Scholar]
- Serre CM, Farlay D, Delmas PD, Chenu C. (1999). Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25:623-629. [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M. (2008). Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci 106:542-546. [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M, Kondo A. (2005). The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin Ther Targets 9:931-940. [DOI] [PubMed] [Google Scholar]
- Wada S, Kojo T, Wang YH, Ando H, Nakanishi E, Zhang M, et al. (2001). Effect of loading on the development of nerve fibers around oral implants in the dog mandible. Clin Oral Implants Res 12:219-224. [DOI] [PubMed] [Google Scholar]
- Wang TM, Hsu JF, Jee WS, Matthews JL. (1993). Evidence for reduced cancellous bone mass in the spontaneously hypertensive rat. Bone Miner 20:251-264. [DOI] [PubMed] [Google Scholar]
- Zhang W, Thorén P. (1998). Hyper-responsiveness of adrenal sympathetic nerve activity in spontaneously hypertensive rats to ganglionic blockade, mental stress and neuronglucopenia. Pflugers Arch 437:56-60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.