Abstract
We conducted a cross-sectional analysis to evaluate the relationship between serum antibody titers against 19 selected oral microorganisms and measures of hyperglycemia in a large, nationally representative data set. The study population consisted of 7,848 participants from the National Health and Nutrition Examination Survey III (1988-1994) who were at least 40 yrs old, with complete serum IgG antibody data against 19 oral microorganisms. The 19 antibody titers were grouped into 4 categories via cluster analysis—orange-red, yellow-orange, orange-blue, and red-green—named to reflect predominant antibody titers against microorganisms in Socransky’s classification scheme for oral microbes. Linear regression models weighted for complex survey design were used in which fasting blood glucose, fasting insulin, and HbA1c were outcomes and antibody cluster scores were exposures, adjusting for potential confounders. Higher orange-red cluster scores were associated with increased hyperglycemia, while higher orange-blue cluster scores were related with decreased hyperglycemia. A 1-unit-higher orange-red cluster score was associated with 0.46 mg/dL higher fasting blood glucose (p = .0038), and a 1-unit-higher orange-blue cluster score was associated with 0.34% lower HbA1c (p = .0257). Groups of antibody titers against periodontal microorganisms were associated with hyperglycemia independent of known risk factors.
Keywords: periodontal microorganisms, antibody titer, periodontal diseases, diabetes, prediabetic state, HbA1c
Introduction
Periodontal infection is associated with hyperglycemia and diabetes (Demmer et al., 2012; Borgnakke et al., 2013). Approximately 11% of U.S. adults have diabetes, and 39% have moderate to severe periodontal disease (Eke et al., 2012). Diabetes prevalence is approximately 4 times higher in people with the most severe periodontal damage (Choi et al., 2011), and periodontal treatment in diabetes generally improves glycemic control (Engebretson and Kocher, 2013); however, a recent clinical trial of nonsurgical periodontal treatment showed otherwise (Engebretson et al., 2013).
There is emerging evidence to support the hypothesis that specific groups of periodontal microorganisms are associated with systemic outcomes. In a longitudinal analysis, carotid intimal medial thickness increase was attenuated with decreased counts of a selected group of oral microorganisms (Desvarieux et al., 2013). Some studies have reported differences in the oral microorganism profile of individuals with and without type 2 diabetes (Ebersole et al., 2008; Makiura et al., 2008), while others have not (Yuan et al., 2001; da Cruz et al., 2008; Field et al., 2012). Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella spp., Fusobacterium spp., and a few other organisms have been associated with type 2 diabetes (Ebersole et al., 2008; Makiura et al., 2008; Casarin et al., 2013; Zhou et al., 2013). The studies evaluating this relationship had small sample sizes and were heterogeneous with respect to method of microbial assessment, age, severity of periodontal disease, degree of diabetes control, and treatment of both periodontal disease and diabetes.
IgG antibody titers are produced in response to infections and remain elevated over a period of 30 mo even after periodontal treatment (Papapanou et al., 2004), and P. gingivalus and A. actinomycetemcomitans were found stable over 15 yrs among individuals who had periodontitis (Lakio et al., 2009). IgG antibody titers against oral microorganisms may therefore represent a cumulative measure of periodontal infection. In 2008, the Centers for Disease Control and Prevention released IgG antibody titer values for oral microorganisms (Vlachojannis et al., 2010) from analysis of stored serum samples of individuals who participated in the National Health and Nutrition Examination Survey III (NHANES III), conducted between 1988 and 1994. The analyses in this report are based on those data. The aim of this study was to evaluate the relationship between serum antibody titers against 19 selected microorganisms and measures of hyperglycemia in a large, nationally representative sample of U.S. adults.
Materials & Methods
Data Source
We conducted a cross-sectional analysis of the NHANES III data, a nationally representative sample of the noninstitutionalized U.S. population, collected through multistage probability cluster sampling. The data included information on a variety of health risks and behaviors, diabetes, periodontal disease, and clinical and antibody information related to periodontal disease (Ezzati et al., 1992).
The study population consisted of NHANES III participants who were at least 40 yrs old, with complete serum IgG antibody data against 19 oral microorganisms. Records of these individuals were linked with relevant dental, medical, socioeconomic, anthropometric, laboratory, and nutritional information available in NHANES III. All data for this report are available at http://www.cdc.gov/nchs/nhanes/nh3data.htm.
Study Population
Of 33,994 participants, 14,464 were at least 40 yrs old; 11,448 completed interviews; 9,379 underwent examinations; and 8,153 provided blood samples, which were analyzed for IgG antibody titer levels against a broad panel of 19 periodontal bacteria (Vlachojannis et al., 2010). Individuals were excluded if they reported taking insulin (mostly type 1 diabetes) or had gestational diabetes, leaving 7,848 participants in the final sample.
IgG Assay for Periodontal Bacteria
Sera from 8,153 NHANES III participants aged 40+ yrs were tested for IgG antibodies against 19 oral bacterial species via the “checkerboard” immunoassay technique at the Oral and Diagnostic Sciences Laboratory, Columbia University College of Dental Medicine; results were reported in gravimetric units. Details are described in NHANES III documentation (Centers for Disease Control and Prevention, 2008). The strains used to prepare whole cell antigenic extracts for checkerboard immunoassay are provided in the Appendix.
Cluster Formation and Naming the Clusters
Serum IgG antibody titer values for each bacterial species were natural log transformed and standardized by dividing by the log-transformed population standard deviation. To form antibody clusters, we used data from individuals with HbA1c ≥ 5.7 (n = 4,424). Standardized z scores of IgG antibodies against 19 periodontal bacteria were included in cluster analysis to derive 4 mutually distinct groups of IgG antibodies against periodontal bacteria, as described in the Figure. After deriving the clusters in this group, we calculated cluster scores for everybody in the population (healthy, prediabetes, diabetes) by summing z scores for each antibody titer (n = 7,874). For instance, one cluster consisted of P. melaninogenica, P. intermedia, P. nigrescens, and P. gingivalis. The z scores of these antibody titers were summed to obtain a score for that cluster.
Figure.
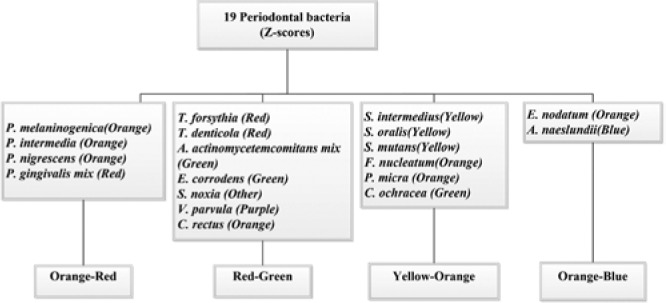
Composition of 4 mutually exclusive clusters formed via cluster analysis of z scores of serum antibody titers against 19 periodontal bacteria. Naming of clusters was done through Socransky’s microbial color complexes, shown in parentheses.
To name the clusters, we adapted Socransky’s color coding for periodontal bacteria as follows (Socransky and Haffajee, 2002, 2005): Cluster 1 included antibodies against 4 organisms, of which 3 were from Socransky’s orange complex and one was from the red complex; we named that cluster orange-red. Based on this approach, the 4 clusters were named orange-red, red-green, yellow-orange, and orange-blue, as shown in the Figure.
In Socransky’s scheme, organisms in the red and orange clusters are related to periodontal disease; yellow and purple cluster organisms are associated with a healthy periodontal state; blue cluster organisms (including the Actinomyces sp.) are found in both periodontal disease and healthy states; and green complex organisms are weakly related to periodontal disease (Socransky and Haffajee, 2002, 2005).
Exposure Measures
Cluster scores were computed for each individual in the study by summing the standardized z scores of IgG titers against oral microorganisms in each of the 4 clusters. These 4 cluster scores were the main exposure variables used in this analysis.
Outcome Measures
The 3 outcomes, which were log transformed, were fasting plasma insulin (n = 7,812), fasting plasma glucose (n = 7,812), and HbA1c percentage (n = 7,833).
Covariate Information
Covariates included age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), income-to-poverty ratio (≤1.5, >1.5 to ≤3.0, and >3.0), years of formal education (≤6 yrs, 7 to 12 yrs, ≥13 yrs), smoking and alcohol intake (current, past, never), physical activity metabolic equivalents (active, ≥6; moderate, ≥4 to <6; less active, <4), central adiposity (≥101.6 cm for males and ≥88.9 cm for females) (Choi et al., 2011), and diet (daily intake of calories; grams of carbohydrate, protein, fat, and fiber). Body mass index was computed from measured height and weight by dividing weight in kilograms by height in meters squared. Annual visits to the dentist was a binary variable (yes/no).
Statistical Methods
SAS 9.3 (SAS Institute, Cary, NC) was used for data management and statistical analyses. SAS survey procedures were used with sample weights, cluster, and strata variables provided by the Center for Disease Control and Prevention to account for the complex sampling design. The threshold for statistical significance was 0.05.
Descriptive statistics were estimated via procedures for complex surveys in SAS (proc surveymeans and proc surveyfreq) (Table 1). Linear regression models were run with log-transformed fasting plasma glucose, HbA1c, and fasting insulin as outcomes and with the 4 z score clusters as the predictors. The second model was adjusted for age and sex. The third model was further adjusted for race and ethnicity, income:poverty ratio, smoking, drinking alcohol, physical activity, fiber intake (continuous), dentist visits, waist circumference, and body mass index (continuous) (Table 2). These results were further stratified by teeth present (dentate and edentulous) (Table 3) and periodontal status, as defined by Eke et al. (2012): (1) no or mild periodontal destruction or (2) moderate or severe periodontal destruction (Table 4).
Table 1.
Characteristics of Study Population: National Health and Nutrition Examination Survey III (1988-1994)
| Characteristic | Healthy (n = 3,424) | Prediabetes (n = 3,334) | Diabetes (n = 1,090) |
|---|---|---|---|
| Age, yrs | 53.85 (0.3) | 59.5 (0.6) | 61.7 (0.6) |
| Sex | |||
| Male | 42.7 | 50.2 | 46.8 |
| Female | 57.3 | 49.8 | 53.2 |
| Race/ethnicity | |||
| Non-Hispanic whites | 85.3 | 76.9 | 71.6 |
| Non-Hispanic blacks | 6.1 | 11.3 | 14.6 |
| Mexican American | 2.6 | 3.9 | 6.0 |
| Others | 5.9 | 7.9 | 7.7 |
| Education, yrs | |||
| ≤6 | 4.7 | 8.5 | 12.3 |
| 7-12 | 50.4 | 57.6 | 62.2 |
| ≥13 | 44.9 | 33.9 | 25.4 |
| Income:poverty ratio | |||
| Lower, ≤1.5 | 15.0 | 22.0 | 30.2 |
| Middle, ≤3.0 | 28.6 | 30.5 | 31.5 |
| Higher, >3.0 | 56.4 | 47.4 | 38.3 |
| Smoking | |||
| Current | 41.6 | 35.1 | 37.2 |
| Past | 41.7 | 46.4 | 47.3 |
| Never | 16.7 | 18.5 | 15.5 |
| Alcohol intake | |||
| Current | 13.9 | 15.3 | 20.0 |
| Past | 34.0 | 42.2 | 48.9 |
| Never | 52.0 | 42.5 | 31.1 |
| Physical activity | |||
| Sedentary | 54.8 | 49.3 | 45.6 |
| Moderately active | 12.3 | 13.4 | 11.1 |
| Vigorously active | 8.0 | 7.4 | 6.7 |
| Missing | 24.9 | 29.8 | 36.6 |
| Missing teeth | |||
| 0 | 53.1 | 51.7 | 50.5 |
| 1-5 | 37.1 | 34.7 | 29.7 |
| 6-10 | 7.7 | 9.3 | 12.1 |
| >10 | 2.1 | 4.3 | 7.8 |
| Annual visits to dentist | |||
| Yes | 59.1 | 43.9 | 38.4 |
| No | 40.9 | 56.1 | 61.6 |
| Waist circumference | |||
| Normal | 60.0 | 45.2 | 23.8 |
| Elevated | 40.0 | 54.8 | 76.2 |
| Nutrition, g/d | |||
| Dietary fibers | 17.2 (0.3) | 16.4 (0.3) | 16.2 (0.5) |
| Total carbohydrate | 250.5 (4.1) | 243.9 (4.1) | 222.7 (5.1) |
| Proteins | 76.6 (1.0) | 77.8 (1.3) | 75.0 (2.2) |
| Total fats | 77.0 (1.3) | 75.7 (1.6) | 70.4 (2.8) |
| Body mass index, kg/m2 | 26.4 (0.1) | 27.8 (0.2) | 30.1 (0.3) |
| Score | |||
| Orange-red | −0.5 (0.1) | −0.3 (0.1) | 0.1 (0.2) |
| Red-green | −0.1 (0.3) | 0.02 (0.3) | −0.2 (0.5) |
| Yellow-orange | −0.01 (0.2) | 0.004 (0.2) | 0.06 (0.3) |
| Orange-blue | 0.2 (0.1) | 0.07 (0.1) | −0.04 (0.1) |
Values with parentheses indicate mean (SE). Stand-alone values indicate percentages.
Table 2.
Association between Cluster Scores and Fasting Blood Glucose, Glycemic Hemoglobin, and Fasting Insulin
| Orange-Red |
Red-Green |
Yellow-Orange |
Orange-Blue |
|
|---|---|---|---|---|
| eβ − 1 (95% CI) | eβ − 1 (95% CI) | eβ − 1 (95% CI) | eβ − 1 (95% CI) | |
| Fasting blood glucose | ||||
| Model 1 | 0.60 (0.32, 0.88) | 0.02 (−0.29, 0.33) | −0.21 (−0.54, 0.12) | −0.72 (−1.09, −0.35) |
| Model 2 | 0.51 (0.22, 0.81) | −0.08 (−0.38, 0.23) | −0.10 (−0.42, 0.23) | −0.49 (−0.87, −0.11) |
| Model 3 | 0.43 (0.14, 0.72) | −0.04 (−0.34, 0.25) | −0.07 (−0.40, 0.25) | −0.38 (−0.81, 0.06) |
| Model 4 | 0.46 (0.15, 0.76) | −0.08 (−0.38, 0.22) | −0.02 (−0.36, 0.31) | −0.35 (−0.79, 0.10) |
| HbA1c | ||||
| Model 1 | 0.34 (0.15, 0.55) | 0.03 (−0.16, 0.22) | −0.05 (−0.27, 0.17) | −0.68 (−0.98, −0.39) |
| Model 2 | 0.51 (0.22, 0.81) | −0.08 (−0.38, 0.23) | −0.10 (−0.42, 0.23) | −0.49 (−0.87, −0.11) |
| Model 3 | 0.17 (−0.06, 0.39) | −0.04 (−0.19, 0.13) | 0.11 (−0.09, 0.31) | −0.30 (−0.59, −0.01) |
| Model 4 | 0.17 (−0.07, 0.40) | −0.02 (−0.18, 0.15) | 0.09 (−0.10, 0.29) | −0.34 (−0.64, −0.04) |
| Fasting insulin | ||||
| Model 1 | 1.34 (0.40, 2.29) | 0.61 (−0.09,1.31) | −1.03 (−1.98, −0.07) | −0.89 (−2.06, 0.29) |
| Model 2 | 1.21 (0.27, 2.16) | 0.51 (−0.19, 1.21) | −0.89 (−1.84, 0.07) | −0.67 (−0.82, 0.48) |
| Model 3 | 0.60 (−0.05, 1.25) | 0.61 (−0.09,1.31) | −0.77 (−1.61, 0.08) | −0.82 (−1.90, 0.27) |
| Model 4 | 0.69 (0.002, 1.39) | 0.47 (−0.26, 1.22) | −0.58 (−1.48,0.34) | −0.69 (−0.80, 0.43) |
Back-transformed to natural units by exponentiation log-transformed estimates. CI, confidence interval. Bold indicates p < .05.
Orange-red: P. melaninogenica, P. intermedia, P. nigrescens, P. gingivalis. Red-green: T. forsythia, T. denticola, A. actinomycetemcomitans, E. corrodens, S. noxia, V. parvula, C. rectus. Yellow-orange: S. intermedius, S. oralis, S. mutans, F. nucleatum, P. micra (previously M. micros), C. ochracea. Orange-blue: E. nodatum, A. naeslundii.
Model 1: Crude association. Model 2: Age and sex adjusted. Model 3: Adjusted further for age, sex, education, race/ethnicity, income:poverty ratio, smoking, alcohol, physical activity, fiber intake, dentist visits, body mass index, and waist circumference. Model 4: Adjusted further for protein intake, carbohydrate intake, total fat, total calories, fiber intake, and dentist visits, including the variables in model 3.
Table 3.
Association between Cluster Scores and Fasting Blood Glucose, Glycemic Hemoglobin, and Fasting Insulin in the Dentate and Edentulous Population
| Orange-Red |
Red-Green |
Yellow-Orange |
Orange-Blue |
|
|---|---|---|---|---|
| eβ − 1 (95% CI) | eβ − 1 (95% CI) | eβ − 1 (95% CI) | eβ − 1 (95% CI) | |
| Dentate population (n = 5,430) | ||||
| Fasting blood glucose | ||||
| Model 1 | 0.53 (0.27, 0.80) | 0.16 (−0.19, 0.50) | −0.31 (−0.66, 0.05) | −0.97 (−1.42, −0.51) |
| Model 2 | 0.37 (0.09, 0.64) | 0.05 (−0.27, 0.37) | −0.16 (−0.49, 0.17) | −0.71 (−1.18, −0.24) |
| Model 3 | 0.22 (−0.07, 0.51) | 0.10 (−0.23, 0.43) | −0.17 (−0.52, 0.18) | −0.47 (−1.00, 0.06) |
| Model 4 | 0.26 (−0.04, 0.56) | 0.07 (−0.26, 0.39) | −0.12 (−0.48, 0.24) | −0.48 (−1.01, 0.06) |
| HbA1c | ||||
| Model 1 | 0.38 (0.19, 0.57) | 0.10 (−0.10, 0.30) | −0.10 (−0.35, 0.14) | −0.81 (−1.20, −0.44) |
| Model 2 | 0.26 (0.08, 0.44) | 0.02 (−0.15, 0.20) | −0.004 (−0.24, 0.24) | −0.56 (−0.92, −0.20) |
| Model 3 | 0.02 (−0.20, 0.24) | −0.01 (−0.18, 0.17) | 0.12 (−0.11, 0.40) | −0.30 (−0.65, −0.42) |
| Model 4 | 0.01 (−0.21, 0.24) | 0.01 (−0.16, 0.18) | 0.11 (−0.12, 0.35) | −0.37 (−0.73, −0.01) |
| Fasting insulin | ||||
| Model 1 | 1.37 (0.49, 2.26) | 0.86 (0.13, 1.60) | −1.31 (−2.23, −0.38) | −1.58 (−2.93,−0.20) |
| Model 2 | 1.10 (0.22, 2.0) | 0.71 (−0.005, 1.44) | −1.08 (−2.0, −0.16) | −1.30 (−2.67, 0.04) |
| Model 3 | 0.33 (−0.34, 1.0) | 0.85 (0.09, 1.61) | −0.93 (−1.80, −0.06) | −1.01 (−2.26, 0.25) |
| Model 4 | 0.48 (−0.25, 1.21) | 0.70 (−0.18, 1.5) | −0.71 (−1.64, 0.22) | −0.90 (−2.18, 0.40) |
| Edentulous population (n = 1,484) | ||||
| Fasting blood glucose | ||||
| Model 1 | 1.15 (0.49, 1.81) | −0.59 (−1.11,−0.07) | 0.04 (−0.65, 0.74) | 0.06 (−0.63, 0.75) |
| Model 2 | 1.11 (0.47, 1.76) | −0.69 (−1.21,−0.17) | 0.18 (−0.52, 0.87) | 0.14 (−0.54, 0.82) |
| Model 3 | 0.97 (0.45, 1.50) | −0.66 (−1.10,−0.23) | 0.45 (−0.07, 0.97) | 0.11 (−0.50, 0.71) |
| Model 4 | 0.98 (0.46, 1.51) | −0.68 (−1.11,−0.25) | 0.47 (−0.06, 1.00) | 0.13 (−0.48, 0.74) |
| HbA1c | ||||
| Model 1 | 0.59 (0.08, 1.09) | −0.09 (−0.44, 0.25) | −0.12 (−0.64, 0.40) | −0.28 (−0.85, 0.30) |
| Model 2 | 0.57 (0.06, 1.08) | −0.15 (−0.50, 0.21) | −0.05 (−0.57, 0.47) | −0.24 (−0.81, 0.34) |
| Model 3 | 0.38 (−0.04, 0.80) | −0.14 (−0.48, 0.21) | 0.17 (−0.26, 0.60) | −0.30 (−0.81, 0.20) |
| Model 4 | 0.35 (−0.06, 0.77) | −0.07 (−0.40, 0.26) | 0.11 (−0.30, 0.52) | −0.25 (−0.74, 0.24) |
| Fasting insulin | ||||
| Model 1 | 1.52 (−0.60, 3.70) | −0.18 (−2.02, 1.70) | −0.48 (−2.83, 1.93) | 2.93 (0.93, 4.98) |
| Model 2 | 1.67 (−0.46, 3.84) | −0.09 (−1.99, 1.84) | −0.61 (−2.97, 1.82) | 2.88 (0.82, 4.98) |
| Model 3 | 1.07 (−0.45, 2.61) | −0.46 (−1.76, 0.86) | 0.31 (−1.61, 2.26) | 1.11 (−0.81, 3.07) |
| Model 4 | 1.03 (−0.55, 2.62) | −0.43 (−1.77, 0.93) | 0.26 (−1.69, 2.25) | 1.33 (−0.69, 3.39) |
Back-transformed to natural units by exponentiation log transformed estimates. CI, confidence interval. Bold indicates p < .05.
Orange-red: P. melaninogenica, P. intermedia, P. nigrescens, P. gingivalis. Red-green: T. forsythia, T. denticola, A. actinomycetemcomitans, E. corrodens, S. noxia, V. parvula, C. rectus. Yellow-orange: S. intermedius, S. oralis, S. mutans, F. nucleatum, P. micra (previously M. micros), C. ochracea. Orange-blue: E. nodatum, A. naeslundii.
Model 1: Crude association. Model 2: Age and sex adjusted. Model 3: Adjusted further for age, sex, education, race/ethnicity, income:poverty ratio, smoking, alcohol, physical activity, dentist visits, body mass index, and waist circumference. Model 4: Adjusted further for protein intake, carbohydrate intake, total fat, total calories, fiber intake, and dentist visits, including the variables in model 3.
Table 4.
Association between Cluster Scores and Fasting Blood Glucose, Glycemic Hemoglobin, and Fasting Insulin Stratified by Periodontal Destruction in Dentate Population
| Orange–Red |
Red–Green |
Yellow–Orange |
Orange–Blue |
|
|---|---|---|---|---|
| eβ − 1 (95% CI) | eβ − 1 (95% CI) | eβ − 1 (95% CI) | eβ − 1 (95% CI) | |
| No periodontal destruction (n = 3,059) | ||||
| Fasting blood glucose | ||||
| Model 1 | 0.39 (0.03, 0.75) | 0.07 (−0.38, 0.52) | −0.22 (−0.61, 0.18) | −0.89 (−1.44,−0.33) |
| Model 2 | 0.22 (−0.14, 0.58) | −0.00 (−0.42, 0.42) | −0.11 (−0.47, 0.26) | −0.58 (−1.17, 0.02) |
| Model 3 | 0.08 (−0.23, 0.40) | 0.04 (−0.35, 0.43) | −0.13 (−0.49, 0.22) | −0.24 (−0.84, 0.36) |
| Model 4 | 0.10 (−0.24, 0.44) | 0.04 (−0.37, 0.45) | −0.10 (−0.47, 0.27) | −0.22 (−0.82, 0.38) |
| HbA1c | ||||
| Model 1 | 0.32 (0.09, 0.55) | −0.006 (−0.26, 0.25) | −0.002 (−0.33, 0.32) | −0.69 (−1.13,−0.25) |
| Model 2 | 0.21 (−0.01, 0.43) | −0.04 (−0.26, 0.18) | 0.05 (−0.24, 0.35) | −0.39 (−0.80, 0.02) |
| Model 3 | −0.02 (−0.28, 0.23) | −0.05 (−0.25, 0.15) | 0.15 (−0.11, 0.40) | −0.09 (−0.49, 0.31) |
| Model 4 | −0.07 (−0.31, 0.17) | −0.01 (−0.22, 0.20) | 0.12 (−0.14, 0.38) | −0.17 (−0.58, 0.25) |
| Fasting insulin | ||||
| Model 1 | 1.45 (−0.34, 3.26) | 0.50 (−0.27, 1.28) | −0.74 (−1.74, 0.27) | −1.88 (−3.52,−0.21) |
| Model 2 | 1.17 (−0.59, 2.96) | 0.39 (−0.40, 1.18) | −0.55 (−1.56, 0.47) | −1.49 (−3.13, 0.17) |
| Model 3 | 0.80 (−0.15, 1.77) | 0.62 (−0.06, 1.31) | −0.73 (−1.69, 0.25) | −0.91 (−2.34, 0.54) |
| Model 4 | 0.90 (−0.03, 1.83) | 0.46 (−0.27, 1.19) | −0.46 (−1.46, 0.56) | −0.57 (−2.11, 1.0) |
| Moderate or severe periodontal destruction (n = 2,371) | ||||
| Fasting blood glucose | ||||
| Model 1 | 0.39 (0.03, 0.75) | 0.07 (−0.38, 0.52) | −0.22 (−0.61, 0.18) | −0.89 (−1.44,−0.33) |
| Model 2 | 0.59 (0.21, 0.97) | 0.19 (−0.43, 0.82) | −0.31 (−1.05, 0.44) | −0.85 (−1.53,−0.16) |
| Model 3 | 0.46 (0.03, 0.89) | 0.23 (−0.49, 0.96) | −0.27 (−1.14, 0.62) | −0.92 (−1.72, −0.11) |
| Model 4 | 0.48 (−0.01, 0.97) | 0.17 (−0.54, 0.88) | −0.18 (−1.04, 0.69) | −0.92 (−1.77, −0.06) |
| HbA1c | ||||
| Model 1 | 0.39 (0.07, 0.70) | 0.26 (−0.16, 0.68) | −0.25 (−0.74, 0.25) | −0.75 (−1.32,−0.18) |
| Model 2 | 0.33 (−0.006, 0.66) | 0.18 (−0.23, 0.60) | −0.15 (−0.64, 0.34) | −0.69 (−1.25, −0.12) |
| Model 3 | 0.13 (−0.24, 0.51) | 0.13 (−0.36, 0.62) | 0.01 (−0.53, 0.56) | −0.67 (−1.26, −0.08) |
| Model 4 | 0.16 (−0.26, 0.59) | 0.11 (−0.38, 0.60) | 0.05 (−0.51, 0.61) | −0.69 (−1.33,−0.05) |
| Fasting insulin | ||||
| Model 1 | 1.06 (−0.48, 2.63) | 1.67 (−0.10, 3.48) | −2.57 (−4.73,−0.37) | −0.26 (−2.33, 1.86) |
| Model 2 | 0.95 (−0.61, 2.52) | 1.69 (−0.02, 3.43) | −2.54 (−4.62, −0.41) | −0.43 (−2.52, 1.71) |
| Model 3 | −0.37 (−1.73, 1.002) | 1.14 (−0.57, 2.88) | −1.29 (−3.42, 0.90) | −1.03 (−2.88, 0.86) |
| Model 4 | −0.04 (−1.43, 1.38) | 1.02 (−0.65, 2.72) | −1.21 (−3.31, 0.95) | −1.28 (−3.18, 0.64) |
Back-transformed to natural units by exponentiation log-transformed estimates. CI, confidence interval. Bold indicates p < .05.
Orange-red: P. melaninogenica, P. intermedia, P. nigrescens, P. gingivalis. Red-green: T. forsythia, T. denticola, A. actinomycetemcomitans, E. corrodens, S. noxia, V. parvula, C. rectus. Yellow-orange: S. intermedius, S. oralis, S. mutans, F. nucleatum, P. micra (previously M. micros), C. ochracea. Orange-blue: E. nodatum, A. naeslundii.
Model 1: crude association. Model 2: Age and sex adjusted. Model 3: Adjusted further for age, sex, education, race/ethnicity, income:poverty ratio, smoking, alcohol, physical activity, dentist visits, body mass index, and waist circumference. Model 4: Adjusted further for protein intake, carbohydrate intake, total fat, total calories, fiber intake, and dentist visits including the variables in model 3.
Results
In this sample, 9.6% of individuals had diabetes, and 36.6% had prediabetes. Individuals with prediabetes or diabetes were more likely to be older, male, nonwhite, with <12 yrs of formal education, belonging to the lower and middle-income categories; they were more likely to be past or current smokers and alcohol drinkers, have higher adiposity measures, consume less fiber, miss >6 teeth, and be less likely to visit a dentist yearly (Table 1).
Antibody clusters were associated with measures of hyperglycemia. A 1-unit-higher orange-red cluster score corresponded with 0.46 mg/dL higher fasting plasma glucose (p = .0038), and 1.69 µU/mL higher fasting insulin (p = .0494) after multivariable adjustment (Table 2). In contrast, a 1-unit-higher orange-blue cluster score was associated with 0.34% lower HbA1c (p = .0257). Red-green and yellow-orange clusters were not associated with any of the 3 hyperglycemia outcome measures (Table 2). Among the dentate population, the orange-blue cluster remained inversely associated with HbA1c and the orange-red cluster positively associated with fasting insulin. The association between the orange-red cluster and fasting glucose was positive in the edentulous population and positive but nonsignificant in the dentate population (Table 3). Among individuals with moderate or severe periodontal destruction, the orange-blue cluster remained inversely associated with HbA1c and the orange-red cluster positively associated with fasting insulin. These associations were qualitatively similar among individuals without periodontal disease, albeit they were not statistically significant (Table 4).
Discussion
Antibody clusters were associated with measures of hyperglycemia independent of other risk factors. Orange-red antibody cluster scores were positively correlated with fasting plasma glucose and fasting insulin, and orange-blue antibody cluster scores were negatively correlated with HbA1c, after accounting for other risk factors in multivariable analyses.
The orange-red cluster of antibodies included P. gingivalis, which has been found to be increased among individuals with diabetes (Ebersole et al., 2008; Makiura et al., 2008). IgG antibody levels against P. gingivalis are highest among individuals with periodontal disease. The IgG levels decline following periodontal treatment; however, they level out but still are higher than levels among individuals who never had periodontal disease (Kudo et al., 2012). IgG antibodies against P. gingivalis are a marker of periodontal disease among individuals without diabetes (Kudo et al., 2012) and may also be an indicator of periodontal disease in people with diabetes. Another explanation of these findings may be that as P. gingivalis is linked with systemic inflammation (Hayashi et al., 2010), which can impair insulin action and lead to hyperglycemia (Gregor and Hotamisligil, 2011). Periodontal treatment has been shown to reduce antibody titers against P. gingivalis (Okada et al., 2013) and improve glycemic control in people with diabetes (Sgolastra et al., 2013). In hyperglycemia, advanced glycation end products are deposited in the periodontal tissues (Lalla and Papapanou, 2011), which can lead to increased inflammation and periodontal destruction and possibly increased levels of P. gingivalis and its corresponding antibody titers.
The orange-blue cluster includes A. naeslundii and E. nodatum antibodies; A. naeslundii has been associated with a healthy state in oral (Socransky and Haffajee, 2002, 2005) and systemic conditions (Desvarieux et al., 2013), albeit through incompletely understood mechanisms. Even though the presence of E. nodatum and T. denticola in the mouth has been associated with periodontal disease (Haffajee et al., 2006), IgG antibodies against E. nodatum (orange complex), F. nucleatum (orange complex), and T. denticola (red complex) were higher in controls as compared with cases of periodontal disease (Papapanou et al., 2000). Higher antibody levels against these organisms may prevent subsequent colonization of these organisms in the mouth and thus be protective against new periodontal lesions, which may explain this finding.
This study has limitations. First, because of the cross-sectional design it was not possible to determine whether antibody titers contributed to hyperglycemia or were a consequence of it. For the same reason, the individuals in the study are prone to survivorship bias because those with more serious disease either died or did not participate in the survey. If the relationship between oral microorganisms and hyperglycemia were indeed causal, this bias would attenuate the associations that we detected. Second, we did not adjust for clinically defined periodontal disease (e.g., clinical attachment loss), because proliferation of periodontal microorganisms precedes periodontal destruction. Clinically defined, periodontal disease is thus on the causal pathway between oral microorganisms and the outcomes, and adjusting for it would cause bias (VanderWeele, 2009). The results were qualitatively similar after stratification by periodontal status. Moreover, we previously reported that clinical attachment loss and periodontal pocket depth were positively associated with diabetes and impaired fasting glucose in these data (Choi et al., 2011). Third, we were able evaluate only 19 of the many oral microorganisms in the mouth. Fourth, we included edentulous individuals in the main analysis because they have elevated IgG antibodies as a result of long-term prior exposure to oral microorganisms. Excluding edentulous individuals did not materially alter the results (Table 3). Fifth, although the data were not recent (i.e., 1988-1994) and prevalence of disease has changed (Eke et al., 2012), this should not influence the relation between serum antibodies and measures of hyperglycemia. Last, because this is an observational study, unmeasured confounding is possible, even though we accounted for a number of factors in the analyses.
The strengths of this study were as follows: First, this was a large representative population-based sample, reducing the likelihood of false-negative results. To our knowledge, this is the largest study to evaluate the relation between titers against oral microorganisms and hyperglycemia. Second, the antibody titers (Papapanou et al., 2001; Centers for Disease Control and Prevention, 2008) and the outcomes were measured in a standardized way. Third, we adjusted for a number of potential confounders, thereby reducing the chances of bias; moreover, the results were consistent in all analyses. Fourth, we grouped the antibody titers against oral microorganisms via cluster analysis. This method uses the correlations among different variables to form mutually exclusive groups. This was an appropriate method to classify antibodies against the 19 oral microorganisms because the microorganisms are correlated and their roles not yet clearly defined. We obtained cluster scores by summing z scores of the titers. We did this because we were interested in evaluating the relative (rather than absolute) contributions of the titers to the overall score. Forming the clusters also reduced the data, thereby minimizing the chance of spurious results from multiple testing (Wang et al., 2007). Finally, in the multivariable models, we evaluated the clusters together. Thus, a 1-unit increase in the orange-red titer score represented a corresponding change in a measure of hyperglycemia, holding the other clusters constant. With this approach, we were able to evaluate the composition antibody titers in relation to the outcome. This measure is biologically important because oral microorganisms are ubiquitous and changes in microbial composition occur relative to other organisms in the mouth (Desvarieux et al., 2013; Socransky & Haffajee, 2002, 2005).
Groups of antibody titers against periodontal microorganisms were associated with hyperglycemia independent of known risk factors. Antibody titers against periodontal microorganisms may characterize the relation between periodontal disease and hyperglycemia.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
Author Contributions: A.T.M. conceived the idea, wrote the first draft, directed the analyses, interpreted the results, and revised the manuscript. D.S. analyzed the data, interpreted the results, and revised the manuscript. C.C., Y.C., L.H., and J.Z. participated in analyses, helped with interpretation, and revised the manuscript.
The authors received funding from the American Diabetes Association (1-13-MUl-08).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. (2013). Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol 84(4):135S-152S. [DOI] [PubMed] [Google Scholar]
- Casarin RC, Barbagallo A, Meulman T, Santos VR, Sallum EA, Nociti FH, et al. (2013). Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J Periodontal Res 48:30-36. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2008). NHANES III: antibodies to periodontal pathogens. URL accessed on 5/14/2014 at: ftp://ftp.cdc.gov/pub/health_statistics/NCHS/nhanes/nhanes3/30a/spsdeppx.pdf.
- Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT. (2011). Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care 34:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz GA, de Toledo S, Sallum EA, Sallum AW, Ambrosano GM, de Cássia Orlandi Sardi J, et al. (2008). Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol 79:1150-1157. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, Jr., et al. (2012). Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999-2004. Diabetes Care 35:2235-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. (2013). Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the oral infections and vascular disease epidemiology study. J Am Heart Assoc 2:e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JL, Holt SC, Hansard R, Novak MJ. (2008). Microbiologic and immunologic characteristics of periodontal disease in Hispanic americans with type 2 diabetes. J Periodontol 79:637-646. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914-920. [DOI] [PubMed] [Google Scholar]
- Engebretson S, Kocher T. (2013). Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol 40(Suppl 14):153-163. [DOI] [PubMed] [Google Scholar]
- Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. (2013). The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 310:2523-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. (1992). Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2 113:1-35. [PubMed] [Google Scholar]
- Field CA, Gidley MD, Preshaw PM, Jakubovics N. (2012). Investigation and quantification of key periodontal pathogens in patients with type 2 diabetes. J Periodontal Res 47:470-478. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. (2011). Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415-445. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Teles RP, Socransky SS. (2006). Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol 21:269-282. [DOI] [PubMed] [Google Scholar]
- Hayashi C, Madrigal AG, Liu X, Ukai T, Goswami S, Gudino CV, et al. (2010). Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J Innate Immun 2:334-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo C, Naruishi K, Maeda H, Abiko Y, Hino T, Iwata M, et al. (2012). Assessment of the plasma/serum IgG test to screen for periodontitis. J Dent Res 91:1190-1195. [DOI] [PubMed] [Google Scholar]
- Lakio L, Antinheimo J, Paju S, Buhlin K, Pussinen PJ, Alfthan G. (2009). Tracking of plasma antibodies against Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis during 15 years [published online August 3, 2009]. J Oral Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. (2011). Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7:738-748. [DOI] [PubMed] [Google Scholar]
- Makiura N, Ojima M, Kou Y, Furuta N, Okahashi N, Shizukuishi S, et al. (2008). Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol Immunol 23:348-351. [DOI] [PubMed] [Google Scholar]
- Okada M, Kobayashi T, Ito S, Yokoyama T, Abe A, Murasawa A, et al. (2013). Periodontal treatment decreases levels of antibodies to Porphyromonas gingivalis and citrulline in patients with rheumatoid arthritis and periodontitis. J Periodontol 84:e74-e84. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlén G. (2000). “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol 71:885-897. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud AM, Sandros J, Dahlén G. (2001). Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J Clin Periodontol 28:103-106. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, Dahlén G. (2004). Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J Clin Periodontol 31:985-990. [DOI] [PubMed] [Google Scholar]
- Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. (2013). Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol 84:958-973. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. (2002). Dental biofilms: difficult therapeutic targets. Periodontol 2000 28: 12-55. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. (2005). Periodontal microbial ecology. Periodontol 2000 38: 135-187. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. (2009). Mediation and mechanism. Eur J Epidemiol 24:217-224. [DOI] [PubMed] [Google Scholar]
- Vlachojannis C, Dye BA, Herrera-Abreu M, Pikdöken L, Lerche-Sehm J, Pretzl B, et al. (2010). Determinants of serum IgG responses to periodontal bacteria in a nationally representative sample of US adults. J Clin Periodontol 37:685-696. [DOI] [PubMed] [Google Scholar]
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. (2007). Statistics in medicine: reporting of subgroup analyses in clinical trials. N Engl J Med 357:2189-2194. [DOI] [PubMed] [Google Scholar]
- Yuan K, Chang CJ, Hsu PC, Sun HS, Tseng CC, Wang JR. (2001). Detection of putative periodontal pathogens in non-insulin-dependent diabetes mellitus and non-diabetes mellitus by polymerase chain reaction. J Periodontal Res 36:18-24. [DOI] [PubMed] [Google Scholar]
- Zhou M, Rong R, Munro D, Zhu C, Gao X, Zhang Q, et al. (2013). Investigation of the effect of type 2 diabetes mellitus on subgingival plaque microbiota by high-throughput 16S rDNA pyrosequencing. PLoS One 8:e61516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


