Abstract
The sympathetic nervous system (SNS) regulates bone resorption through β-2 adrenergic receptor (Adrb2). In orthodontic tooth movement (OTM), mechanical force induces and regulates alveolar bone remodeling. Compressive force-associated osteoclast differentiation and alveolar bone resorption are the rate-limiting steps of tooth movement. However, whether mechanical force can activate Adrb2 and thus contribute to OTM remains unknown. In this study, orthodontic nickel-titanium springs were applied to the upper first molars of rats and Adrb1/2-/- mice to confirm the role of SNS and Adrb2 in OTM. The results showed that blockage of SNS activity in the jawbones of rats by means of superior cervical ganglion ectomy reduced OTM distance from 860 to 540 μm after 14 d of force application. In addition, the injection of nonselective Adrb2 agonist isoproterenol activated the downstream signaling of SNS to accelerate OTM from 300 to 540 μm after 7 d of force application. Adrb1/2-/- mice showed significantly reduced OTM distance (19.5 μm) compared with the wild-type mice (107.6 μm) after 7 d of force application. Histopathologic analysis showed that the number of Adrb2-positive cells increased in the compressive region of periodontal ligament after orthodontic force was applied on rats. Mechanistically, mechanical compressive force upregulated Adrb2 expression in primary-cultured human periodontal ligament cells (PDLCs) through the elevation of intracellular Ca2+ concentration. Activation of Adrb2 in PDLCs increased the RANKL/OPG ratio and promoted the peripheral blood mononuclear cell differentiation to osteoclasts in the cocultured system. Upregulation of Adrb2 in PDLCs promoted osteoclastogenesis, which accelerated OTM through Adrb2-enhanced bone resorption. In summary, this study suggests that mechanical force-induced Adrb2 activation in PDLCs contributes to SNS-regulated OTM.
Keywords: orthodontics, biomechanical phenomena, beta-2 adrenergic receptors, sympathetic nervous system, bone remodeling, osteoclast
Introduction
β-2 adrenergic receptor (Adrb2), 1 of the 3 postsynaptic β adrenergic receptors, is important in sympathetic nervous system (SNS)–regulated bone remodeling that involves osteoblast-mediated bone formation and resorption (Takeda et al., 2002; Elefteriou et al., 2005). β adrenergic receptor agonist isoproterenol (ISO) treatment triggers an osteoclastogenic response to accelerate bone loss (Takeda et al., 2002). Adrb2-/- mice show a higher bone mass phenotype than the wild type (WT) by decreasing the expression of osteoclast differentiation factor RANKL in osteoblast progenitor cells (Elefteriou et al., 2005). Bones are mechanosensitive organs remodeled with functional mechanical loading (Biewener, 1985; Huiskes et al., 2000). In orthodontic tooth movement (OTM), alveolar bone remodeling is induced and regulated by constant exertion of mechanical force. Tooth movement is started and facilitated by the compressive force-associated osteoclast formation and bone resorption (Immermann and Compere, 1954). Force-stimulated local aseptic periodontal inflammation has an important function in the adaptive response of bone to OTM (Cooper and Sims, 1989; Garlet et al., 2007; Iwasaki et al., 2009). Recent studies report that selective Adrb2 antagonist butoxamine reduces SNS-accelerated OTM by preventing alveolar bone loss (Kondo et al., 2013; Sato et al., 2014). However, whether mechanical force can directly affect the activity of Adrb2 and thus regulate bone remodeling in OTM remains unclear. Moreover, the contributions of SNS and Adrb2 signaling in bone mechanoadaptive response remain controversial (de Souza et al., 2005; Marenzana et al., 2007).
The periodontal ligament (PDL) is a layer of soft tissue between the tooth and the alveolar bone that provides the necessary microenvironment for cells involved in the alveolar bone remodeling of OTM. Mechanical force acts on PDL cells (PDLCs) and induces the secretion of inflammatory factors, such as PGE2 (Kang et al., 2010), and osteoclastogenesis by upregulating RANKL (Kanzaki et al., 2002). As PDL is derived from neural crest cells (Dupin and Sommer, 2012), PDLCs may express neural receptor Adrb2. Therefore, force-induced Adrb2 in PDLCs may be involved in the bone remodeling of OTM.
In this study, we hypothesize that force-induced activation of Adrb2 in PDLCs may contribute to osteoclast-mediated OTM. To address this hypothesis, we explored whether orthodontic force could induce the activation of Adrb2 in PDLCs and consequently promote OTM through enhanced osteoclastogenesis in the OMT model of rats and Adrb1/2-/- mice. We further examined the underlying mechanism of force-induced activation of Adrb2 in primary-cultured human PDLCs.
Materials & Methods
Animals and Treatments
Sprague-Dawley rats (male, 8 wk old), Adrb1/2-/- mice (003810, male, 8-10 wk old; Jackson Laboratory), and WT mice (129, male, 8-10 wk old) were used in this study. All the animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Peking University (LA2013-92).
To set up the OTM animal model, orthodontic nickel-titanium coiled springs (0.2 mm in thickness, 1 mm in diameter, 5 mm in length; Smart Technology, Beijing, China) were ligated between the right maxillary first molar and the incisors of the rats (Appendix Fig. 1A; Dunn et al., 2007). The springs were activated to deliver an orthodontic force of 60 g.
We designed independent experiments and chose different time periods to observe OTM distance because of the different purposes of each experiment and our preliminary data. To test the role of SNS on OTM, the right superior cervical ganglions of the rats were removed or sham-operated in 2 groups: force group and force + superior cervical ganglionectomy (SCGx) group (n = 8). The effectiveness of SCGx was confirmed by ipsilateral eyelid ptosis (Matthews and Quilliam, 1964) (Appendix Fig. 2). Three days after the operation, orthodontic force was applied to both groups for 14 d. To confirm the role of Adrb2 in OTM, 1 d after the application of orthodontic force, 2 groups of rats (force and force + ISO, n = 10) were intraperitoneally injected with vehicle or nonselective Adrb2 agonist ISO (20 mg/kg bodyweight; 15627, Sigma), respectively, for 6 consecutive days.
Orthodontic force was applied to the 2 groups of mice (Adrb1/2-/- group and WT group, n = 14) to reveal the osteoclastogenic function of Adrb2 in OTM. The spring (1 mm in length) was bonded between the molar and the incisors of the mice by using flowable restorative resin (3M ESPE, USA) to deliver a force of 30 g for 7 d (Appendix Fig. 1B).
Tartrate-resistant Acid Phosphatase Staining
The medium number of transverse serial sections (4.5 μm) from the corresponding group was stained for tartrate-resistant acid phosphatase (TRAP) using a leukocyte acid phosphatase kit (387A, Sigma) according to the manufacturer’s protocol. TRAP-positive multinucleated (> 3 nuclei) cells that attached to the alveolar bone surface (Parfitt et al., 1987) mesial to the middle palatal roots were counted (n = 5).
Cell Culture and Treatments
All protocols used to obtain human tissue samples were approved by the Ethical Guidelines of Peking University. The protocols were performed with appropriate informed consent (PKUSSIRB-201311103). Human PDLCs were isolated from PDL of normal orthodontic extracted bicuspids as previously reported (Seo et al., 2004). PDLCs isolated from 3 different individuals were pooled together and used in this study with 3 to 4 passages. Human orofacial bone marrow–derived cells (OMCs) were isolated using a previously described method (Yamaza et al., 2011).
PDLCs were treated with various doses of ISO (0-1 μM) alone or combined with Adrb2 selective antagonist ICI 118,551 (0.01-0.1 μM; I127, Sigma) for 6 hr to investigate the promotion of osteoclast differentiation. To knock down Adrb2 expression, PDLCs were transfected with Adrb2 small interfering (si) RNA and scramble siRNA for 48 hr before ISO stimulation.
Mechanical Stimulation on Primary-cultured Cells
Static compressive force was applied to PDLCs and OMCs in vitro using the uniform method (Mitsui et al., 2005) in a culture medium containing 6 mM of Ca2+. In brief, a layer of glass cover and additional metal weights were placed on top of an 80% confluent cell layer in 6-well plates. Cells were subjected to different continuous compressive forces ranging from 0 to 2 g/cm2 for 6 hr or at 1.5 g/cm2 for different durations ranging from 0 to 12 hr.
To reveal the role of intracellular Ca2+ in force-induced Adrb2 upregulation, PDLCs were pretreated with intracellular calcium chelator BAPTA-AM (5 μM; A1076, Sigma) for 30 min in 6 mM of Ca2+ medium and subjected to compressive force (1.5 g/cm2, 6 hr).
Peripheral Blood Mononuclear Cell and PDLC Co-culture
PDLCs were seeded into 12-well plates at 5 × 103 cells/well. CD11b+ peripheral blood mononuclear cells were added to PDLCs at 5 × 105 cells/well after 1 d. The cells were co-cultured in a medium containing M-CSF (30 ng/mL) and sRANKL (50 ng/mL; Elefteriou et al., 2005). The cells were then treated with 0.1 μM of ISO or vehicle. After 3 wk, the cells were fixed and stained for TRAP. TRAP-positive multinucleated (> 3 nuclei) cells were counted in 5 visual fields at 20× magnification in each well (n = 3).
Statistical Analyses
Data were presented as mean ± standard deviation. Statistical analyses were performed using Student’s t-test or 1-way analysis of variance (SPSS 19.0); p < .05 was considered statistically significant.
Results
SNS-regulated OTM Partially Depends on Adrb2
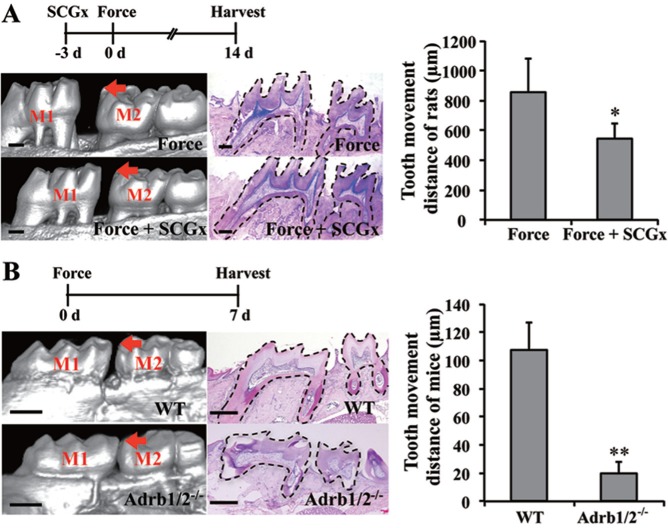
To confirm the function of SNS in OTM, the superior cervical ganglia of the rats were removed to block SNS activity in the jawbones. The tooth movement distance on the force-applied side increased to 860 μm after 14 d of OTM in the force group. Tooth movement distance of the SCGx group (540 μm) decreased compared with that of the force group (p < .05; Fig. 1A). Hematoxylin and eosin staining showed the integrity of the periodontal tissue around the 2 molars after tooth movement (Fig. 1), which suggests that orthodontic force-induced tooth movement partially depends on SNS.
Figure 1.
Sympathetic nervous system–regulated orthodontic tooth movement (OTM) partially depends on β-2 adrenergic receptor (Adrb2). (A, B) Analysis of the OTM distance by micro–computed tomography scanning and hematoxylin and eosin staining. (A) Superior cervical ganglionectomy (SCGx) decreased OTM distance after 14 d of force application in rats (n = 8). (B) OTM distance decreased in the Adrb1/2-/- group compared to the WT group after 7 d of force application in mice (n = 14). WT, wild type. Hematoxylin and eosin staining shows the integrity of the periodontal tissue around the 2 molars after tooth movement. The bar indicates 500 μm. M1, the first molar. M2, the second molar. Arrows indicate OTM distance. * p < .05. ** p < .001.
To reveal whether Adrb2 contributed to SNS-regulated OTM, Adrb2 was activated by ISO in the rat OTM animal model. Force-induced increase in tooth movement distance in the force group (300 μm) was further promoted by the administration of ISO (540 μm, p < .001, Appendix Fig. 1D). Orthodontic force was applied to Adrb1/2-/- and WT mice. The tooth movement distance on the force-applied side increased to 107.6 μm after 7 d of OTM in WT mice (Fig. 1B). However, tooth movement distance significantly decreased in the knockout mice (19.5 μm) compared with that in WT (p < .001). Adrb2 promotes osteoclastogenic response, and Adrb1 contributes to osteoblastogenic response (Pierroz et al., 2012). We suggest Adrb2 to play an important role in OTM as compressive force-associated bone resorption in the direction of the tooth movement limits the rate of OTM (Masella and Meister, 2006).
Mechanical Force Upregulates the Adrb2 Expression in PDLCs
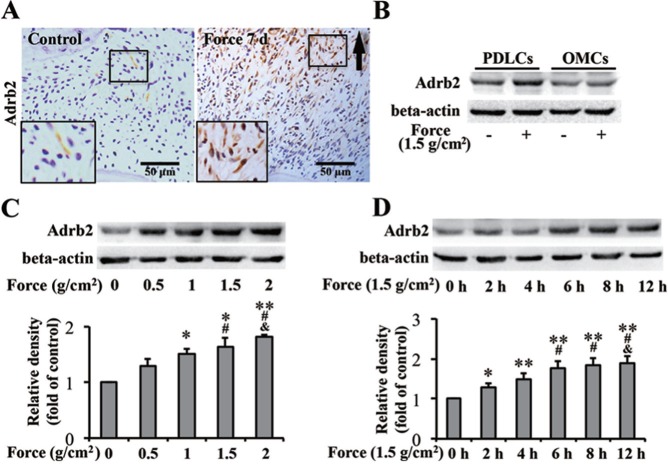
Histopathologic examination revealed Adrb2-positive fibroblast-like PDLCs in the compressive region of PDL in the force group after the application of orthodontic force for 7 d in rats. Few positive cells were observed in the control group (Figure 2A). This finding suggests that mechanical stimulation could upregulate Adrb2 expression in PDLCs.
Figure 2.
Mechanical force upregulates β-2 adrenergic receptor (Adrb2) expression in periodontal ligament cells (PDLCs). (A) Immunohistochemistry of Adrb2. Orthodontic force increased the number of Adrb2-postive PDLCs in rats. Arrows indicate the direction of tooth movement. Large boxed areas show higher-magnification views of the small boxes. (B-D) Western blot of Adrb2. (B) Higher Adrb2 expression was induced in PDLCs than in orofacial bone marrow-derived cells in vitro after the application of force for 6 hr. (C) Adrb2 expression in PDLCs was upregulated with increasing force-treated intensity for 6 hr. * p < .05 vs. 0 g/cm2 group. # p < .05 vs. 0.5 g/cm2 group. & p < .05 vs. 1 g/cm2 group. ** p < .01 vs. 0 g/cm2 group. (D) Adrb2 expression in PDLCs was upregulated as force-treated time was prolonged. * p < .05 vs. 0-hr group. # p < .05 vs. 2-hr group. & p < .05 vs. 4-hr group. ** p < .01 vs. 0-hr group. Beta-actin served as internal control for equal loading. The data are representative of 3 independent experiments.
Adrb2 expression has been detected in osteoblasts. However, we found that orthodontic force had more significant effects on PDLCs than OMCs in terms of upregulating Adrb2 expression (Appendix Figure 3A). To confirm this phenomenon, PDLCs and OMCs were subjected to static compression in vitro. The upregulation of Adrb2 was higher in PDLCs than in OMCs after the application of force (Figure 2B). Moreover, Adrb2 expression in PDLCs showed an increasing trend with the increase in force-treated intensity or time (Figure 2C, D), whereas no significant increase was found in OMCs (Appendix Figure 3B, C). The results were consistent with in vivo observation, confirming that Adrb2 is expressed in PDLCs and that mechanical force upregulates the expression of Adrb2 in PDLCs.
Force-induced Adrb2 Upregulation Partially Depends on the Increase in Intracellular Ca2+ Concentration
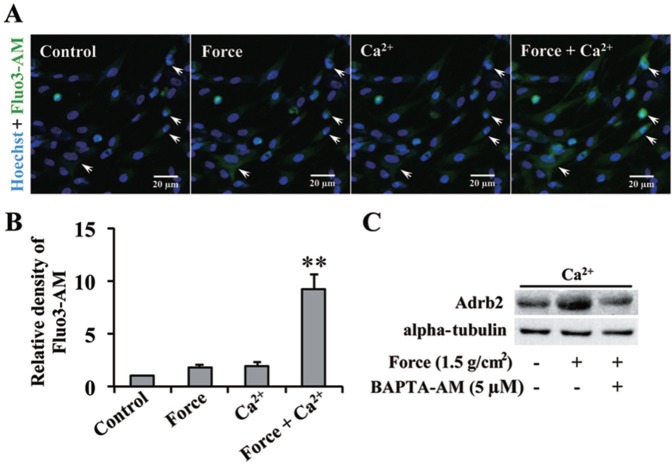
We further explored the underlying mechanism of force-induced Adrb2 upregulation in PDLCs. Figure 3A, B shows the dynamic changes in the intracellular Ca2+ concentration in the same field of view. Before force or exogenous Ca2+ was applied, intracellular Ca2+ concentration could barely be detected. After separately applying force or treating the cells with Ca2+, we found no evident change in intracellular Ca2+ concentration. In comparison, the green intracellular Ca2+ signal was significantly enhanced after a combined treatment of force and exogenous Ca2+ was applied. BAPTA-AM, used to suppress intracellular Ca2+ concentration, decreased the force-induced upregulation of Adrb2 protein (Figure 3C). Therefore, the force-induced increase in intracellular Ca2+ concentration has an important function in the force-induced Adrb2 upregulation.
Figure 3.
Force-induced β-2 adrenergic receptor (Adrb2) upregulation in periodontal ligament cells (PDLCs) depends on intracellular Ca2+ concentration. (A) Immunofluorescence of intracellular Ca2+. Fluo3-AM indicates intracellular Ca2+ (green). Nuclei were counterstained with Hoechst (blue). Arrows indicate the dynamic changes in the fluorescence intensity of cells. (B) Relative density of intracellular Ca2+ signal. Force-stimulated PDLCs in HBSS with 10 mM of Ca2+ presented significantly higher intracellular Ca2+ concentration compared with the control (Ca2+-free HBSS), Force (Ca2+-free HBSS), and Ca2+ groups. ** p < .01. (C) Western blot of Adrb2. Force-induced Adrb2 upregulation was reversed by BAPTA-AM. Alpha-tubulin served as internal control for equal loading. The data are representative of 3 independent experiments.
Adrb2 Activation in PDLCs Promotes RANKL/OPG Ratio and Osteoclast Differentiation
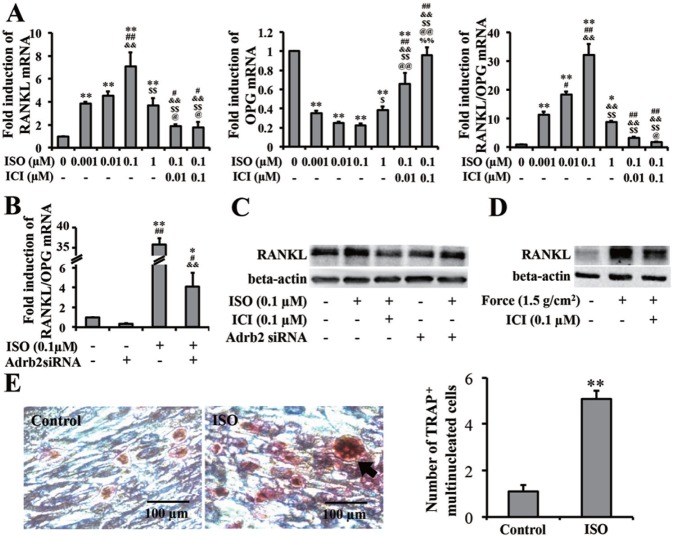
Although mechanical force upregulated the expression of Adrb2, the function of force-induced Adrb2 in PDLCs remains unclear. RANKL mRNA was upregulated by ISO treatment. ICI 118,551 partially reversed the ISO-induced upregulation of RANKL. The OPG mRNA showed a different trend from that of RANKL. The mRNA ratio of RANKL/OPG increased when the cells were treated with ISO and decreased when treated with ICI 118,551 together (Figure 4A).
Figure 4.
β-2 adrenergic receptor (Adrb2) in periodontal ligament cells (PDLCs) promotes osteoclast differentiation. (A, B) Real-time polymerase chain reaction data of RANKL/OPG mRNA ratio in PDLCs. (A) ISO upregulated RANKL/OPG mRNA ratio, which was reversed by ICI. ICI, ICI 118,551; ISO, isoproterenol. * p < .05 vs. the control group. # p < .05 vs. 0.001-μM ISO group. & p < .05 vs. 0.01-μM ISO group. $ p < .05 vs. 0.1-μM ISO group. @ p < .05 vs. 1-μM ISO group. % p < .05 vs. 0.1-μM ISO + 0.01-μM ICI group. ** p < .01 vs. the control group. ## p < .01 vs. 0.001-μM ISO group. && p < .01 vs. 0.01-μM ISO group. $$ p < .01 vs. 0.1-μM ISO group. @@ p < .01 vs. 1-μM ISO group. %% p < .01 vs. 0.1-μM ISO + 0.01-μM ICI group. (B) Pretransfection of Adrb2 siRNA downregulated ISO-induced RANKL/OPG mRNA ratio. * p < .05 vs. the control group. # p < .05 vs. 0.1-μM ISO group. & p < .05 vs. Adrb2 siRNA group. ** p < .01 vs. the control group. ## p < .01 vs. 0.1-μM ISO group. && p < .01 vs. Adrb2 siRNA group. (C, D) Western blot of RANKL in PDLCs. (C) RANKL protein expression depends on Adrb2 in PDLCs. RANKL expression was evaluated by Western blot. (D) Force-promoted RANKL expression was partially decreased by Adrb2 antagonist ICI pretreatment. Beta-actin served as the internal control for equal loading. (E) TRAP staining of osteoclasts in PBMCs cocultured with PDLCs. Arrows represent TRAP-positive multinucleated osteoclasts. Treatment with ISO increased osteoclasts. OSC, osteoclasts. n = 3. ** p < .01. The data are representative of 3 independent experiments.
To confirm the enhancing effect of Adrb2 on the mRNA ratio of RANKL/OPG, we used Adrb2 siRNA to knock down Adrb2 expression in PDLCs (Appendix Figure 4). After endogenetic Adrb2 was supressed, the upregulation of the mRNA ratio of RANKL/OPG by ISO was almost eliminated (Figure 4B). Western blot showed that ISO treatment increased the expression of RANKL but was reversed when ICI 118,551 was added. The increase in RANKL expression became subtle after Adrb2 siRNA transfection was performed, although the same ISO dose was also administered (Figure 4C).
Furthermore, we showed that force-induced RANKL upregulation in PDLCs was partially decreased by Adrb2 antagonist ICI 118,551 pretreatment (Figure 4D). Therefore, force-induced upregulation of RANKL partially depended on Adrb2 in PDLCs.
We used a PDLCs/CD11b+ peripheral blood mononuclear cells coculture system to further validate the enhancing capacity of Adrb2 in PDLCs in osteoclast differentiation. The ISO-stimulated group yielded more TRAP-positive multinucleated cells. This finding indicates that PDLCs could promote osteoclast differentiation in vitro through Adrb2 signaling (p < .001; Figure 4E).
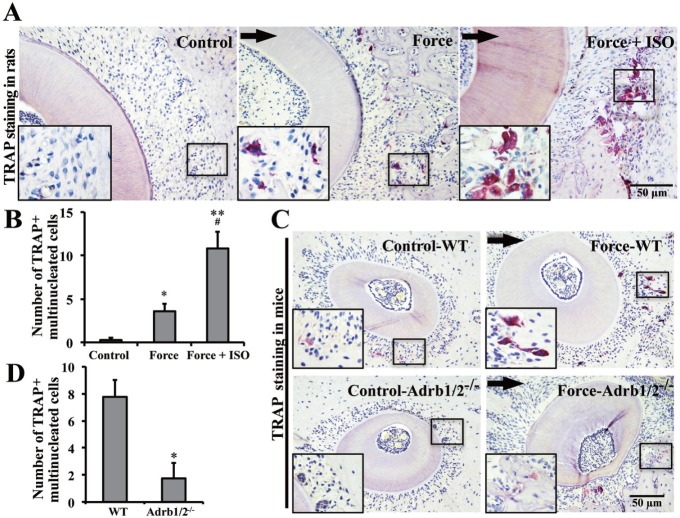
Orthodontic Force-induced Osteoclast Differentiation Partially Depends on Adrb2
TRAP staining was conducted to confirm the function of Adrb2 in osteoclast differentiation during OTM. TRAP-positive multinucleated cells were barely detected in the specific position of the control group in rats. However, the number of TRAP-positive multinucleated cells increased after orthodontic force was applied (p < .05). ISO treatment further increased the orthodontic force-induced osteoclast differentiation (p < .05; Figure 5A, B). The number of orthodontic force-induced TRAP-positive multinucleated cells decreased in Adrb1/2-/- mice compared with that in WT mice (p < .05; Figure 5C, D). Our data suggested that osteoclast differentiation in OTM partially depended on force-induced Adrb2.
Figure 5.
Orthodontic force-induced osteoclast differentiation partially depends on β-2 adrenergic receptor (Adrb2). (A) Tartrate-resistant acid phosphatase (TRAP) staining for osteoclasts in rats with orthodontic force application for 7 d. Arrows indicate the direction of tooth movement. Large boxed areas show higher-magnification views of the small boxes. (B) Semiquantification of TRAP+ multinucleated cells. The Force + ISO group presented the highest number of TRAP-positive multinucleated osteoclasts, followed by the Force and control groups. n = 5. * p < .05 vs. the control group. ** p < .01 vs. the control group. # p < .05 vs. Force group. (C) TRAP staining for osteoclasts in mice with orthodontic force application for 7 d. (D) Semiquantification of TRAP+ multinucleated cells. Orthodontic force-induced osteoclast formation was decreased in the Adrb1/2-/- group compared with that in the wild-type group. n = 5. * p < .05.
Discussion
We demonstrated that mechanical force-induced Adrb2 expressed in PDLCs contributed to SNS-regulated OTM by modulating osteoclast differentiation. First, OTM was reduced by SNS blockage and accelerated by ISO injection in rats. Adrb1/2-/- mice also showed reduced OTM distance. Second, force induced the activation of Adrb2 in PDLCs through the increase in intracellular Ca2+ concentration. Third, the activation of Adrb2 in PDLCs promoted osteoclast differentiation by increasing the RANKL/OPG ratio. Our results provide new insights to understand force-induced remodeling of alveolar bone in OTM.
Force-induced Adrb2 contributed to osteoclast differentiation and SNS-regulated OTM. In this study, the effect of sympathectomy on OTM was demonstrated for the first time by directly removing the superior cervical ganglion. Consequently, OTM distance was decreased by SCGx. Our result is consistent with that of a previous study in which sympathetic signaling is found to regulate experimental tooth movement (Kondo et al., 2013). ISO promoted osteoclast differentiation and OTM in rats. Adrb1/2-/- mice showed a decrease in osteoclast differentiation and OTM distance. Although OTM distance may also be affected by other SNS-regulated activities, such as masticatory movement and alveolar bone density (Passatore and Roatta, 2007; Sato et al., 2014), our data provide a novel mechanism by which SNS-regulated OTM is mediated by force-induced Adrb2 activation. In previous studies, animal models were used to investigate the function of SNS in bone mechanoadaptive responses, such as tail suspension and sciatic neurectomy. However, hardly any SNS involvement was found (de Souza et al., 2005; Marenzana et al., 2007; Marenzana and Chenu, 2008). Orthodontic force, as an exerted mechanical stimulus, could induce the corresponding change in alveolar bone remodeling visually indicated by the distance of tooth movement. Thus, the OTM animal model used in this study was relatively effective in examining the bone mechanoadaptive responses. The regulatory function of SNS and Adrb2 in bone remodeling under mechanical microenvironment was supported by our results. Therefore, the mechanism by which these responses affect the OTM process should be determined.
Adrb2 is known to be expressed in osteoblasts and various osteogenic cell lines, such as UMR 106, SaoS-2, and MG63, serving as the mediator of SNS regulated bone remodeling (Aitken et al., 2009; Elefteriou et al., 2005; Wong, 1979). We found that Adrb2 was highly expressed in PDLCs after 7 d of orthodontic force application in vivo. In addition, Adrb2 expression was further confirmed in isolated and expanded PDLCs derived from the neural crest where the SNS originated. PDL-derived stem cells express neural crest cell–specific markers and can be induced to form neural crest-like cells (Coura et al., 2008; Dupin and Coelho-Aguiar, 2013; Fortino et al., 2014; Xiaojie et al., 2014), which implies a close connection between PDLCs and SNS in Adrb2 expression. Static compressive force in vitro upregulated the expression of Adrb2 in PDLCs as force was increased and force-treated time was prolonged. A previous study reported that glucocorticoids increase Adrb2 expression in osteoblasts (Ma et al., 2011). In this study, we showed that mechanical stimulation could also promote Adrb2 expression and indicated that cells in physiologic mechanical environments could be involved in the corresponding Adrb2-mediated biological responses, such as bone remodeling, through force-induced Adrb2. In this study we found the force-induced upregulation of Adrb2 in PDLCs but found no significant increase of Adrb2 expression in OMCs, which suggests that PDLCs-expressed Adrb2 may be more sensitive to mechanical stimulation than OMCs. During orthodontic treatment, mechanical force loaded on tooth is transduced to the PDL, and then PDLCs respond to the stimuli and induce a chain of biological responses (Lekic and McCulloch, 1996). Generally, ankylosed teeth, in which the tooth root is directly connected with the alveolar bone, cannot be moved by orthodontic force because of the lack of PDL (Kanzaki et al., 2002). Our results supported the significant role of PDLCs played in OTM. Different cell types, microenvironments, and sources of development may contribute to the different responses of PDLCs and OMCs to mechanical force. However, more evidence is needed to reveal how OMCs influence the SNS-regulated OTM.
β adrenergic receptors play a major role in the regulation of cardiac function. Upon sympathetic stimulation, Adrb2 can stimulate cAMP production and enhance Ca2+ channel activity in the heart (Fajardo et al., 2013; Harvey and Hell, 2013). In this study, we found that force-induced upregulation of Adrb2 was eliminated by the intracellular Ca2+ chelator BAPTA-AM, suggesting that force-induced Adrb2 in PDLCs partially depended on the increase in intracellular Ca2+. Intracellular Ca2+ is essential in several signal pathways, such as MAPK, CREB, and NF-κB pathways, which are reported to be Adrb2-positive transcriptional regulators (Aksoy et al., 2001; Yeh et al., 2012). Ca2+ induces the stimulation of adenylyl cyclase and the elevation of intracellular cAMP levels (Watson et al., 2000). cAMP response element-binding protein can positively regulate Adrb2 expression (Collins et al., 1990). The results may help understand the mechanism by which force-induced increase in intracellular Ca2+ concentration contributes to the upregulation of Adrb2.
In the downstream region of the PDLCs-expressed Adrb2, Adrb2 activation by ISO increased the RANKL/OPG ratio, which was decreased by Adrb2-selective antagonist ICI 118,551 and Adrb2 siRNA transfection. Our results are consistent with those of a previous study, which reported that Adrb2 favors bone resorption by increasing the expression of osteoclast differentiation factor RANKL in osteoblast progenitor cells (Elefteriou et al., 2005). Mechanical stimulation also intensified the Adrb2-regulated upregulation of RANKL protein expression. The results of the cocultured PDLCs and peripheral blood mononuclear cells confirmed that the activation of Adrb2 in PDLCs promoted osteoclast formation. Adrb1 and Adrb2 signaling exert opposite effects on bone remodeling. Adrb2 promotes bone resorption, whereas Adrb1 contributes to bone formation. In the double-knockout animal model, the concomitant loss of Adrb1 may counteract the effect of Adrb2 deficiency on bone (Pierroz et al., 2012). In OTM, the rate-limiting step is the compression-associated osteoclasts formation (Masella and Meister, 2006). We found that Adrb1/2-/- mice showed decreased osteoclast differentiation and OTM distance, suggesting that the Adrb2 deficiency-induced decrease of osteoclast formation plays a major role in this process. In addition, selective antagonist and siRNA of Adrb2 reduced osteoclatogenic response in PDLCs. This finding suggests that Adrb2 in PDLCs contributes to the compression-associated osteoclast formation in OTM. However, bone homeostasis is a dynamic change between bone resorption and formation, and bone apposition may present in the compression site. Whether PDLCs express Adrb1 and play osteoblastogenic function in OTM still needs further study.
In conclusion, mechanical force upregulated Adrb2 expression in PDLCs through the force-induced increase in the intracellular Ca2+ concentration. The activation of Adrb2 in PDLCs contributed to osteoclast differentiation through the RANKL/OPG system and promoted SNS-regulated OTM. The results may help understand the mechanism by which mechanical force affects the alveolar bone remodeling of OTM.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by grants from the National Natural Science Foundation of China (grant no. 81300897), and International S&T cooperation program of China (grant no. 2013DFB30360).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aitken SJ, Landao-Bassonga E, Ralston SH, Idris AI. (2009). Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch Biochem Biophys 482:96-103. [DOI] [PubMed] [Google Scholar]
- Aksoy MO, Bin W, Yang Y, Yun-You D, Kelsen SG. (2001). Nuclear factor-kappa B augments beta(2)-adrenergic receptor expression in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 281:L1271-L1278. [DOI] [PubMed] [Google Scholar]
- Biewener AA. (1985). Bone and its functions: the mechanical adaptations of bones. Science 227:629-630. [DOI] [PubMed] [Google Scholar]
- Collins S, Altschmied J, Herbsman O, Caron MG, Mellon PL, Lefkowitz RJ. (1990). A cAMP response element in the beta 2-adrenergic receptor gene confers transcriptional autoregulation by cAMP. J Biol Chem 265:19330-19335. [PubMed] [Google Scholar]
- Cooper SM, Sims MR. (1989). Evidence of acute inflammation in the periodontal ligament subsequent to orthodontic tooth movement in rats. Aust Orthod J 11:107-109. [PubMed] [Google Scholar]
- Coura GS, Garcez RC, de Aguiar CB, Alvarez-Silva M, Magini RS, Trentin AG. (2008). Human periodontal ligament: a niche of neural crest stem cells. J Periodontal Res 43:531-536. [DOI] [PubMed] [Google Scholar]
- de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. (2005). Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res 20:2159-2168. [DOI] [PubMed] [Google Scholar]
- Dunn MD, Park CH, Kostenuik PJ, Kapila S, Giannobile WV. (2007). Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone 41:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Coelho-Aguiar JM. (2013). Isolation and differentiation properties of neural crest stem cells. Cytometry A 83:38-47. [DOI] [PubMed] [Google Scholar]
- Dupin E, Sommer L. (2012). Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol 366:83-95. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. (2005). Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514-520. [DOI] [PubMed] [Google Scholar]
- Fajardo G, Zhao M, Urashima T, Farahani S, Hu DQ, Reddy S, et al. (2013). Deletion of the beta2-adrenergic receptor prevents the development of cardiomyopathy in mice. J Mol Cell Cardiol 63:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortino VR, Chen RS, Pelaez D, Cheung HS. (2014). Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol 229:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet TP, Coelho U, Silva JS, Garlet GP. (2007). Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci 115:355-362. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Hell JW. (2013). CaV1.2 signaling complexes in the heart. J Mol Cell Cardiol 58:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. (2000). Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405:704-706. [DOI] [PubMed] [Google Scholar]
- Immermann EW, Compere EL. (1954). Bone metastases of neurogenic tumors of the sympathetic nervous system in children. Q Bull Northwest Univ Med Sch 28:138-142. [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Chandler JR, Marx DB, Pandey JP, Nickel JC. (2009). IL-1 gene polymorphisms, secretion in gingival crevicular fluid, and speed of human orthodontic tooth movement. Orthod Craniofac Res 12:129-140. [DOI] [PubMed] [Google Scholar]
- Kang YG, Nam JH, Kim KH, Lee KS. (2010). FAK pathway regulates PGE production in compressed periodontal ligament cells. J Dent Res 89:1444-1449. [DOI] [PubMed] [Google Scholar]
- Kanzaki H, Chiba M, Shimizu Y, Mitani H. (2002). Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 17:210-220. [DOI] [PubMed] [Google Scholar]
- Kondo M, Kondo H, Miyazawa K, Goto S, Togari A. (2013). Experimental tooth movement-induced osteoclast activation is regulated by sympathetic signaling. Bone 52:39-47. [DOI] [PubMed] [Google Scholar]
- Lekic P, McCulloch CA. (1996). Periodontal ligament cell population: the central role of fibroblasts in creating a unique tissue. Anat Rec 245:327-341. [DOI] [PubMed] [Google Scholar]
- Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F. (2011). Beta2-adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 152:1412-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenzana M, Chenu C. (2008). Sympathetic nervous system and bone adaptive response to its mechanical environment. J Musculoskelet Neuronal Interact 8:111-120. [PubMed] [Google Scholar]
- Marenzana M, De Souza RL, Chenu C. (2007). Blockade of beta-adrenergic signaling does not influence the bone mechano-adaptive response in mice. Bone 41:206-215. [DOI] [PubMed] [Google Scholar]
- Masella RS, Meister M. (2006). Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop 129:458-468. [DOI] [PubMed] [Google Scholar]
- Matthews EK, Quilliam JP. (1964). Effects of central depressant drugs upon acetylcholine release. Br J Pharmacol Chemother 22:415-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui N, Suzuki N, Maeno M, Mayahara K, Yanagisawa M, Otsuka K, et al. (2005). Optimal compressive force induces bone formation via increasing bone sialoprotein and prostaglandin E(2) production appropriately. Life Sci 77:3168-3182. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. (1987). Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595-610. [DOI] [PubMed] [Google Scholar]
- Passatore M, Roatta S. (2007). Modulation operated by the sympathetic nervous system on jaw reflexes and masticatory movement. Arch Oral Biol 52:343-346. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Bonnet N, Bianchi EN, Bouxsein ML, Baldock PA, Rizzoli R, et al. (2012). Deletion of beta-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res 27:1252-1262. [DOI] [PubMed] [Google Scholar]
- Sato T, Miyazawa K, Suzuki Y, Mizutani Y, Uchibori S, Asaoka R, et al. (2014). Selective beta2-adrenergic antagonist butoxamine reduces orthodontic tooth movement. J Dent Res 93:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149-155. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. (2002). Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305-317. [DOI] [PubMed] [Google Scholar]
- Watson EL, Jacobson KL, Singh JC, Idzerda R, Ott SM, DiJulio DH, et al. (2000). The type 8 adenylyl cyclase is critical for Ca2+ stimulation of cAMP accumulation in mouse parotid acini. J Biol Chem 275:14691-14699. [DOI] [PubMed] [Google Scholar]
- Wong GL. (1979). Induction of metabolic changes and down regulation of bovine parathyroid hormone-responsive adenylate cyclase are dissociable in isolated osteoclastic and osteoblastic bone cells. J Biol Chem 254:34-37. [PubMed] [Google Scholar]
- Xiaojie L, Dapeng L, Ping G, Yan D, Gang S. (2014). Biological behavior of neurally differentiated periodontal ligament stem cells on different titanium implant surfaces. J Biomed Mater Res A 102:2805-2812. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Ren G, Akiyama K, Chen C, Shi Y, Shi S. (2011). Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res 90:317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CK, Chandrasekar B, Lin AL, Dang H, Kamat A, Zhu B, et al. (2012). Cellular signals underlying beta-adrenergic receptor mediated salivary gland enlargement. Differentiation 83:68-76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.