Abstract
Bone marrow–derived mesenchymal stem/progenitor cells (BMSCs) are commonly used in regeneration therapy. The current primary source of BMSCs is the iliac crest; however, the procedure is associated with various burdens on the patient, including the risk of pain and infection. Hence, the possibility to collect BMSCs from other, more accessible, sources would be an attractive approach. It is well known that stem cells migrate from surrounding tissues and play important roles in wound healing. We thus hypothesized that stem/progenitor cells could be isolated from granulation tissue in the dental socket, and we subsequently collected granulation tissue from dog dental socket 3 d after tooth extraction. After enzyme digestion of the collected tissue, the cells forming colonies constituted the dental socket–derived stem/progenitor cells (dDSCs). Next, dDSCs were compared with dog BMSCs (dBMSCs) for phenotype characterization. A flow cytometric analysis showed that dDSCs were positive for CD44, CD90, and CD271 but negative for CD34 and CD45, similar to dBMSCs. dDSCs also exhibited osteogenic, adipogenic, and chondrogenic differentiation ability, similar to dBMSCs, with a higher capacity for colony formation, proliferation, and motility than dBMSCs. In addition, an in vivo ectopic bone formation assay showed that dDSCs and dBMSCs both induced hard tissue formation, although only dDSCs formed a fibrous tissue-like structure connected to the newly formed bone. Finally, we tested the ability of dDSCs to regenerate periodontal tissue in a one-wall defect model. The defects in the dDSC-transplanted group (β-TCP/PGA/dDSCs) were regenerated with cementum-like and periodontal ligament-like tissues and alveolar bone, whereas only bony tissue was observed in the control group (β-TCP/PGA). In conclusion, we identified and characterized a population of stem/progenitor cells in granulation tissue obtained from the dental socket that exhibited several characteristics similar to those of BMSCs. Dental sockets could therefore be a novel source for isolating stem/progenitor cells from bone.
Keywords: stem cells, granulation tissue, dental socket, bone marrow–derived stem cells, wound healing, dental tissue–derived mesenchymal stem cells
Introduction
Stem cells are characterized by their capacity for self-renewal and ability to differentiate into other cell types. Embryonic stem cells and induced pluripotent stem cells are widely known for their capability for pluripotent differentiation (Evans and Kaufman, 1981; Martin, 1981; Takahashi and Yamanaka, 2006). However, ethical issues and the potential risk of tumorigenesis remain major concerns, limiting their clinical application (Schuldiner et al., 2003; Carvalho and Ramalho-Santos, 2013). Yet, adult tissue-derived stem cells, which play important roles in the response to injury and physiologic maintenance of organs (Klein and Simons, 2011), are considered to be a safer source of stem/progenitor cells and thus face less ethical barriers, having already been used in several clinical treatments, including therapy for spinal cord injury and heart disease in humans (Mazzini et al., 2006; Moviglia et al., 2006). Bone marrow is one of the most common sources of mesenchymal stem/progenitor cells (MSCs; Tang et al., 2006); however, major limitations in the utilization of bone marrow–derived MSCs (BMSCs) include the potential risk of infection and pain at the donor site (most frequently the iliac crest) as well as the relatively limited ability of these cells to expand in vitro (Catacchio et al., 2013).
During routine dental practice, tooth extraction is frequently performed as a consequence of extensive caries and/or advanced periodontal disease or as a particular need during orthodontic treatment. Following tooth extraction, the healing dental socket is temporarily an opened site of bone containing granulation tissue, which may be a more accessible source for isolating stem/progenitor cells. Additionally, a condition of wound healing is particularly attractive, as previous reports have shown that MSCs migrate to sites of injury to accelerate wound healing (Karp and Leng Teo, 2009; Ueda et al., 2014). Indeed, our results revealed a high number of cells positive for the MSC marker CD146 in the dental socket 3 d after tooth extraction. Therefore, we hypothesized that a particular stem/progenitor cell population could be isolated from the healing socket. Further experiments confirmed a successful isolation of the dental socket–derived cells (DSCs), which demonstrated characteristics similar to MSCs from long bones.
Materials & Methods
Animals
A total of six one-year-old female Toyo beagle dogs (Oriental Yeast, Tokyo, Japan) were used in the experiments. Prior to the experimental procedures, the dogs were anesthetized via intramuscular injection of a mixture of xylazine (8 mg/kg; Bayer, Tokyo, Japan) and ketamine (80 mg/kg; Sankyo, Tokyo, Japan). Local anesthesia with 2% xylocaine containing 1/80,000 epinephrine was additionally provided before tooth extraction or collection of granulation tissue from the socket. The animals were kept in single cages with water and nonsolid food. Animals were euthanized with deep anesthesia, followed by intracardiac injection of pentobarbital.
Five eight-week-old female SCID/nude mice (Balb/c nu/nu; CLEA, Tokyo, Japan) were used for ectopic bone formation experiments, and eight-week-old female C57BL/6 mice were employed in the bone fracture and tooth extraction models. Prior to the surgical procedures, general anesthesia was induced via initial inhalation of isoflurane (Isoflu; Dainippon Sumitomo Pharma Co., Osaka, Japan) or intraperitoneal injection of a mixture of xylazine and ketamine.
The animals were treated according to the guidelines for animal research of Okayama University Dental School as well as the principles of the Declaration of Helsinki. The research protocol was approved by the ethics committee for animal experiments at Okayama University (OKU-2013125, OKU-2012421). The study conformed with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for preclinical procedures.
Isolation of Canine Cells
The maxillary second and third premolars were extracted from the dogs, and granulation tissue was collected from the dental socket after 3 d (dog DSC [dDSC]) and 10 d (dDSCs-X). Additionally, we recollected the granulation tissue from the same socket at day 6—that is, 3 d after the first sampling (dDSC-repeat [dDSC-r])—to evaluate the possibility to recollect DSCs from the same socket. The granulation tissues were minced and digested in a mixture of collagenase type I and dispase for 45 min at 37°C, as previously reported (Sonoyama et al., 2006), and the isolated cells were filtered through a 70-µm strainer and cultured in basal medium consisting of α-MEM (Life Technologies, Gaithersburg, MD, USA), 15% fetal bovine serum (FBS; Life Technologies), 100 µM of L-ascorbic acid 2-phosphate (WAKO, Tokyo, Japan), 2 mM of L-glutamine (Life Technologies), 100 U/mL of penicillin and 100 µg/mL of streptomycin (SIGMA, St. Louis, MO, USA) at 37°C in 5% CO2. Dog BMSCs (dBMSCs) were harvested from the femur of the same animals by flushing the marrow and then filtered and seeded in culture dishes. dDSCs and dBMSCs at first-to-third passage were used for the experiments.
Multipotent Differentiation Capacity
The multipotent differentiation capacity of dDSCs toward osteogenesis was tested in vitro, as previously described (Ono et al., 2008; Hara et al., 2013a). Adipogenic differentiation of dDSCs was induced via a commercially available canine adipocyte differentiation medium (Cell Applications, Inc., San Diego, CA, USA). After 21 d of culture, lipid droplets were stained with oil red O.
dDSCs were induced to differentiate into the chondrogenic lineage via a pellet culture system. The chondrogenic medium comprised low-glucose D-MEM (Life Technologies) supplemented with 1% FBS, 5% ITS solution (BD Biosciences, San Jose, CA, USA), 50 µM of ascorbic acid, 100 µM of dexamethasone, and 10 ng/mL of TGF-β3 (R&D, Minneapolis, MN, USA) for 21 d. The pellets were then fixed with 4% paraformaldehyde (PFA) and embedded in paraffin for histologic analysis. Sections of 5 µm in thickness were prepared and stained with alcian blue to detect glycosaminoglycans.
Real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
Total cellular RNA was extracted with RNeasy (Qiagen, Hilden, Germany) according to the manufacturer’s protocol, and cDNA was synthesized with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA; Hara et al., 2013b). The primer list is shown in Appendix Table 1. Real-time RT-PCR was performed with KAPA SYBR FAST (Kapa Biosystems, Inc., Wilmington, MA, USA), and the expression levels of the target genes were normalized to that of ribosomal protein s29 (s29) for the murine cells and that of GAPDH for the canine cells.
CFU-F Assay
To evaluate the colony-forming ability, 5 × 105 cells were seeded on 6-cm dishes and cultured for 2 wk (Friedenstein et al., 1976). The cells were then fixed and stained with 0.1% toluidine blue / 2% PFA in phosphate-buffered saline for 24 hr. Aggregates of more than 50 cells were counted as colonies.
Flow Cytometric Analysis
The cells were dissociated via Accutase (Innovative Cell Technologies Inc., San Diego, CA, USA) and washed twice with 1% FBS containing phosphate-buffered saline, followed by incubation with the following antibodies: monoclonal mouse anti-canine CD14-FITC (BD), monoclonal mouse anti-canine CD34-FITC (Thermo Scientific, Waltham, MA, USA), monoclonal rat anti-mouse CD44-APC (BD), monoclonal rat anti-canine CD45-FITC (Thermo Scientific), monoclonal mouse anti-human CD90-FITC (Dako, Glostrup, Denmark), monoclonal mouse anti-canine CD271-FITC (Miltenyi Biotec, Bergisch Gladbach, Germany), and the respective isotype-matched negative control antibodies on ice for 30 min. After washing, the cells were analyzed with the MACSQuant Analyzer, and the data were assessed using the FlowJo software program (Tree Star, Ashland, OR, USA).
Cell Motility Assay
The degree of cell motility was evaluated with the Oris Cell Migration Assay kit (Platypus Technologies, Madison, WI, USA), according to the manufacturer’s protocol. Briefly, 3 × 105 cells were seeded into each well and cultured for 6 hr, then fixed with 4% PFA and stained with phalloidin (Life Technologies). Images of the cells were captured with fluorescence microscopy (Biozero BZ-8000, KEYENCE Corp., Osaka, Japan), further binarized with Photoshop (Adobe Systems, San Jose, CA, USA), and analyzed with the Image J software package (National Institutes of Health, Bethesda, MD, USA).
Cell Proliferation Assay
The cells (1 × 104) were seeded onto 96-well plates with α-MEM containing 10% FBS. Twenty-four hours after seeding, an MTS assay (CellTiter 96 AQueous One Solution; Promega, Madison, WI, USA) was carried out to determine the number of viable cells, according to the manufacturer’s instructions.
Telomerase Activity
Telomerase activity was detected via the Quantitative Telomerase Detection Kit (US Biomax, Inc., Rockville, MD, USA) and a real-time RT-PCR machine (Chromo4, Bio-Rad), according to the manufacturer’s protocol.
Experimental Models Based on Mice
The mouse maxillary first molar was extracted in 2 steps. The teeth were first luxated with a 19-G needle and then removed with tweezers under a microscope.
For ectopic bone formation assay, 3 × 106 cells were mixed with 40 mg of β-TCP (OSferion60; Olympus Terumo Biomaterials Corp., Tokyo, Japan) and implanted in the dorsal subcutis of mice, as previously described (Ono et al., 2011). After 8 wk, the transplants were harvested and fixed, then decalcified and embedded in paraffin, after which serial 5-µm sections were cut and stained with hematoxylin and eosin.
Canine Model of Periodontal Tissue Regeneration
First, fourth mandibular premolars were extracted to provide adequate space for the future bone surgical defects. After 3 mo, one-wall infrabony defects (5 × 5 mm) were created on the mesial side of the mandibular first molars in each dog. The root cementum was completely removed mechanically with dental curettes (scaling) and physicochemically with 2 min of dental surface treatment with 19% EDTA. dDSCs were cultured on temperature-responsive culture dishes (35 mm in diameter; UpCell, CellSeed, Tokyo, Japan) with osteogenic induction medium for 1 wk to reach overconfluence. Three-layered cell sheets were constructed with polyglycolic acid (Neoveil, PGA Felt-Sheet Type, 0.15 mm in thickness; Gunze, Tokyo, Japan), according to a previous report (Iwata et al., 2009) and transplanted onto the exposed root surface site of the same donor (autologous transplantation). Polyglycolic acid sheets alone were transplanted as a control. Finally, the infrabony defects were filed with β-TCP, and the gingival flaps were sutured tightly. There was no randomization in the group assignment. Eight weeks after transplantation, samples were collected, fixed with 4% PFA, embedded in paraffin, and sectioned for a further histologic analysis with hematoxylin and eosin or Azan staining.
Immunohistochemistry
The murine maxilla and femur specimens were excised, immediately frozen, and immersed in cryosection medium for sectioning, according to a previously described method (Kawamoto and Shimizu, 2000). For immunohistochemical analysis, sections were blocked with 5% goat serum and then immunolabeled with primary antibody for CD146 (1:100, Abcam, San Francisco, CA, USA) or stage-specific embryonic antigen 4 (SSEA-4) or the isotype-matched negative control antibody at 4°C overnight. The target proteins were visualized with goat anti-rabbit IgG Alexa-488 (1:1000, Life Technologies) under a fluorescence microscope.
Statistical Analysis
An unpaired Student’s t test was used for the statistical analysis (Prism 5, GraphPad Software, Inc., La Jolla, CA, USA). A p value < .05 was considered to be statistically significant.
Results
Histologic Evaluation of Dental Socket Healing Following Tooth Extraction in the Mice
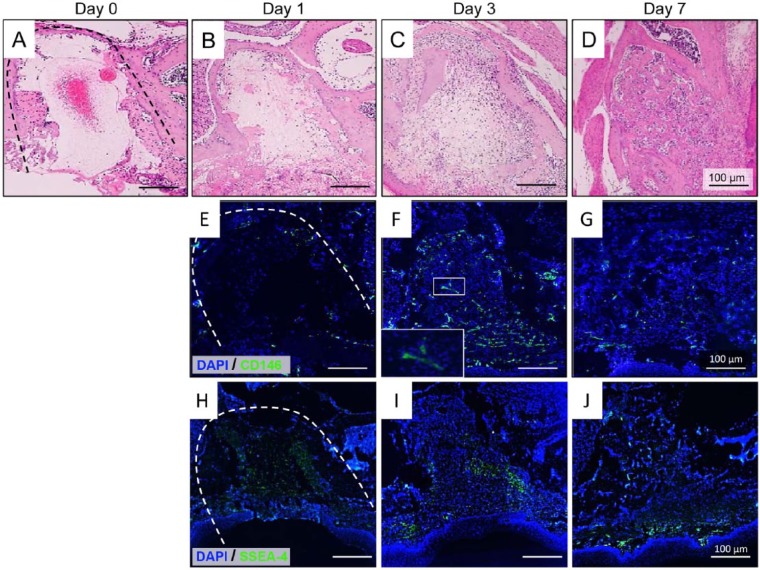
First, to better understand the process of wound healing in the dental socket, we extracted the maxillary first molars in mice and performed a time-dependent histologic analysis. At day 1 after extraction, the dental sockets were filled mainly with fibrin clots, with only a few CD146+ and SSEA-4+ cells (Fig. 1B, 1E, 1H). Of note, 3 d after tooth extraction, a large number of inflammatory cells and cells positive for CD146 and SSEA-4 was observed in the dental sockets (Fig. 1C, 1F, 1I), in accordance with the findings of previous studies showing an increase in stem/progenitor cells at the site of healing (Karp and Leng Teo, 2009; Ueda et al., 2014). On the seventh day, the sockets became filled with trabecular bone, indicating the final stage (remodeling) of healing, and the number of CD146+ and SSEA-4+ cells decreased dramatically (Fig. 1D, 1G, 1J). To confirm whether these findings could also be observed in long bones, we created a bone fracture model using mouse femurs and confirmed an increase in the number of CD146+ cells 3 d after fracture (Appendix Fig. 1A-1D). Of note, mouse BMSCs obtained from the fractured femurs also exhibited a significant increase in telomerase activity and expression levels of the early stem cell marker genes OCT-4 and NANOG compared with that observed in mouse BMSCs derived from normal bone (Appendix Fig. 1E, 1F).
Figure 1.
Histologic analysis of mouse dental socket after tooth extraction. (A-D) Images of hematoxylin and eosin staining of the dental sockets in mice at 0 (A), 1 (B), 3 (C), and 7 d (D) after tooth extraction. Initially, the dental socket was gradually filled with granulation tissue, and trabecular bone was observed 7 d after tooth extraction. Dotted line shows the margin of the dental socket. (E-G) Immunofluorescence staining for CD146 in the dental socket at 1 (E), 3 (F), and 7 d (G) after tooth extraction. Note the higher number of CD146-positive cells in the dental socket at 3 d after tooth extraction. (H-J) Immunofluorescence staining for stage-specific embryonic antigen 4 (SSEA-4) in the dental socket at day 1 (H), 3 (I), and 7 (J) after tooth extraction. All images are representative of 3 independent experiments.
These data indicate that (1) a pool of stem/progenitor cells with an enhanced stem cell phenotype accumulates during wound healing; (2) granulation tissue from dental sockets may be a unique source for isolating these particular stem/progenitor cells; and (3) the third day after tooth extraction provides more suitable timing for isolating stem/progenitor cells from the healing site. Nevertheless, because of the great difficulty in isolating mouse DSCs, we attempted to isolate and characterize DSCs from dogs.
Isolation and Characterization of dDSCs
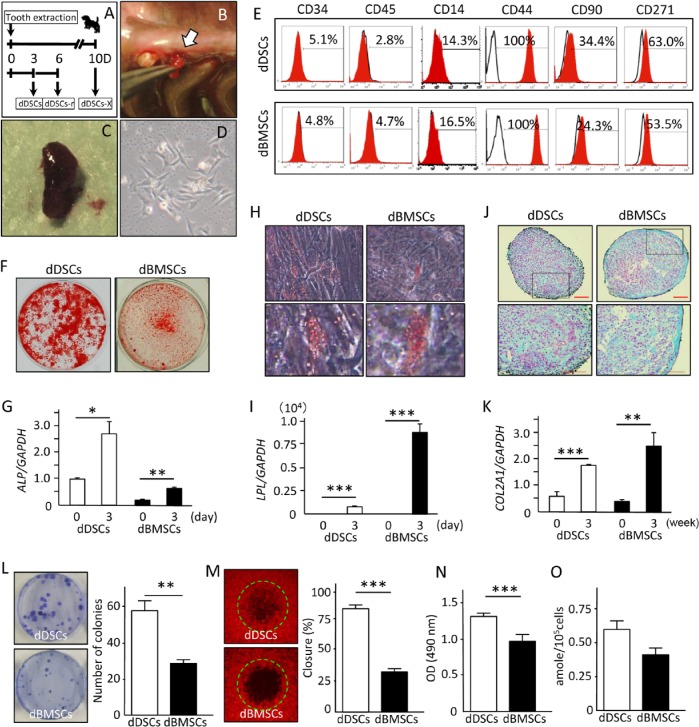
Because of species-related differences in the speed of wound healing, we also analyzed the healing process in the dental socket in a dog model of tooth extraction (Appendix Fig. 2). The dental sockets were filled with fibrin 3 d after tooth extraction, then gradually filled with granulation tissue over the following days. On day 14, trabecular bone was observed in the dental socket, indicating the final stage of healing. Based on these histologic data, we hypothesized that stem/progenitor cells could be isolated from granulation tissue during the initial 10 d of wound healing. Indeed, we were able to successfully isolate and culture dDSCs collected on day 3 but not day 10 (dDSCs-X; data not shown). Additionally, we collected and were able to successfully culture cells from the same dental socket (dDSCs-r) 3 d after the first sampling (Fig. 2A-2D).
Figure 2.
Isolation and characterization of the dog mesenchymal stem/progenitor cells. (A) Schematic illustration of the experimental design for collecting dental socket–derived cells (DSCs). (B, C) Photograph of the granulation tissue collected from the dental socket 3 d after tooth extraction. (D) DSC morphology at the first passage. (E) Cell surface characterization of dog DSCs (dDSCs) and dog bone marrow–derived mesenchymal stem/progenitor cells (dBMSCs) with flow cytometric analysis. (F-K) Mouse dental socket–derived cells (mDSCs) and dBMSCs were cultured in specific induction medium for 21 d to induce cell differentiation into osteo-, adipo-, and chondrogenic lineages and then stained with alizarin red S (F), oil red O (H), or alcian blue (J). Expression levels of the differentiation marker genes ALP (G), LPL (I), and COL2A1 (K) for osteo-, adipo-, and chondrogenesis, respectively, were evaluated by real-time reverse transcription polymerase chain reaction analysis. Four independent experiments with at least 3 wells were performed with cells obtained from 3 dogs. Scale bar (J), 100 µm. (L) Images and quantitative results of the CFU-F assay showing a significantly higher number of colonies in the dDSCs. Two independent experiments were performed in quadruplicate, with cells from 2 dogs. (M) The degree of cell motility was evaluated with the OrisCell Migration Assay kit 6 hr after cell plating. Images of each well were captured with fluorescence microscopy, and the closing ratio was measured with the NIH image software program. Experiments were performed in quadruplicate, with cells from 2 dogs. (N) MTS assay showing a significantly higher proliferation ability of dDSCs. Data representative of 2 independent experiments with 6 wells per experiment, from cells of 2 dogs. (O) Telomerase assay showing a higher but not statistically significant activity in dDSCs than in dBMSCs. Data are representative of 3 independent experiments performed with cells from 3 dogs. * p < .05. ** p < .01. *** p < .001. t test.
Next, we performed a series of experiments to characterize dDSCs and dDSCs-r. A flow cytometric analysis showed that dDSCs and dDSCs-r were positive for CD44, CD90, and CD271 but negative for hematopoietic stem cell markers CD34 and CD45, as well as the macrophage marker CD14, similar to dBMSCs (Fig. 2E, Appendix Fig. 3A). A small fraction of dDSCs and dBMSCs were positive for CD14, demonstrating that some of the cells were monocytes or macrophages. dDSCs and dDSCs-r were further induced to differentiate into osteogenic, adipogenic, and chondrogenic lineages. Similar to dBMSCs, dDSCs and dDSCs-r stained positively for alizarin red S, oil red O, and alcian blue, with corresponding increases in the expression levels of key genes, ALP, LPL, and COL2A1, respectively, indicating the efficient capacity for multipotent differentiation of dDSCs (Fig. 2F-2K) and dDSCs-r (Appendix Fig. 3B-3D).
Since DSCs consist of a heterogeneous cell population, we also attempted to characterize single-cell colonies. Twelve single colonies of dDSCs and dBMSCs were isolated and induced to differentiate into osteogenic and adipogenic lineages (Appendix Table 2). Similar to dBMSCs, 6 of the 12 dDSCs colonies differentiated into both osteogenic and adipogenic lineages or the adipogenic lineage alone. However, the rate of osteogenic differentiation was higher in dDSCs (75%) than dBMSCs (50%), which could partially explain the higher expression of ALP and stronger staining for alizarin red observed in the dDSCs (Fig. 2F, 2G).
We subsequently evaluated the colony-forming ability (CFU-F assay), cell motility, cell proliferation, and telomerase activity of dDSCs and dDSCs-r. The CFU-F assay is a method for determining the incidence of clonogenic MSCs. Interestingly, dDSCs formed a significantly higher number of colonies than dBMSCs (Fig. 2L). Likewise, dDSCs exhibited a significantly higher degree of motility and proliferation than dDSCs (Fig. 2M, 2N). Yet, although dDSCs demonstrated a higher telomerase activity than dBMSCs, the difference was not statistically significant (Fig. 2O). Similar findings were observed for dDSCs-r (Appendix Fig. 3F-3I).
In vivo Ectopic Bone Formation Assay and Canine Periodontal Regeneration Model
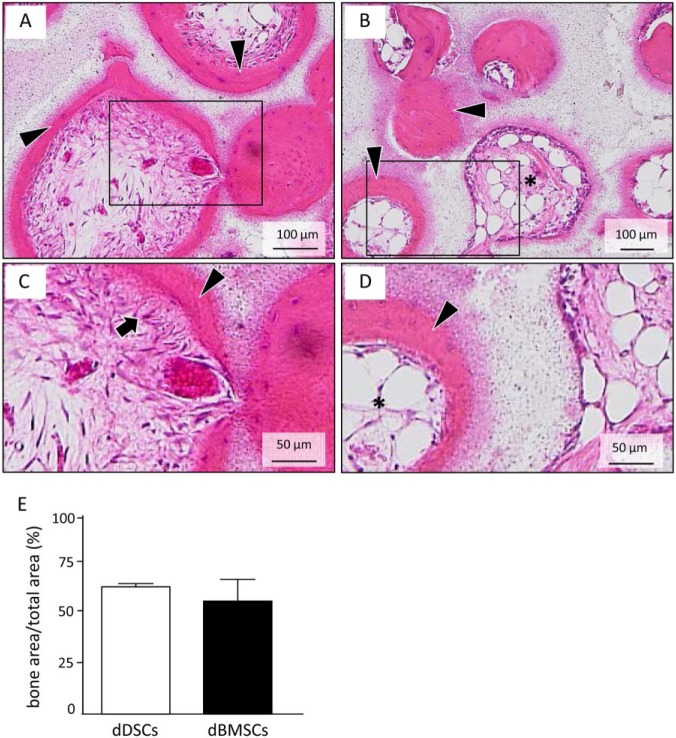
To evaluate and compare the osteogenic potential of dDSCs in vivo, cells were transplanted with β-TCP subcutaneously in immunocompromised mice. As predicted according to our in vitro data, ectopic bone-like tissue was observed on the surface of the β-TCP in both the dDSC- and dBMSC-transplanted groups. Interestingly, fibrous tissue connected with the newly formed bone was observed in only the dDSC-transplanted group (Fig. 3A, 3C), whereas adipose tissue was detected in the center of the mineralized region in the dBMSC-transplanted group (Fig. 3B, 3D).
Figure 3.
Ectopic bone formation assay in vivo: dog dental socket–derived cells (dDSCs) were implanted with β-TCP under the skin on the back of nude mice. After 8 wk, samples were harvested and analyzed by histology. (A-D) Hematoxylin and eosin staining of the transplanted cell/β-TCP samples. In both dDSCs (A, C) and dog bone marrow–derived mesenchymal stem/progenitor cells (dBMSCs; B, D), newly formed mineralized tissue was observed on the surface of the carrier (A, B). Sharpey’s fiber-like tissue (arrows) was observed connected with newly formed bone-like tissue in the dDSC group (C). Yet, adipose tissue (asterisk) was observed in the inner portion of the bone-like tissue formed by dBMSCs (D). All images are representative of 3 independent experiments. (E) Quantitation of newly formed bone in dDSC- and dBMSC-transplanted group.
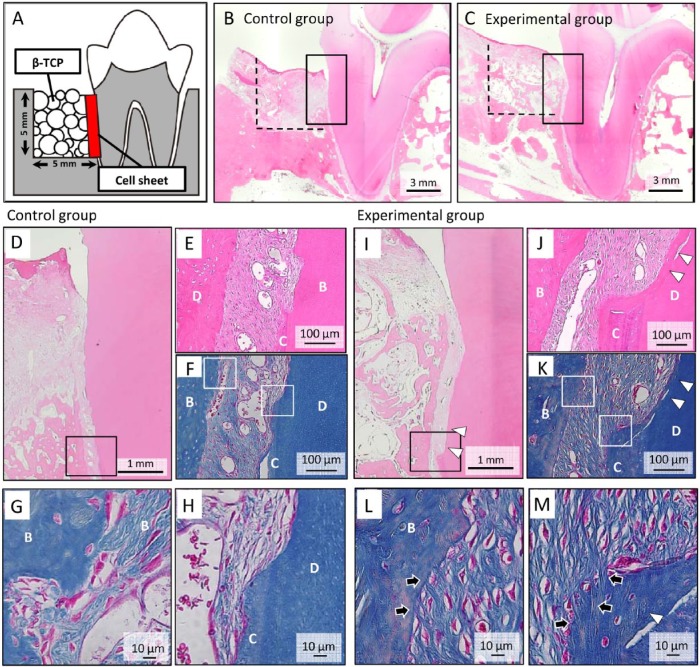
Based on these findings, we hypothesized that dDSCs may have the potential to regenerate the periodontal ligament in a large animal experimental model. Figure 4A shows a schematic illustration of the bone defects and dDSC transplantation in a cell sheet. One-wall bone defects were surgically created, and 3-layered dDSC sheets were subsequently transplanted onto the scaled tooth root surface, after which the bone defects were filled with porous β-TCP. A histologic analysis conducted after 8 wk showed newly formed bone in both the control and experimental groups (Fig. 4B, 4C), whereas newly formed cementum-like tissue was observed in the experimental group only (Fig. 4D-4F, 4I-4K). Additionally, higher-magnification images of Azan-stained sections showed Sharpey’s fiber-like tissue connecting the newly formed bone and cementum-like tissue in the experimental group only (Fig. 4G, 4H, 4L, 4M).
Figure 4.
Periodontal tissue regeneration with autologous transplantation of dog dental socket–derived cells (dDSCs). (A) Schematic illustration of a one-wall periodontal defect in the dog mandible. (B-E) After 3-layered dDSC sheets with polyglycolic acid were transplanted onto the surface of the tooth root, β-TCP was implanted into the one-wall bone defect. A histologic analysis was performed 2 mo after transplantation (B, D-H: control group; C, I-M: experimental group). (D, I) Higher magnification of the squared areas in Figure 4B and 4C, respectively. A greater amount of newly formed bone was observed in dDSC-transplanted group than that in the control group. The squares indicate the areas shown in Figure 4E (hematoxylin and eosin staining) and 4F (Azan staining) or 4J (hematoxylin and eosin staining) and 4K (Azan staining) at a higher magnification. (H, G) Higher magnification of Figure 4F. (L, M) Higher magnification of Figure 4K. Sharpey’s fiber-like tissue (arrow) connecting the newly formed bone and cementum-like tissue (arrowhead) was observed in the experimental group. B, bone; C, cementum; D, dentin.
Discussion
Bone marrow has been regarded as one of the rich sources of adult MSCs (Mazzini et al., 2006; Moviglia et al., 2006); however, BMSCs represent a very small fraction (0.001%-0.01%) of the total population of cells in the marrow (Pittenger et al., 1999). Previous reports have demonstrated an increase in the stem/progenitor cell population during wound healing (Ng et al., 2008; Sasaki et al., 2008; Ueda et al., 2014). Some of these progenitor cells migrate to the site of healing due to the chemoattractant properties of cytokines. Additionally, we previously demonstrated that tumor necrosis factor α, but not interleukin 1 or 6, increases the expression of stem cell markers of dental pulp-derived MSCs (Ueda et al., 2014) and that tumor necrosis factor α–treated DPSCs exhibit an increased telomerase activity, as well as capacity for migration, proliferation, and differentiation (Ueda et al., 2014). Therefore, during the initial stage of wound healing, the inflammatory state may be of fundamental importance for both the attraction and modulation of stemness of MSCs. Since it would be very difficult to induce sites of inflammation in long bones to isolate a higher number of more active stem/progenitor cells, the DSC population is particularly attractive as a unique cell source for application in MSC-based regenerative therapy.
In addition to inflammatory conditions, the developmental origin of the cells may have an effect on the differential properties of DSCs compared with BMSCs. Previous studies characterizing and comparing craniofacial MSCs and BMSCs have shown that dental tissue–derived MSCs display higher rates of proliferation, colony formation, and osteogenic differentiation than BMSCs (Alge et al., 2010; Tamaki et al., 2013). These characteristics are in accordance with the present data obtained for dDSCs. Since dDSCs were able to form a fiber-like structure in the ectopic bone formation assay and regenerate periodontal tissue in the one-wall bone defect model in the current study, we assume that dDSCs contain periodontal ligament–derived MSCs. Nevertheless, the characteristics of the heterogenic population of dDSCs requires further investigation to identify the exact origin of the cells, which may include cells not only from the remaining periodontal ligament and/or apical papilla but also from the surrounding alveolar bone and/or circulating MSCs.
In this study, we utilized a canine model to demonstrate the stem cell properties of DSCs. A major limitation of this work was the inability to characterize dDSCs in detail because of the low number of antibodies with reactivity to canine cells. Therefore, future studies with human cells may enable researchers to obtain a better characterization of the DSC population as well as a better understanding of the biological properties (including the immunomodulatory properties) of DSCs, which may eventually increase the potential number of applications of these cells in regenerative medicine.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was partially supported by a Grant-in-Aid for Scientific Research to T.K. (22249064) and K.M. (22390366) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also partially supported by Organ Technologies Inc.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, et al. (2010). Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med 4:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AS, Ramalho-Santos J. (2013). How can ethics relate to science? The case of stem cell research. Eur J Hum Genet 21:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catacchio I, Berardi S, Reale A, De Luisi A, Racanelli V, Vacca A, et al. (2013). Evidence for bone marrow adult stem cell plasticity: properties, molecular mechanisms, negative aspects, and clinical applications of hematopoietic and mesenchymal stem cells transdifferentiation. Stem Cells Int 2013:589139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154-156. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Gorskaja JF, Kulagina NN. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267-274. [PubMed] [Google Scholar]
- Hara ES, Ono M, Eguchi T, Kubota S, Pham HT, Sonoyama W, et al. (2013a). miRNA-720 controls stem cell phenotype, proliferation and differentiation of human dental pulp cells. PLoS One 8:e83545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara ES, Ono M, Kubota S, Sonoyama W, Oida Y, Hattori T, et al. (2013b). Novel chondrogenic and chondroprotective effects of the natural compound harmine. Biochimie 95:374-381. [DOI] [PubMed] [Google Scholar]
- Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, et al. (2009). Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 30:2716-2723. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206-216. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Shimizu M. (2000). A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem Cell Biol 113:331-339. [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. (2011). Universal patterns of stem cell fate in cycling adult tissues. Development 138:3103-3111. [DOI] [PubMed] [Google Scholar]
- Martin GR. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78:7634-7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Boccaletti R, et al. (2006). Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res 28:523-526. [DOI] [PubMed] [Google Scholar]
- Moviglia GA, Fernandez Vina R, Brizuela JA, Saslavsky J, Vrsalovic F, Varela G, et al. (2006). Combined protocol of cell therapy for chronic spinal cord injury: report on the electrical and functional recovery of two patients. Cytotherapy 8:202-209. [DOI] [PubMed] [Google Scholar]
- Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, et al. (2008). PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112:295-307. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubota S, Fujisawa T, Sonoyama W, Kawaki H, Akiyama K, et al. (2008). Promotion of hydroxyapatite-associated, stem cell-based bone regeneration by CCN2. Cell Transplant 17:231-240. [DOI] [PubMed] [Google Scholar]
- Ono M, Inkson CA, Kilts TM, Young MF. (2011). WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res 26:193-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180:2581-2587. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Itskovitz-Eldor J, Benvenisty N. (2003). Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells 21:257-265. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663-676. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Nakahara T, Ishikawa H, Sato S. (2013). In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 101:121-132. [DOI] [PubMed] [Google Scholar]
- Tang J, Xie Q, Pan G, Wang J, Wang M. (2006). Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg 30:353-361. [DOI] [PubMed] [Google Scholar]
- Ueda M, Fujisawa T, Ono M, Hara ES, Pham HT, Nakajima R, et al. (2014). A short-term treatment with tumor necrosis factor-alpha enhances stem cell phenotype of human dental pulp cells. Stem Cell Res Ther 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.