Abstract
Reactionary dentin formation is an adaptive secretory response mediated by odontoblasts to moderate dentin injury. The implications of this process for neuroimmune interactions operating to contain pathogens have not been fully appreciated. The purpose of the present study was to describe the relationship between reactionary dentinogenesis, the neurogenic changes of dental pulp innervation, and dendritic cell recruitment to caries progression, using a comparative immunohistochemical approach in human teeth from young adult individuals. Reactionary dentin formation during dentin caries progression is associated with changes in the integrity of junctional complexes within the odontoblast layer. Diminished coexpression of Cx43 and zonula occludens 1 implies a reduced level of intercellular connectivity between odontoblasts. Dentin caries also causes overexpression of growth-associated protein 43, a modulator of neural plasticity that promotes extensive sprouting of nerve endings into the reactionary dentin matrix. At the same time, an elevated number of HLA-DR-positive dendritic cells infiltrate the odontoblast layer and subsequently invade reactionary dentin formed underneath the early caries-affected regions. Simultaneous odontoblast layer remodeling, nerve fiber sprouting, and activation of dendritic cells during caries progression suggest a coordinated neuroimmune response to fight caries pathogen invasion and to promote dentin-pulp healing. We propose that reactionary dentin formation hinders pathogen invasion and supports defensive neuroimmune interactions against infection. The eventual understanding of this complex scenario may contribute to the development of novel approaches to dental caries treatment.
Keywords: odontoblast, Cx43, ZO-1, dendritic cell, nociceptor, pain
Introduction
Biological understanding of dental pulp defense mechanisms against caries pathogens is crucial for the development of better caries treatments (Bjorndal, 2002). Reactionary dentin formation is considered an adaptive secretory response by odontoblasts to moderate dentin injury (Smith et al., 1995; Goldberg and Smith, 2004; Charadram et al., 2012). However, its implications for the local neuroimmune response fighting pathogens have not been fully appreciated. In mature teeth, the dentin-pulp complex forms a dynamic functional unit where dentinogenic, sensory, and defense mechanisms converge (Pashley, 1996; Byers et al., 2003). Dentinogenic activity is mediated by the odontoblast, a terminally differentiated postmitotic cell with a long-lived secretory condition (Couve et al., 2012). Mature odontoblasts are highly polarized columnar cells at the dentin-pulp interface and constitute a physiologic barrier at the periphery of the dental pulp (Couve et al., 2013; Ikeda and Suda, 2013). Odontoblasts are the first line of defense against dentin pathogen invasion. Important changes occur during the formation of reactionary dentin in the organization of the odontoblast layer and are evident at the dentin-pulp interface beneath carious infected dentin regions (Smith et al., 1995; Charadram et al., 2012).
The odontoblast layer also supports a terminal network of nociceptors branching from the vast sensory innervation of the dental pulp (Byers et al., 2003). It has been demonstrated that bacterial pathogens directly activate nociceptors, which constitute one of the principal mechanisms leading to neurogenic inflammation and pain (Chiu et al., 2013). In response to noxious stimuli and dentin caries, sensory nerves within the dental pulp undergo neuroplastic changes, including the sprouting of terminal branches (Sakurai et al., 1999; Byers et al., 2003).
Furthermore, within the dental pulp, dendritic cells are a major population of resident immune cells, predominantly located within the subodontoblast and paraodontoblast regions (Yoshiba et al., 1996; Jontell et al., 1998; Ohshima et al., 1999). Dendritic cells are potent antigen-presenting cells that initiate and modulate immune responses by sampling, processing, and presenting antigens from foreign pathogens to T cells (Banchereau and Steinman, 1998). As essential components of the innate and adaptive immune responses against pathogen invasion, they are crucial for the preservation of dental pulp vitality during pathogenic injuries caused by caries infection (Hahn and Liewehr, 2007; Goldberg et al., 2008; Farges et al., 2013).
Dental caries is a chronic infectious disease mediated by a complex and dynamic bacterial biomass that affects the mineralized tissues of the tooth. Bacterial invasion of dentinal tubules is a well-described phenomenon and the main cause of the inflammatory response of the dental pulp (Cooper et al., 2010). Therefore, the detection of caries pathogens is crucial to neutralize and eliminate injurious stimuli, to preserve dentin-pulp complex functionality, and to promote the healing of the dental pulp. Prior evidence suggests that the defensive process mediated by odontoblasts against caries pathogens occurs in parallel with immune responses at the dentin-pulp complex (Hahn and Liewehr, 2007; Veerayutthwilai et al., 2007; Horst et al., 2011; Farges et al., 2013). Furthermore, nociceptor sprouting has been characterized as an early neural reaction to dentin injury, related to reactionary but not reparative dentinogenesis (Byers et al., 2003). However, the role of reactionary dentinogenesis as an adaptive process that facilitates immune responses to dental caries pathogens is still poorly understood, and the physiopathologic dynamics among affected odontoblasts, neurogenic changes, and immune cells have not been related spatiotemporally to the dental caries process. The purpose of the present study was to describe the relationship among the formation of reactionary dentin by activated odontoblasts, the neurogenic changes in nerve endings of the dentin-pulp complex, and dendritic cell recruitment to caries progression, using a comparative immunohistochemical and microscopical approach in human teeth from young adult individuals.
Materials & Methods
Tissue and Sample Preparation
Fifteen healthy molars and 28 with occlusal caries lesions were obtained from young adult patients (18-28 yr). Teeth were extracted under local anesthesia for clinical indications and donated under signed consent according to the protocols of the Dental School Ethics Committee of the University of Valparaíso. Extracted teeth were immersed in fixative solution (4% paraformaldehyde in phosphate buffered saline, 0.5% picric acid, pH 7.4), and the crowns were separated from the roots. After 2 hr, the crowns were bisected along the mesiodistal axis with an Isomet low-speed diamond saw (Buehler, Lake Bluff, IL, USA). To define the histopathologic extent of dentin caries lesions, all samples were classified by visual examination via the International Caries Detection and Assessment System scoring criteria (Ismail et al., 2013). Dentin caries lesions were classified as follows: moderate caries, 15 teeth with scores of 3 or 4 (lesions extending to the outer or middle third of dentin), and severe caries, 13 teeth with scores of 5 or 6 (cavitated lesions affecting the inner third of dentin). Carious teeth were selected for active lesions characterized by bacterial invasion of dentinal tubules and significant changes in the odontoblast layer (Bjorndal, 2002). Samples were decalcified for 3 mo in 4% EDTA, pH 7.4.
Immunohistochemistry and Imaging
Demineralized crown samples were cryoprotected successively in 15% and 30% sucrose in phosphate buffered saline (pH 7.4) for 24 hr and frozen in tissue-freezing medium (Tissue-Tek, Sakura Finetek, Torrance, CA, USA); 20-µm-thick coronal dental pulp sections were cut with a cryostat (Leica CM-1900, Nussloch, Germany) at −20°C. The sections were collected and mounted on poly-L-lysine-covered microscope slides, rehydrated in phosphate buffered saline, and incubated for 1 hr in blocking solution containing 1% bovine serum albumin, 1% horse serum, and 0.3% Triton X-100. The primary and secondary antibodies used in the present study and their respective dilutions are shown in the Appendix Table. The primary antibodies were diluted in blocking solution and applied over night at 4°C. The secondary antibodies were applied for 1 hr at room temperature. All tissues sections were counterstained with DAPI and mounted in Fluomount mounting medium (Dako Industries, Carpenteria, CA, USA). Bacterial presence within dentinal tubules was confirmed by DAPI fluorescence. Immunolabeled sections of the coronal dental pulp were imaged with a confocal microscope (Nikon C1 plus, Nikon, Japan) with 409-, 488-, and 543-nm laser lines. Image stacks were processed with CZ software (Nikon, Japan) and displayed as maximum-intensity projection with ImageJ software (NIH, Bethesda, MD, USA). Adjustments of brightness and contrast were done with Adobe Photoshop CS4 (Adobe Systems, Mountain View, CA, USA).
Results
Reactionary dentin was observed in all carious samples at the dentin-pulp interface in moderate to severe carious lesions (Figs. 1 -3). A highly developed nerve fiber plexus was observed in the peripheral dental pulp beneath caries lesions, and sprouting axon endings appeared immersed within the collagen fibers of the reactionary dentin. At the same time, immunocompetent cells identified as dendritic cells by their immunoreactivity against HLA-DR were associated with neuronal components within the dentin-pulp complex.
Figure 1.
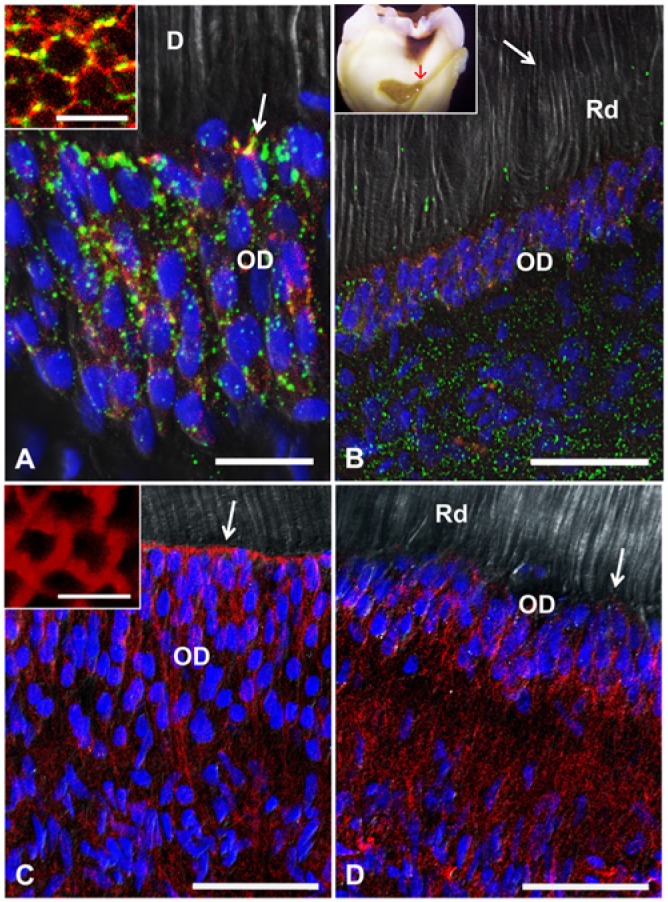
Confocal images of human odontoblasts from the coronal pulp. (A, B) Double immunolabeling for Cx43 (green) and zonula occludens 1 (red). (A) In healthy odontoblasts, Cx43 appears as discrete spots between cells and in larger profiles at the distal odontoblast-predentin-dentin interface (arrow). In oblique sections of the peripheral dental pulp, zonula occludens 1 is observed throughout the cell membrane at the level of the terminal web, with Cx43-immunoreactive profiles (inset). (B) Under caries conditions, odontoblasts beneath reactionary dentin display reduced Cx43 expression, mainly at the level of the terminal web. The boundary between orthodentin and reactionary dentin is indicated by the arrow. The inset of (B) shows a molar with moderate dentin caries (International Caries Detection and Assessment System score 4), indicating the area from which the samples were obtained (red arrow). (C, D) Phalloidin labeling of the actin cytoskeleton (red). (C) Healthy odontoblasts display a strong reaction at the terminal web (arrow). Oblique sections reveal actin localization beneath the cell membrane (inset). (D) In odontoblasts close to carious dentin, the terminal web is almost absent (arrow). Nuclei are labeled with DAPI. Scales: A, 20 µm; B-D, 50 µm; insets, 10 µm. D, dentin; OD, odontoblast layer; Rd, reactionary dentin.
Figure 2.
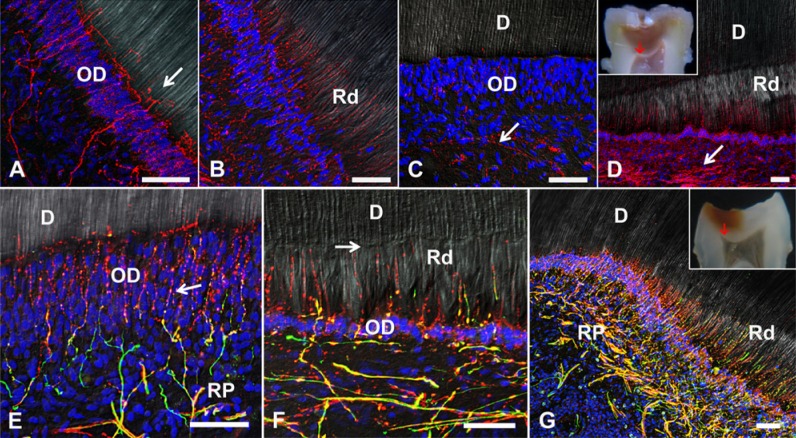
Nerve fibers innervating the dentin-pulp complex within the coronal dental pulp. (A, B) βIII-tubulin immunoreactivity (red). (A) Within a healthy section of dentin-pulp complex, a well-developed branched subodontoblast nerve plexus extends axon profiles through the odontoblast layer (OD) forming a complex terminal network at the predentin-dentin interface (arrow). (B) The nerve plexus beneath carious dentin appears highly sprouted with numerous terminal axons extending radially into the reactionary dentin matrix (Rd). (C, D) Growth-associated protein 43 (GAP-43) immunolabeling (red). (C) GAP-43 expression within the healthy dentin-pulp complex is seen as sparse labeling, mainly in axons from the Raschkow plexus (arrow). (D) In a severely carious molar with International Caries Detection and Assessment System score 5 (inset, red arrow indicates sample area), the axons of the Raschkow plexus are highly reactive for GAP-43, and labeling comprises fine axons projecting through a thinned odontoblast layer into the Rd. (E-G) Double immunolabeling of neurofilament (green) and βIII-tubulin (red). (E) In the healthy dentin-pulp complex, nerve fibers forming the Raschkow plexus (RP) coexpress neurofilament and βIII-tubulin, branch at the subodontoblast layer and form numerous varicosities between the odontoblasts (arrow). Nerve fibers extend vertically though the OD and form a complex terminal network at the predentin-dentin interface. (F) Rd underneath a moderate carious lesion contains sprouted nerve terminals that coexpress neurofilaments and βIII-tubulin, extending from a thinned OD toward the calciotraumatic line (arrow). (G) In a tooth with International Caries Detection and Assessment System score 5 caries (inset, red arrow indicates sample area), sprouting of the Raschkow plexus is evident as radial nerve fibers project into Rd. Scales: 50 µm.
Figure 3.
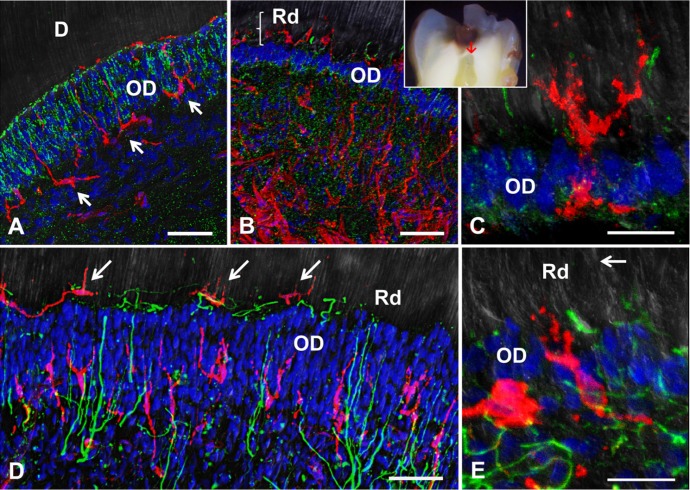
Confocal immunolabeling of HLA-DR-reactive immune cells located at the dentin-pulp complex. (A-C) Double labeling for Cx43 (green) and HLA-DR (red). (A) In a healthy tooth, the expression pattern of Cx43 within the odontoblast layer is highly ordered, surrounding the cells and forming a prominent line of gap junctions at the apical interface with dentin (D). Arrows indicate HLA-DR-positive dendritic cells. (B) In a moderately carious tooth (International Caries Detection and Assessment System score 4, inset; sample area indicated by red arrow), Cx43 is highly expressed within the dentin-pulp complex, with evident changes within the odontoblast layer (OD). (C) Under high magnification, it is possible to observe HLA-DR-positive cells resembling dendritic cells passing through the OD. (D, E) Double immunolabeling for neurofilament (green) and HLA-DR (red). (D) In moderate carious dentin lesions within the dentin-pulp complex beneath the infected dentin, enlarged sprouted axons are projected through the OD in close association with dendritic cell profiles. Some HLA-DR-positive dendritic cells appear nested within nerve fiber endings (arrow). (E) Dendritic cells in close association with fine axon endings within the narrow OD. The arrow indicates the boundary of reactionary dentin (Rd). Scales: A, B, D: 50 µm; C, E: 20 µm.
Odontoblast Alterations beneath Caries Lesions
In healthy human teeth, odontoblasts of the coronal dental pulp are highly polarized columnar cells arranged in a crowded layer. Double immunolabeling for Cx43 and zonula occludens 1 (ZO-1) revealed significant differences between healthy and carious conditions (Fig. 1A, B). Immunoreactivity of healthy mature odontoblasts displayed high levels of Cx43 expression at the distal portion of the cells, the transition region between the odontoblast cell body and its process. Homogeneous Cx43 immunoreactivity of lower intensity is evident throughout the odontoblast layer (Fig. 1A). At the distal region, the expression of ZO-1 forms a circumferential belt in which disclike Cx43-positive profiles appear inserted. In transverse sections obtained from the distal odontoblast layer, double immunolabeling reveals annular structures formed by ZO-1 with putative gap junctions (Fig. 1A, arrow and inset). Odontoblasts underneath the interface with reactionary dentin in carious lesions are reduced in size and lose their columnar shape, and Cx43 expression is reduced. The normal annular arrangement of Cx43 and ZO-1 is not observed, while diffuse ZO-1 labeling appears within the reduced odontoblast palisade (Fig. 1B). Rhodamine phalloidin labeling of cytoskeletal actin filaments in healthy odontoblasts revealed that actin is mainly arranged at the cortical cell domain, forming the terminal web at the distal region of the cells (Fig. 1C, arrow and inset). On the contrary, in altered odontoblasts beneath carious lesion, the terminal web was reduced or absent (Fig. 1D).
Nerve Fibers within the Dentin-Pulp Complex in Healthy and Carious Teeth
Myelinated and unmyelinated axons are peripherally arranged within the subodontoblast region of the dentin-pulp complex forming the Raschkow plexus and project fine nerve endings into the odontoblast layer and predentin-dentin interface (Fig. 2). Significant differences of nerve fiber density and the neural innervation pattern are evident in carious conditions versus healthy teeth. In the latter, βIII-tubulin immunoreactivity labels nerve fibers branching from the subodontoblast region through the odontoblast layer, forming a complex terminal network at the dentin-pulp interface (Fig. 2A), while in carious teeth, nerve fibers beneath infected dentin regions appear highly sprouted into the reactionary dentin matrix (Fig. 2B). Furthermore, the expression of growth-associated protein 43 (GAP-43), a general marker of neuronal plasticity, is significantly increased at the dentin-pulp complex beneath moderate to severe carious lesions, with dense neuronal sprouting (Fig. 2C, 2D). Neurofilament (NF200) is mainly present in myelinated axons, while βIII-tubulin is expressed in most of the fine nerve endings present within the odontoblast layer and the terminal network at the predentin-dentin interface. These fine axons emerge as strings of varicosities, which are presumed to be active nociceptor sites (Fig. 2E). Most axon endings traverse the odontoblast layer and project into reactionary dentin (Fig. 2B, 2F). In severe dentin carious lesions, the neurogenic response is even stronger, producing an extended network of axons at the peripheral dental pulp under caries-affected regions (Fig. 2G).
Immunohistochemistry of Dendritic Cells in the Dental Pulp
In healthy and carious teeth, dendritic cells are easily detected by labeling HLA-DR (Fig. 3). Dendritic cells are mainly distributed at the peripheral dental pulp within the subodontoblast and paraodontoblast region, extending large processes into the odontoblast layer and occasionally inserting dendrites into the predentin matrix (Fig. 3A). In carious teeth underneath moderate and severe dentin lesions, an increase of HLA-DR-positive immunolabeled cells within the dentin-pulp complex is evident, projecting dendrites into the adjacent reactionary dentin domains (Fig. 3B). Moreover, dendritic cells cross the reduced odontoblast layer and become resident within reactionary dentin (Fig. 3C). Dendritic cell invasion into reactionary dentin matrix in moderate dentin lesions is observed with nerve fiber sprouting (Fig. 3D), and an increased innervation density is evident in the immediate surroundings of dendritic cells within reactionary dentin (Fig. 3E).
Discussion
Invasion of dentinal tubules by caries pathogens triggers defense and repair mechanisms within the dentin-pulp complex to contain pathogen spread and reduce dental pulp tissue damage. The data obtained in this study suggest that odontoblasts situated beneath infected dentinal tubules secrete reactionary dentin, accompanied by neuronal sprouting and immune cell invasion into the reactionary collagen matrix. This scenario is most evident at the borders of the dental pulp interface beneath caries lesions, where dentinal tubule infection is still limited (Sakurai et al., 1999; Yoshiba et al., 2003). Mature odontoblasts are highly communicated by gap junctions. Intercellular junctional complexes formed by tight and gap junctions are mostly apically located to maintain cell polarity and the physiologic barrier integrity of the odontoblast layer (Couve et al., 2013; Ikeda and Suda, 2013). Previous studies established that the integrity of the odontoblast layer is affected by dental caries pathogens and restorative procedures (Turner et al., 1989). The present study demonstrates that once caries pathogens initiate the invasion of dentinal tubules, the odontoblast layer barrier beneath the carious lesion is reduced in thickness and junctional complexes are modified, affecting the patterns of gap junctions and the terminal web. Mature odontoblasts show a strong expression of the scaffold protein ZO-1 at the apical junctional complex (Joao and Arana-Chavez, 2003). Changes in the junctional complex and the cellular polarity of odontoblasts could be the result of modifications in the expression of ZO-1, since downregulation of ZO-1 has been associated with disassembly of intercellular junctions (Rhett et al., 2011). Regulation of the permeability of the odontoblast barrier might facilitate detection and contain invasion of dentin caries pathogens by immune cells.
Regularly spaced immune-competent cells (e.g., dendritic cells) act as sentinels against pathogen invasion at the dentin-pulp interface. Once activated by diffusible bacterial compounds, dendritic cells initiate an innate immune response against caries pathogens and their migration into the predentin-dentin interface toward the bacterial aggressor. The activation of these cells is an early event in caries progression and implies changes at the odontoblast layer barrier to allow their passage into the predentin-dentin interface. Likewise, it has been demonstrated that dendritic cells open tight junctions between epithelial cells and send dendrites across the epithelial barrier to detect pathogens in gut and upper respiratory epithelium (Rescigno et al., 2001; Kojima et al., 2013).
In the present study, we confirm that the neurogenic response to dentin caries progression comprises nerve fiber sprouting into the reactionary dentin matrix. During caries progression, the early detection of bacterial components at the dentin-pulp interface is a crucial event, mediated in part by TLR2 and TLR4 receptors expressed by odontoblasts (Veerayutthwilai et al., 2007; Horst et al., 2011). Moreover, TLR4 is expressed by nociceptors of the dental pulp, which are able to mediate severe inflammatory responses (Wadachi and Hargreaves, 2006). Our understanding of dental pulp pathogenesis under bacterial injury and the activation of immune responses within the dentin-pulp complex is still incomplete (Veerayutthwilai et al., 2007; Farges et al., 2013). Evidently, crosstalk between the nervous and immune systems is required for an effective defense against invading pathogens (Sakurai et al., 1999). Along these lines of evidence, sensory denervation of rat molars has been shown to reduce the survival and healing rate of exposed dental pulp tissue (Byers and Taylor, 1993) and trigger a significant reduction in immunocompetent cell recruitment after dentin injury (Fristad et al., 1995). In addition to slowing down pathogen progression into the dental pulp, reactionary dentin formation provides a supportive matrix for nerve sprouting into the caries-affected area. The increase in nerve fiber density in caries-affected regions may represent the physiologic substrate of the increased pain sensation in response to extensive and deep dentin carious lesions. The expression of GAP-43 in axons traversing the dental pulp is consistent with this notion, since GAP-43 is required for the sprouting of injured nerve fibers during regenerative processes (Aigner et al., 1995; Jimenez-Andrade and Mantyh, 2012). In this respect, the dentin-pulp complex behaves as other highly innervated tissues, in which the sprouting of nerve fibers is associated with the initial immune response to bacterial injury, activating neurogenic pain.
The neurogenic changes observed at the sites of reactionary dentin formation could be related to an increased synthesis of neuropeptides to promote inflammatory responses against caries pathogens (Byers et al., 2003; Sakurai et al., 1999). This interpretation is in line with the generally accepted notion that the crosstalk between the nervous system and the initial immune response is critical for tissue defense (Chiu et al., 2012). An increased liberation of neuropeptides has also been suggested to explain changes in pain symptoms associated with pulpitis progression, a classic neurogenic inflammatory response to caries injury (Henry and Hargreaves, 2007; Chiu et al., 2012). While the level of dentin infection determines the severity of the neuroimmune response in the first place, the persistence of dentin infection can aggravate dental pulp conditions, causing severe pain with the formation of local abscesses or dental pulp necrosis. The coexistence of neural and immune responses described here in the caries-infected human dentin is in correspondence with the formation of reactionary dentin by odontoblasts. Correlated odontoblast layer remodeling, nerve fiber sprouting, and activation of dendritic cells during caries progression suggest that these events form part of a coordinated neuroimmune response required to detect and contain caries pathogen invasion and to promote dentin-pulp healing. The early neuroimmune response needs to be taken into account in the development of novel approaches to dental caries treatment.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the Chilean government through FONDECYT grant Nos. 1141281 and 1120513 and the Millennium Institute CINV.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, et al. (1995). Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83:269-278. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. (1998). Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- Bjorndal L. (2002). Dentin and pulp reactions to caries and operative treatment: biological variables affecting treatment outcome. Endodontic Topics 2:10-23. [Google Scholar]
- Byers MR, Taylor PE. (1993). Effect of sensory denervation on the response of rat molar pulp to exposure injury. J Dent Res 72:613-618. [DOI] [PubMed] [Google Scholar]
- Byers MR, Suzuki H, Maeda T. (2003). Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc Res Tech 60:503-515. [DOI] [PubMed] [Google Scholar]
- Charadram N, Farahani RM, Harty D, Rathsam C, Swain MV, Hunter N. (2012). Regulation of reactionary dentin formation by odontoblasts in response to polymicrobial invasion of dentin matrix. Bone 50:265-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ. (2012). Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15:1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. (2010). Inflammation-regeneration interplay in the dentine-pulp complex. J Dent 38:687-697. [DOI] [PubMed] [Google Scholar]
- Couve E, Osorio R, Schmachtenberg O. (2012). Mitochondrial autophagy and lipofuscin accumulation in aging odontoblasts. J Dent Res 91:696-701. [DOI] [PubMed] [Google Scholar]
- Couve E, Osorio R, Schmachtenberg O. (2013). The amazing odontoblast: activity, autophagy, and aging. J Dent Res 92:765-772. [DOI] [PubMed] [Google Scholar]
- Farges JC, Alliot-Licht B, Baudouin C, Msika P, Bleicher F, Carrouel F. (2013). Odontoblast control of dental pulp inflammation triggered by cariogenic bacteria. Front Physiol 4:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad I, Heyeraas KJ, Kvinnsland IH, Jonsson R. (1995). Recruitment of immunocompetent cells after dentinal injuries in innervated and denervated young rat molars: an immunohistochemical study. J Histochem Cytochem 43:871-879. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Smith AJ. (2004). Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med 15:13-27. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Farges JC, Lacerda-Pinheiro S, Six N, Jegat N, Decup F, et al. (2008). Inflammatory and immunological aspects of dental pulp repair. Pharmacol Res 58:137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CL, Liewehr FR. (2007). Innate immune responses of the dental pulp to caries. J Endod 33:643-651. [DOI] [PubMed] [Google Scholar]
- Henry MA, Hargreaves KM. (2007). Peripheral mechanisms of odontogenic pain. Dent Clin North Am 51:19-44. [DOI] [PubMed] [Google Scholar]
- Horst OV, Horst JA, Samudrala R, Dale BA. (2011). Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Suda H. (2013). Odontoblastic syncytium through electrical coupling in the human dental pulp. J Dent Res 92:371-375. [DOI] [PubMed] [Google Scholar]
- Ismail AI, Tellez M, Pitt NB, Ekstrand KR, Ricketts D, Longbottom C, et al. (2013). Caries management pathways preserve dental tissues and promote oral health. Community Dent Oral Epidemiol 41:e12-e40. [DOI] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Mantyh PW. (2012). Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 14:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joao SM, Arana-Chavez VE. (2003). Expression of connexin 43 and ZO-1 in differentiating ameloblasts and odontoblasts from rat molar tooth germs. Histochem Cell Biol 119:21-26. [DOI] [PubMed] [Google Scholar]
- Jontell M, Okiji T, Dahlgren U, Bergenholtz G. (1998). Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med 9:179-200. [DOI] [PubMed] [Google Scholar]
- Kojima T, Go M, Takano K, Kurose M, Ohkuni T, Koizumi J, et al. (2013). Regulation of tight junctions in upper airway epithelium. Biomed Res Int 2013:947072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima H, Maeda T, Takano Y. (1999). The distribution and ultrastructure of class II MHC-positive cells in human dental pulp. Cell Tissue Res 295:151-158. [DOI] [PubMed] [Google Scholar]
- Pashley DH. (1996). Dynamics of the pulpo-dentin complex. Crit Rev Oral Biol Med 7:104-133. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. (2001). Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361-367. [DOI] [PubMed] [Google Scholar]
- Rhett JM, Jourdan J, Gourdie RG. (2011). Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22:1516-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Okiji T, Suda H. (1999). Co-increase of nerve fibers and HLA-DR- and/or factor-XIIIa-expressing dendritic cells in dentinal caries-affected regions of the human dental pulp: an immunohistochemical study. J Dent Res 78:1596-1608. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H. (1995). Reactionary dentinogenesis. Int J Dev Biol 39:273-280. [PubMed] [Google Scholar]
- Turner DF, Marfurt CF, Sattelberg C. (1989). Demonstration of physiological barrier between pulpal odontoblasts and its perturbation following routine restorative procedures: a horseradish peroxidase tracing study in the rat. J Dent Res 68:1262-1268. [DOI] [PubMed] [Google Scholar]
- Veerayutthwilai O, Byers MR, Pham TT, Darveau RP, Dale BA. (2007). Differential regulation of immune responses by odontoblasts. Oral Microbiol Immunol 22:5-13. [DOI] [PubMed] [Google Scholar]
- Wadachi R, Hargreaves KM. (2006). Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 85:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba K, Yoshiba N, Iwaku M. (2003). Class II antigen-presenting dendritic cell and nerve fiber responses to cavities, caries, or caries treatment in human teeth. J Dent Res 82:422-427. [DOI] [PubMed] [Google Scholar]
- Yoshiba N, Yoshiba K, Nakamura H, Iwaku M, Ozawa H. (1996). Immunohistochemical localization of HLA-DR-positive cells in unerupted and erupted normal and carious human teeth. J Dent Res 75:1585-1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.