Abstract
Hymenaea courbaril is a leguminous tree species from the neotropical rain forests. Its cotyledons are largely enriched with a storage cell wall polysaccharide (xyloglucan). Studies of cell wall storage polymers have been focused mostly on the mechanisms of their disassembly, whereas the control of their mobilization and the relationship between their metabolism and seedling development is not well understood. Here, we show that xyloglucan mobilization is strictly controlled by the development of first leaves of the seedling, with the start of its degradation occurring after the beginning of eophyll (first leaves) expansion. During the period of storage mobilization, an increase in the levels of xyloglucan hydrolases, starch, and free sugars were observed in the cotyledons. Xyloglucan mobilization was inhibited by shoot excision, darkness, and by treatment with the auxin-transport inhibitor N-1-naphthylphthalamic acid. Analyses of endogenous indole-3-acetic acid in the cotyledons revealed that its increase in concentration is followed by the rise in xyloglucan hydrolase activities, indicating that auxin is directly related to xyloglucan mobilization. Cotyledons detached during xyloglucan mobilization and treated with 2,4-dichlorophenoxyacetic acid showed a similar mobilization rate as in attached cotyledons. This hormonal control is probably essential for the ecophysiological performance of this species in their natural environment since it is the main factor responsible for promoting synchronism between shoot growth and reserve degradation. This is likely to increase the efficiency of carbon reserves utilization by the growing seedling in the understorey light conditions of the rain forest.
The presence and mobilization of xyloglucans following seed germination were first reported in the 19th century for seeds of Impatiens balsamina, nasturtium (Tropaeolum majus), and Cyclamen europaeum (Heinricher, 1888; Reiss, 1889). Later in the 1960s, the botanical distribution of xyloglucans in seeds was reviewed by Kooiman (1960), who used the ability of xyloglucan to stain with iodine, giving a distinctive blue color, as a form of detection.
Seed xyloglucans have a main β-d-(1→4)-glucan backbone branched with α(1→6)-linked d-xylopyranosyl or β-d-galactopyranosyl(1→2)-d-xylopyranosyl residues. Except for the absence of terminal fucosyl units α-l-(1→2)-linked to the branching β-d-galactosyl residues, there is a remarkable similarity between seed reserve xyloglucan and structural xyloglucan from primary cell walls of dicotyledonous tissues (Hayashi, 1989).
The reserve function of xyloglucan in cotyledons has been demonstrated for seeds of nasturtium (Edwards et al., 1985), Tamarindus indica (Reis et al., 1987), Copaifera langsdorffii (Buckeridge et al., 1992), and Hymenaea courbaril (Tiné et al., 2000), where xyloglucan mobilization in vivo is followed by the rise and fall of the activities of four hydrolases: β-galactosidase, endo-β-(1→4)-d-glucanase (or xyloglucan transglycosylase hydrolase [XTH], which is a term used for the gene or protein that can have endo-transglycosylase [XET] or endo-β-hydrolase [XEH; hydrolase] activity [Rose et al., 2002]), α-xylosidase, and β-glucosidase.
Reid and co-workers isolated the four main enzymes responsible for xyloglucan degradation in nasturtium. They are (1) xyloglucan-specific endo-β-(1→4)-d-glucanase or xyloglucan XET (Edwards et al., 1986; Fanutti et al., 1993); (2) a β-galactosidase with high specificity toward xyloglucan (Edwards et al., 1988); (3) a xyloglucan oligosaccharide-specific α-xylosidase or oligoxyloglucan exo-xylohydrolase (Fanutti et al., 1991); and (4) a transglycosylating β-glucosidase (Crombie et al., 1998).
On the basis of these results, together with other studies on the mode of action of the XTH (Edwards et al., 1986; Fanutti et al., 1993), Crombie et al. (1998) proposed a model for xyloglucan mobilization in nasturtium. In this model, the four enzymes are thought to work in a concerted fashion, producing Gal, Glc, and Xyl. In nasturtium, XTH and β-galactosidase are the only enzymes capable of attacking the polymer. Under low concentrations of xyloglucan oligosaccharides (acceptors), XEH activity predominates over XET activity (Fanutti et al., 1993). Thus, when in contact with high Mr xyloglucan, hydrolysis predominates, producing oligosaccharides, which are promptly attacked by the exo-glycosidases (α-xylosidase and β-glucosidase), thereby reducing the polymer to its monosaccharide constituents.
Reis et al. (1987) cytochemically analyzed the digestion of the xyloglucan-containing cell walls of T. indica cotyledons. Using the techniques of iodine staining and a gold probe prepared by complexing Escherichia coli β-galactosidase with gold particles, they were able to study xyloglucan mobilization in cotyledon cell walls at the ultrastructural level with high specificity. They observed the presence of an inner and an outer wall, which are not degraded and become more apparent following xyloglucan mobilization. Furthermore, a fibrous material was left after mobilization, showing that not all the wall is mobilized. They also found that as xyloglucan is degraded, the relative proportions of monosaccharides (Glc:Xyl:Gal) do not change significantly.
In C. langsdorffii, the mobilization of the cotyledon cell wall storage has been observed cytochemically, physiologically, and biochemically by Buckeridge et al. (1992). They have studied two different populations from two different biomes (forest and savannah) and did not find apparent differences in xyloglucan mobilization between seeds from the two environments. Recently, a β-galactosidase was purified from cotyledons of C. langsdorffii (Alcântara et al., 1999). In contrast with nasturtium β-galactosidase, this enzyme showed very high specificity toward certain xyloglucan oligosaccharides and was not active against the polymer. Furthermore, its pH optimum showed a very sharp peak at 3.2, while the optima of the other hydrolases were around 4.5.
In cotyledons of H. courbaril, Tiné et al. (2000) showed that xyloglucan is mobilized after germination and that Glc and Suc are produced concomitant with xyloglucan disassembly. Furthermore, these authors detected the same four xyloglucan-degrading enzyme activities that have been found in nasturtium. They also found evidence for the presence of transglycosylation activity (XET) in this species. As in C. langsdorffii, β-galactosidase activity, as detected using ρ-nitrophenyl galactopyranoside, had a pH optimum at 3.2, whereas the other hydrolases detected were active at 4.5. The results obtained for β-galactosidases of C. langsdorffii and H. courbaril suggest that this enzyme might be one of the important steps in the control of seed storage xyloglucan metabolism in legumes since the retrieval of Gal branches from certain xyloglucan oligosaccharides are essential for the further attack of the other exo-hydrolases of the system (Buckeridge et al., 2000; Tiné et al., 2003).
Although some work has been performed on the mechanism of seed storage xyloglucan mobilization in seeds, very little is known about the control mechanisms involved in the process. The only report was provided by Hensel et al. (1991), who demonstrated that 2,4-dichlorophenoxyacetic acid (2,4-D, a synthetic auxin) was capable of inducing xyloglucan mobilization in detached cotyledons of nasturtium. On the other hand, the effects of auxin on primary cell wall fucosylated xyloglucan have been studied more extensively; the principal effects of the hormone being (1) activation of H+ transport to the extracellular medium (possibly related to activities of ATPases), in which the lowering of local pH favors the action of glycosidases and expansins (Taguchi et al., 1999; Cosgrove, 2000), and (2) activation or alteration of gene expression so that enzymes (mainly glycosidases) have enhanced activities or are synthesized de novo.
In this study, we investigated some aspects of the effect of auxin on the mobilization of xyloglucan in cotyledons of growing seedlings of H. courbaril. Our results indicate that auxin is involved in xyloglucan metabolism, being produced in the shoot of the growing seedling so that the pace of growth possibly controls storage mobilization in the cotyledons.
RESULTS
Storage Mobilization in the Cotyledons and Early Seedling Growth
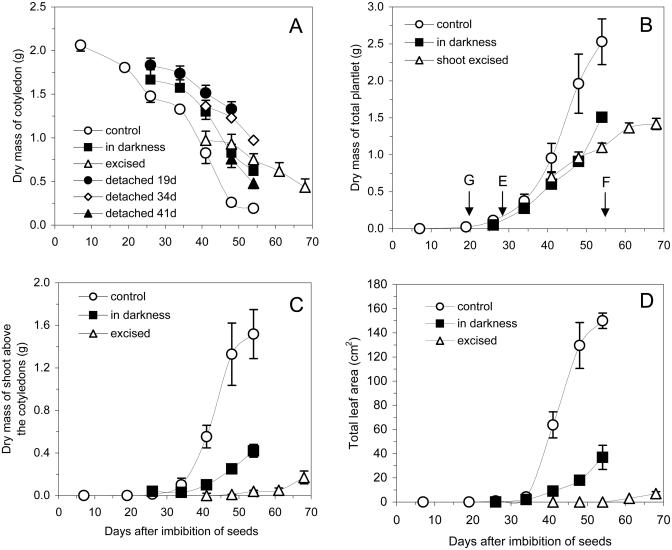
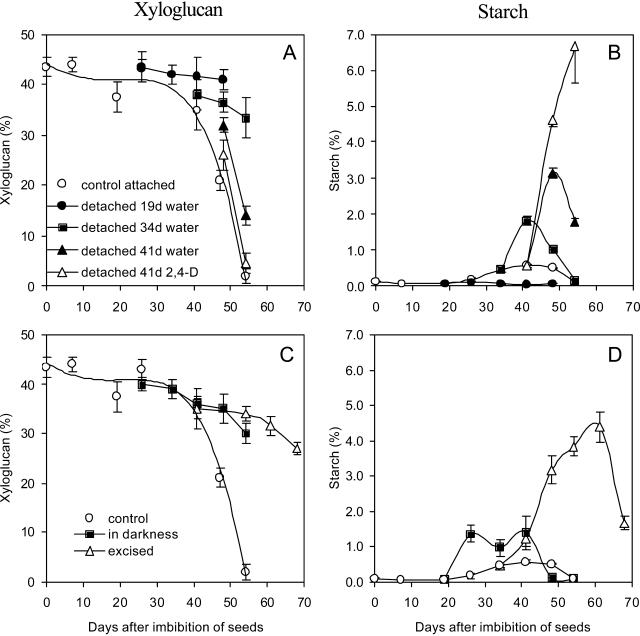
The decay curve of dry mass of the cotyledons of H. courbaril decreased in two phases (Experiment I; see Fig. 7 for details of the experiments I and II). The first step was from 0 to 26 d, when protein bodies and raffinose series oligosaccharides are mobilized (Tiné et al., 2000), and the second was from 34 to 50 d, when xyloglucan is degraded (Figs. 1A and 2A). In detached cotyledons from growing seedlings at different stages of germination/establishment, a decrease in dry mass (Fig. 1A) or xyloglucan content (Fig. 2A) was observed only when it occurred after 34 d. By contrast, when cotyledons were detached at 41 d and incubated in water, xyloglucan degradation was delayed but occurred at a similar rate as that observed in the attached cotyledons (Fig. 2A).
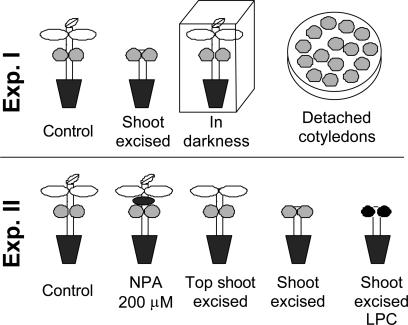
Figure 7.
Schematic representation of the two experiments performed. See “Materials and Methods” for details.
Figure 1.
Dry masses of detached and attached cotyledons (A), whole seedlings (B), the shoot above the cotyledons insertions (C), and total leaf area of seedlings of H. courbaril (D). Seedlings with cotyledons grown under control conditions (control), in the darkness, or with the shoot excised above the cotyledons insertions at 34 d (excised). Cotyledons were detached at 19, 34, and 41 d and kept in water in the darkness. Germination (G), emergence of seedlings (E), and fall of cotyledons (F). Bars represent sd of the mean of five replicates.
Figure 2.
Contents of xyloglucan and starch in detached cotyledons (A and B, respectively; legend in A) and in cotyledons attached to the seedlings (C and D; legend in C) of H. courbaril. The attached cotyledons were from seedlings grown under control conditions (control), in the darkness. or with shoot excised above the cotyledons insertions at 34 d (excised). Cotyledons were detached at 19, 34, and 41 d and kept in water in the darkness or in 10−6 m 2,4-D (41 d only). Bars represent sd of the mean of three composed replicates.
The addition of 10−6 M 2,4-D after 41 d significantly increased the rate and extent of xyloglucan degradation, similar to the extent observed in attached cotyledons (Fig. 2A). Exogenous 2,4-D affected xyloglucan mobilization only at the concentration of 10−6 m and in the same period as the attached cotyledons. Incubation of detached cotyledons in 2,4-D before the period of highest rate of xyloglucan mobilization (after 34 d) or in all periods at 10−4 and 10−5 m failed to evoke any detectable xyloglucan breakdown (data not shown).
The drastic dry mass loss and xyloglucan mobilization in the cotyledons were directly related to the increase in dry mass of the seedling, mainly with the shoot and expansion of eophylls (Figs. 1 and 2A). This relationship was clearer from the observation of seedlings of H. courbaril growing after excision of the shoot or in the darkness. The latter prevented the increase in total leaf area (Fig. 1D) and dry masses of whole seedlings (Fig. 1B) and their shoot (Fig. 1C). Furthermore, darkness promoted a reduction of xyloglucan mobilization (Figs. 1A and 2C).
Another group of seedlings, subjected to excision of the shoot at 34 d (when xyloglucan degradation starts), showed a strong delay in xyloglucan mobilization (Fig. 2C). This inhibitory effect was followed by a significant reduction of seedling growth (Fig. 1B). Approximately 15 d after excision, the shoot started to grow again, producing new leaves (Fig. 1, C and D), an event that was followed by an increase in xyloglucan mobilization (Fig. 2C).
Starch, Soluble Sugars, and Xyloglucan Metabolism
The reduction observed in dry mass and xyloglucan during mobilization was followed by an increase in the amount of starch, mainly in detached cotyledons. Moreover, addition of 10−6 m 2,4-D to detached cotyledons at a period of advanced mobilization (41 d) promoted an even greater increase in starch synthesis (Fig. 2B), suggesting that this phenomenon might be dependent on xyloglucan mobilization.
Seedlings growing in the darkness or with the excision of the shoot showed an induction of starch synthesis, accumulating relatively higher amounts of starch in the cotyledons than the control plants (Fig. 2D). In the excised seedlings, the accumulated starch was mobilized when growth of the shoot restarted.
Analysis of the free sugars showed that in attached cotyledons, Fru and Glc increased at the same time as xyloglucan was degraded, peaking at 48 d and decreasing rapidly afterward (Table I, control seedling). Suc was also present during the germination period, decreasing quickly up to 26 d and increasing again during xyloglucan degradation. When seedlings were grown in the darkness or when the shoot was excised, a strong reduction in the concentration of free sugars was observed (Table I). The resumption of xyloglucan mobilization after excision of shoot was also confirmed by a parallel increase in Glc, Fru, and Suc in the cotyledons (Table I).
Table I.
Contents of Glc, Fru, and Suc (μg mg−1 cotyledon) detected by high performance anion-exchange chromatography in alcohol extracts of detached and attached cotyledons of seedlings of H. courbaril
| Days | Treatments
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Seedling
|
Seedling in Darkness
|
Shoot Excised 34 d
|
Detached 19 d Water
|
Detached 34 d Water
|
Detached 41 d Water
|
Detached 41 d 2,4-D
|
|||||||||||||||
| Glc | Fru | Suc | Glc | Fru | Suc | Glc | Fru | Suc | Glc | Fru | Suc | Glc | Fru | Suc | Glc | Fru | Suc | Glc | Fru | Suc | |
| μg mg cot−1 | |||||||||||||||||||||
| 7 | 0.9 | 1.3 | 5.3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 19 | 0.5 | 1.7 | 5.7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 26 | 0.4 | 1.8 | 0.6 | 0.3 | 1.4 | 0.9 | – | – | – | 0.4 | 1.7 | 8.2 | – | – | – | – | – | – | – | – | – |
| 34 | 1.9 | 5.8 | 0.6 | 0.4 | 3.3 | 3.1 | – | – | – | 0.3 | 1.4 | 7.5 | – | – | – | – | – | – | – | – | – |
| 41 | 5.2 | 10.4 | 3.7 | 2.0 | 6.6 | 3.0 | 3.1 | 5.5 | 4.4 | 0.2 | 0.8 | 4.2 | 1.1 | 5.8 | 24.7 | – | – | – | – | – | – |
| 48 | 22.1 | 23.1 | 6.0 | 1.9 | 4.9 | 4.6 | 4.4 | 8.6 | 1.4 | 0.1 | 0.3 | 0.6 | 1.5 | 7.9 | 22.2 | 4.5 | 20.9 | 41.0 | 18.4 | 32.9 | 33.8 |
| 54 | 16.3 | 13.8 | 5.3 | 2.5 | 7.2 | 7.7 | 2.0 | 7.2 | 2.3 | – | – | – | 0.2 | 0.6 | 0.1 | 8.5 | 31.8 | 3.7 | 0.9 | 5.7 | 0.9 |
| 61 | – | – | – | – | – | – | 4.6 | 9.0 | 4.4 | – | – | – | – | – | – | – | – | – | – | – | – |
| 68 | – | – | – | – | – | – | 6.0 | 12.2 | 4.6 | – | – | – | – | – | – | – | – | – | – | – | – |
The attached cotyledons were from seedlings grown under control conditions, in the darkness, or with shoot excised above the cotyledons insertions at 34 d. The detached cotyledons were isolated at 19, 34, and 41 d after the beginning of seed imbibition and maintained in darkness with water or 2,4-D (10−6 m). All data represent the analyses of the same three samples of cotyledons used for starch determination. −, Not determined.
The analysis of detached cotyledons showed that only those isolated after 34 d were capable of maintaining an increase in free sugars, reaching higher levels when detachment occurred at 41 d (Table I). Likewise, cotyledons treated with 2,4-D at 41 d showed high free-sugar contents during xyloglucan mobilization. It is important to note that isolated cotyledons tended to show a higher Suc to monosaccharide ratio than attached cotyledons.
Xyloglucan Hydrolases
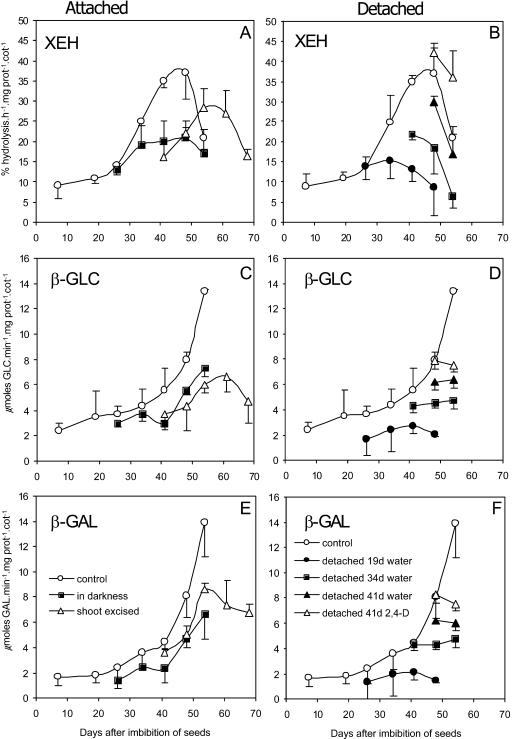
In attached cotyledons, all hydrolase activities related to xyloglucan mobilization increased during the same period of the most intense changes in dry mass and carbohydrate contents. In all treatments, the increase in the activities of XEH, α-xylosidase (assayed only in the second experiment), β-galactosidase, and β-glucosidase occurred 30 d after imbibition (Figs. 3 and 4).
Figure 3.
Specific activities of xyloglucan hydrolases in attached (A, C, and E; legends at E) and detached cotyledons (B, D, and F; legends at F) of H. courbaril during seedling development. The attached cotyledons used were from seedlings grown under control conditions (control), in the darkness. or with shoot excised above the cotyledons insertions (shoot excised). The detached cotyledons were taken from developing seedlings at 19, 34. and 41 d and kept in water in the darkness or in 10−6 m 2,4-D (41 d only). A and B, XEH. C and D, β-glucosidase. E and F, β-galactosidase. The activities detected in attached cotyledons (control) were added to each graph as a reference. Bars represent sd of the mean of three composed replicates.
Figure 4.
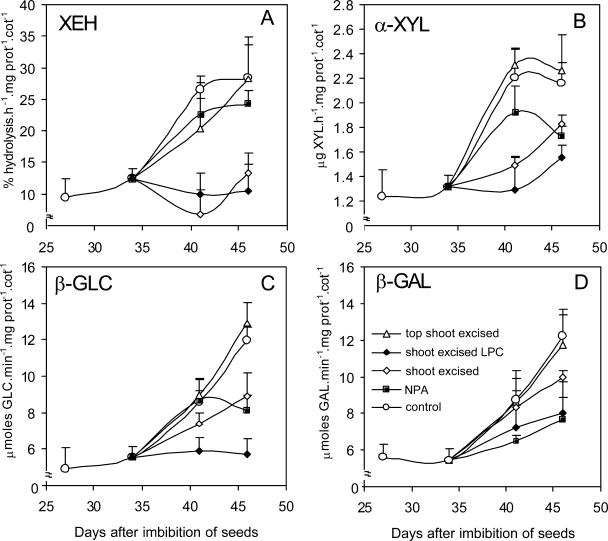
Specific activities of xyloglucan hydrolases in attached cotyledons of seedlings of H. courbaril grown as a control without excision treatment (control) and intact seedlings treated with N-1-naphthylphthalamic acid at 200 μm (NPA); and in shoot excised plants (shoot excised), shoot excised seedlings with light-protected cotyledons (shoot excised LPC) and shoot apex excised seedlings (top shoot excised). A, XEH. B, α-xylosidase. C, β-glucosidase. D, β-galactosidase. The activities detected in attached cotyledons (control) were added to each graph as a reference. Bars represent sd of the mean of three composed replicates.
In cotyledons detached at 19 d (Fig. 7, Experiment I), the activities of the three enzymes did not increase at the same rate as in the attached cotyledons. However, the cotyledons isolated at 34 and 41 d and kept in water thereafter showed a less pronounced increase in activities of both endo- and exo-enzymes in comparison with detached cotyledons incubated in 2,4-D or attached cotyledons (Fig. 3, B, D, and F). This occurred concomitantly with the reduction of xyloglucan and with the increase of free sugars and starch in the cotyledons (Table I; Fig. 2). The enzyme activities in cotyledons detached at 41 d were also stimulated by 10−6 m 2,4-D, in particular XEH activity (Fig. 3B).
The dark-grown seedlings showed reduction of all hydrolase activities in relation to the light-growth seedlings (Fig. 3, A, C, and E). This was also observed after excision of the shoot of the seedling in both experiments (Figs. 3 and 4).
In order to evaluate the isolated effect of auxin on the activity of xyloglucan hydrolases in the presence of sink strength, the following treatments were used: top shoot excision or application of N-1-naphthylphthalamic acid (NPA, an inhibitor of auxin transport). Although after top shoot excision no differences in hydrolase activities had been observed, when NPA was used, with the exception of XEH, all hydrolase activities were strongly reduced (Fig. 4). The exception of this reduction was observed with XEH, which has not been significantly reduced by NPA (Fig. 4A). This reduction in hydrolase activities may be explained by the fact that auxin is produced by the entire shoot, and in our experiments, although excision of the top shoot decreased the level of auxin in the cotyledons, NPA also promoted a strong reduction in indole-3-acetic acid (IAA) contents in the cotyledons in relation to the treatments of top shoot excised and control seedlings (see below).
Endogenous Auxin
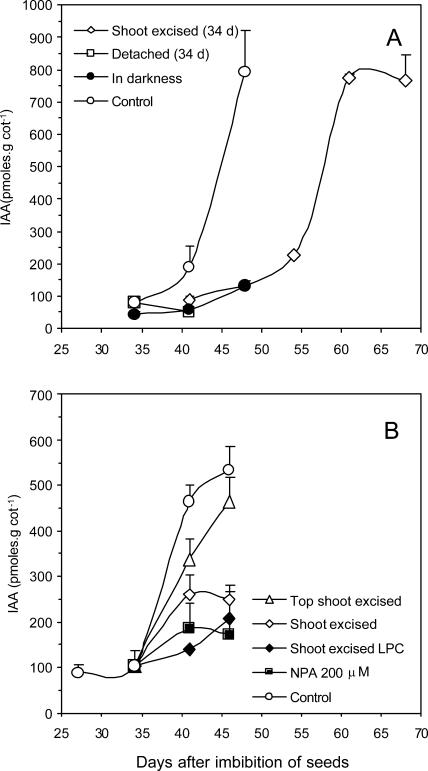
Aiming to test the hypothesis that there is a relationship between auxin increase and xyloglucan mobilization in the cotyledons of H. courbaril, we sought to measure the endogenous levels of auxin in detached or in attached cotyledons of seedlings submitted to shoot (all parts above cotyledons) and top shoot excision (all parts above the eophylls; see Experiment II in Fig. 7) and auxin transport inhibition (NPA). Our results showed that auxin levels increased at the same period of the xyloglucan mobilization in the cotyledons of H. courbaril (Fig. 5). Levels of IAA increased rapidly in cotyledons of intact (control) seedlings (approximately 8-fold), but in cotyledons of seedlings that had their shoot excised at 34 d, the highest IAA level was reached several days later and coincided with the observed regrowth of the shoot (compare Figs. 5A and 1D). On the other hand, in cotyledons detached at 34 d, no increase in IAA was observed (Fig. 5A). A similar behavior was observed for attached cotyledons from seedlings in the dark, from shoot excised kept in the light, or NPA treated (Fig. 5, A and B). Although these results suggest that the cotyledons of H. courbaril appear not to be able to produce endogenous auxin, this possibility cannot be excluded since in the light-kept cotyledons (NPA or shoot excised), a small increase in IAA was observed throughout the experiment (Fig. 5B).
Figure 5.
Concentration of endogenous IAA determined by ELISA (A; Peres et al., 1997) and by GC-SIM-MS (B; Chen et al., 1988). In A, the IAA was measured in the attached cotyledons from intact seedlings of H. courbaril growing in light, darkness, or with shoot excised above the cotyledons insertions (shoot excised) at 34 d. The only isolated cotyledons used as a reference in this case were the ones detached at 34 d and subsequently kept in the darkness. The plant materials used for auxin analyses were selected according to availability of tissue and to maximize the differences observed during the period of analysis. In B, the IAA was also measured in attached cotyledons of intact seedlings of H. courbaril grown in the light and in intact seedlings treated with NPA at 200 μm (NPA 200 μm). In the last technique, the IAA was also evaluated in shoot excised seedlings at 34 d (shoot excised), in shoot excised seedlings with light-protected cotyledons (shoot excised LPC), and in seedlings with the shoot apex excised (top shoot excised). Bars represent sd of the mean of three composed replicates.
DISCUSSION
According to Bewley and Black (1994), there are two possible mechanisms by which the embryonic axis can control storage mobilization in seeds. One is through the action of the axis as a sink organ, metabolizing the products of storage compounds (e.g. Suc), avoiding feedback mechanisms by their end products, and another is through the delivery of a signal (e.g. a hormone) to modulate storage mobilization.
Our results can be used as a direct evidence for the hypothesis of the existence in developing seedlings of H. courbaril of a hormonal signal (auxin) as the principal control of xyloglucan mobilization in this tree species. However, other mechanisms related to metabolic events in the shoot organs (eophyll and leaves), as well as in the cotyledons, appear to participate in a chain of events that are related to the modulation of xyloglucan catabolism and use of its products during seedling growth.
The Importance of Sink Strength, Carbohydrate Metabolism, and Light for Storage Xyloglucan Mobilization
Our results suggest that the embryonic axis apparently plays a role in xyloglucan mobilization by establishment of a sink for its degradation products, mainly in the expanding leaves. In this process, as previously proposed by Chory (1993), light seems to play an important role by stimulating the production of the shoot, which was the main sink to the products of xyloglucan. The reductions of the rate of xyloglucan mobilization by excision of the shoot or by isolation of cotyledons appear to be related to the observed reduction in activity of all xyloglucan hydrolases (Figs. 3 and 4). This might be partially explained by the reduction of sink strength, denoted by the observation of high levels of starch and soluble free sugars in the cotyledons (Fig. 2D; Table I), and a consequent feedback inhibition.
Our results suggest that accumulation of starch might be dependent on xyloglucan mobilization. Indeed, transitory starch has been observed previously in seeds that mobilize galactomannan (Reid, 1971; McCleary, 1983; Buckeridge and Dietrich, 1996), and this has been proposed as an important step in the regulation of carbon flow in leaves (Ludewig et al., 1998). It is thought that transitory starch is synthesized as a response to the production of large amounts of carbon during storage cell wall polysaccharide mobilization (Buckeridge et al., 2000). Although starch has been previously detected in cotyledon of H. courbaril during the period of xyloglucan mobilization (Tiné et al., 2000), the results presented in this study show for the first time, to our knowledge, what appears to be a direct relationship between events of starch accumulation associated with different rates of degradation of xyloglucan in the cotyledons.
According to Rolland et al. (2002), a low level of sugars enhances photosynthesis, reserve mobilization, and export, whereas their abundant presence promotes growth and storage. These authors also highlight the fact that sugar-limited conditions down-regulated biosynthetic activities to conserve energy and protect cells against nutrient stress. This is usually accompanied by up-regulation of starch degradation to sustain metabolic activity (Yu, 1999; Fujiki et al., 2000).
In the cotyledons of H. courbaril, the accumulation of starch may be therefore considered as a temporary consequence of the relationship between source (xyloglucan degradation) and sink (synthesis/transport of Suc to the shoot) intensities. By storing starch transitorily, the seedling may thus avoid potentially adverse effects of accumulation of high concentration of reducing sugars (Geiger et al., 2000).
At the H. courbaril cotyledon cell wall level, the balance between XET and XEH activities is dependent on the oligosaccharide concentration present in the apoplast (Tiné et al., 2000). Furthermore, the β-galactosidase of cotyledons of H. courbaril is strongly inhibited by Gal (Ki = 3.7 mm; Alcântara, 2000). A strong inhibitory effect was also observed on hydrolase activities when cotyledons of shoot excised seedlings were covered with aluminum foil (Fig. 4). These results permit to speculate that darkness has an inhibitory effect on xyloglucan mobilization so that the cotyledons have a mechanism to sense light.
A possible explanation for the effect of light is the observation of presence of active photosynthesis (only electron transport but not CO2 assimilation) in cotyledons of H. courbaril (M.P.M. Aidar, U. Lutgge, L.I.V. Amaral, M.S. Buckeridge, unpublished data). According to recent observations by Rolletschek et al. (2003), photosynthesis contributes with oxygen supply in cotyledons of pea (another legume). Thus, it is possible to speculate that the decrease in the activities of hydrolases in the darkness might be a consequence of lack of oxygen.
Altogether, these observations suggest that xyloglucan mobilization is probably controlled by multiple factors at different levels in cotyledon tissue. There appear to exist points of control acting as local effects of reaction products in the wall (e.g. oligosaccharides and free Gal), up to a more indirect environmental effect of light on the cotyledons themselves.
Auxin and Xyloglucan Mobilization
Considering the second mechanism proposed by Bewley and Black (1994), in which storage mobilization might be controlled by hormones, our experiments have shown that auxin appears to be involved in the control of xyloglucan mobilization in the cotyledons. One piece of evidence favoring this hypothesis is that the concentration of IAA in the cotyledons varied in accordance with the changes in hydrolase activities (Figs. 3–5). Furthermore, this increase in IAA in the cotyledons can be significantly decreased or delayed by (1) excision of the shoot; (2) growth in the darkness; and (3) use of NPA, an inhibitor of polar auxin transport (Jensen et al., 1998; Reed et al., 1998; Muday and DeLong, 2001). As the addition of 10−6 m 2,4-D to detached cotyledons was able to induce an increase in xyloglucan degradation, starch biosynthesis and at the same time promote xyloglucan hydrolase activities, it can also be deduced that these events are possibly under the control of auxin, as has been observed for primary (Hoson, 1993; Valero and Labrador, 1995; Catalá et al., 1997, 2000; Kotake et al., 2000) and storage cell walls (Hensel et al., 1991). Furthermore, as in the case of nasturtium (Hensel et al., 1991), we can speculate that cotyledons of H. courbaril become sensitive to auxin only during the period of xyloglucan mobilization since the incubations of detached cotyledons in 2,4-D before this period failed to evoke any detectable degradation of xyloglucan (data not shown).
Altogether, the results confirm that the IAA present in the cotyledons during xyloglucan mobilization is produced in and transported from the developing shoot, which is considered the main site of auxin biosynthesis (Bartel, 1997). It should be noted that during xyloglucan mobilization, IAA seems to be produced mainly in the expanding eophylls since the excision of the shoot apex had a limited effect on IAA production (Fig. 5B).
The levels of IAA in the cotyledons seem to be strongly associated with polar auxin transport, as we confirmed by NPA treatment, and not with hydrolysis of IAA conjugates as has been described for seedlings of Pinus sylvestris (Ljung et al., 2001). The behavior of NPA-treated seedlings clearly demonstrated that the increase in xyloglucan hydrolase activities was strongly dependent on polar auxin transport from the developing shoot. This treatment promoted a negative regulation of all hydrolase activities (Fig. 4).
Recently, studies have demonstrated the importance of polar auxin transport in many aspects of plant development, such as the expansion of hypocotyl of cucumber (Shinkle et al., 1998), Arabidopsis (Jensen et al., 1998), and tomato (Kraepiel et al., 2001) hypocotyls, as well as the development of lateral roots (Reed et al., 1998) and the differentiation of leaf and cotyledon veins in Arabidopsis (Sieburth, 1999). Polar auxin transport is also considered to be a coordinator of rhythmicity in the extension rate oscillations of the first internode in Arabidopsis (Jouve et al., 1999). However, in most cases, the action of auxin has been described in the context of influencing the metabolism of the primary cell wall in growing herbaceous seedlings.
The single work in which a relationship between auxin and storage cell wall metabolism had been reported was in cotyledons of nasturtium (Hensel et al., 1991), which is also a herbaceous species. To our knowledge, this study is the first evidence of a relationship between endogenous levels of IAA and storage xyloglucan mobilization in the cotyledons of H. courbaril, a shade-tolerant tree from the neotropical rain forests. Among the possibilities for further studies are the influences of IAA on vein establishment in the cotyledons, the expression of cell wall-related genes, and on apoplastic pH, since acid pH optima (3.2–4.5) for the activities of xyloglucan hydrolases have been demonstrated in cotyledons of H. courbaril (Alcântara, 2000; Tiné et al., 2000) and in C. langsdorffii (Alcântara et al., 1999).
Synchronism and Ecological Function
Considering the results in terms of the effects of sink and auxin on carbohydrate metabolism in the cotyledons, it can be suggested that both light and aerial parts have a strong relationship with the catabolism of the storage cell wall xyloglucan and transient accumulation of starch and Suc, as summarized in Figure 6. As H. courbaril is a shade-tolerant species (Souza and Válio, 1999), this kind of correlation highlights the existence of a synchronism among the rates of storage mobilization/product utilization, depending on light intensity or light quality.
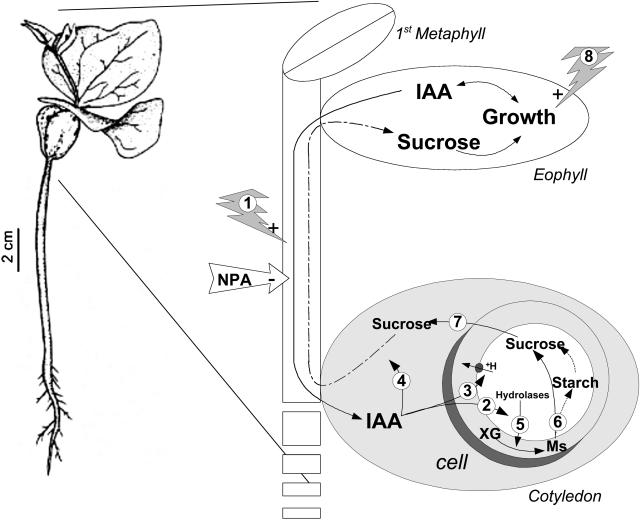
Figure 6.
Representation of the synchronism between events taking place during xyloglucan mobilization and shoot development in developing seedlings of H. courbaril. Auxin (IAA) is produced in expanding leaves (eophyll and first metaphyll) and transported to cotyledons by polar transport, which can be stimulated by red light (1) and inhibited by NPA. In the cotyledons, the IAA may act on the modulation of expression of the genes coding for cell wall hydrolases (2); on +H-pump activity reducing the apoplastic pH (3); or on the establishment of vascular system (4). Following the xyloglucan (XG) degradation (5) in storage cell walls of cotyledons by the concerted action of the hydrolases, monosaccharides (Ms) are transported to the cytoplasm, where they are metabolized (6) to Suc and starch. The Suc produced is driven mainly toward the growing shoot (7). In the shoot, light stimulates (8) growth, using Suc as a carbon backbone, which results in IAA availability to the cotyledons and consequently stimulation of xyloglucan mobilization.
Shinkle et al. (1998) demonstrated that red light is capable of inducing polar transport of auxin in Arabidopsis and cucumber, respectively. However, Kraepiel et al. (2001) showed indirectly that in tomato, the transport was independent of light. Our results showed that darkness strongly inhibited polar auxin transport in seedlings of Hymenaea. Also, in experiments where seedlings were grown under different conditions of red to far red ratios, mobilization was faster in increasing proportions of red light (H.P. Santos and M.S. Buckeridge, unpublished data). This suggests that seedlings of H. courbaril respond to light in a similar way to that of Arabidopsis and cucumber. Considering the results of Shinkle et al. (1998) on the influence of light on auxin polar transport, the hormonal control observed could play an important ecophysiological role in seedlings of H. courbaril by promoting synchronism between growth and reserve degradation under variable light conditions (Fig. 6). This is likely to increase the efficiency of carbon reserve utilization by the growing seedling in the understorey of the rain forest.
Altogether, our results corroborate the hypothesis that the presence of cell wall polysaccharides as storage compounds in cotyledons reflects an ancient functional transfer during evolution, as has been suggested by Buckeridge et al. (2000). This hypothesis states that not only the polymer but also its related metabolism might have been subjected to similar selective pressure during evolution. Therefore, it is reasonable to suggest that certain features of the mechanism of primary cell extension have been maintained in the storage cell wall metabolism during evolution, so that the catabolism of xyloglucan and its control mechanisms resemble the auxin-induced growth of primary cell walls. Our data support this hypothesis.
MATERIALS AND METHODS
Material and Experimental Conditions
In all experiments we employed size-selected seeds (5.5–6.0 g) of Hymenaea courbaril, collected in São João da Boa Vista county (22°00′S; 47°18′W), São Paulo, Brazil. These seeds were stored for 4 years under dry and cold conditions (relative humidity 35%, 8°C) in the Botanical Institute of São Paulo, Brazil. Seeds were scarified with sandpaper on the lateral position in relation to the embryo, surface-sterilized for 15 min in 10-fold-diluted commercial hypochlorite bleach, rinsed, and placed on trays between two sheets of wet paper (at 30°C) until germination was visible (0.5 cm of radicle). Elapsed time in all experiments was registered in relation to the beginning of imbibition, in days (days after imbibition of seeds).
Germinated seeds were placed in pots (1.5 L) with a washed sand:vermiculite mixture (2:1, v/v), and the pots were placed in a growth chamber, under shelves equipped with 10 fluorescent lamps (60 W) and four incandescent lamps (40 W) reaching around 200 μE m−2 s−1 of photosynthetic active light intensity. The photoperiod throughout the experiments was 12-h-light/12-h-dark cycle with constant temperature (25°C) and relative humidity (60%). Every 15 days, 50 mL of a complete Hoagland solution was added to each pot to avoid mineral deficiency.
Experiments and Treatment Descriptions
This study was performed with two complementary experiments, which are summarized in Figure 7. The first one compared the xyloglucan mobilization process in detached and attached cotyledons. The attached cotyledons were followed in intact seedlings (control), in the darkness, and with excision of the shoot above the cotyledon insertion (shoot excised). With these procedures, we intended to characterize the xyloglucan mobilization process in intact plants or in plants growing without light stimulus or shoot sink, as well as to check whether and when the isolated cotyledons are able to start/maintain the mobilization process. With these experiments, it has been possible to test whether cotyledons are able to respond to endogenous factors, including auxin.
The control plants were grown in pots as described above and without growth restrictions. For the dark treatment, the pots were placed into a black paper box under the same growth chamber conditions when the emergence of seedlings started, approximately 19 d. The shoot excision was performed at about 34 d, when the development of eophylls (the first green leaves developed by seedlings) started, by cutting the entire shoot above the cotyledons insertion (epicotyls and eophylls).
Detachment of cotyledons was performed at 7, 19, 26, 34, and 41 d after the beginning of imbibition, from plants grown as in the control treatment. The detachment dates were chosen on the basis of the results obtained by Tiné et al. (2000). These time points were chosen to isolate the cotyledons before, during, and after the start of xyloglucan mobilization. The detached cotyledons were surface-sterilized for 15 s in 70% (v/v) ethanol containing 1% (v/v) polyoxyethilene sorbitan monooleate (Tween 80) and placed in groups of 15 in sterile petri dishes (20 × 140 mm). The solution for incubation (15 mL per dish) was composed of freshly distilled water, 1 mm CaCl2, 300 mg L−1 ampicillin, plus 2,4-D at 10−4, 10−5, 10−6 m or water (as a control). All detached cotyledons (2,4-D treated and water control) were kept in the same growth chamber where seedlings were grown. This was done in order to maintain the same temperature regimes but in darkness to avoid light oxidation of auxin. The change of the incubation solutions and the surface sterilization were performed daily to avoid differences in hormonal concentration and microbial contamination.
The second experiment (Fig. 7) was performed only with attached cotyledons in seedlings following different treatments in order to distinguish between the effects of sink strength, endogenous IAA, and light on xyloglucan mobilization. The treatments were: intact seedlings (intact), shoot excised seedlings (excised), shoot excised seedlings with light-protected cotyledons (excised LPC), shoot apex excised seedlings (top shoot excised), and intact plants treated with NPA at 200 μm (NPA 200 μm). The intact seedlings were grown under the same conditions of light, temperature, and humidity as used for the control plants in the first experiment. Shoot excision was performed as in the first experiment. However, for seedlings that had their top shoot excised, the plants were grown without the apex of the last internode (the second), the remaining sink being the expansion of eophylls. In light-protected cotyledons treatment, the cotyledons were wrapped with aluminum foil before the start of xyloglucan mobilization (approximately 26 d). At the same time, the NPA treatment was performed by applying a ring of lanolin paste containing 200 μm NPA (concentration chosen according to Reed et al., 1998, and Sieburth, 1999). The paste was applied, after warming with hot water, on the epicotyl surface 1 cm above the cotyledon insertion, using the tip of a Pasteur pipette. The amount of paste applied was around 3 mg.
In all experiments, treatment samples of seedlings (Experiment I) and/or cotyledons (Experiments I and II) were collected once a week. After measurements of leaf area, all materials were stored at −80°C.
Dry Mass and Leaf Area of Seedlings
The seedlings of the first experiment were divided into leaves, stems (nodes and internodes), and roots. Leaf area was analyzed using a digital system (Skye Instruments, Llandrindod Wells, UK) and together with the other seedling parts dried at 70°C (72 h) and weighed to determine dry mass. The analyses were performed separately on five seedlings per sample.
Dry Mass of Cotyledons and Xyloglucan Determination
Ten cotyledons per sample (Experiment I) were divided into two groups, in which five were used for dry mass and carbohydrate determinations, and the other five were used for enzyme analyses. Samples of the first group were dried at 70°C (72 h), weighed (dry mass), powdered, and divided into three subsamples (100 mg each). These were subjected to xyloglucan extraction in 30 mL of water at 80°C for 8 h. After filtration through nylon cloth, centrifugation was performed (10,000g, 30 min, 5°C), followed by precipitation with three volumes of ethanol. The precipitate was stored overnight at 5°C, collected by centrifugation, freeze-dried, and weighed. The water-soluble polysaccharides from the cotyledons produced a freeze-dried fluffy material that comprised more than 95% xyloglucan (Buckeridge et al., 1992). The freeze-dried xyloglucan was weighed, and the content was calculated in relation to cotyledon dry mass.
Starch, Suc, and Monosaccharide Analyses
The same powdered cotyledons used for xyloglucan extraction were submitted to starch and soluble sugar measurements. The starch analyses were performed according to an enzymatic technique described by Arêas and Lajolo (1980). One hundred milligrams of each powdered cotyledon was weighed and submitted to four extractions with 0.5 mL of 80% (v/v) ethanol (80°C, 20 min). After each extraction, the insoluble material was pelleted by centrifugation (10,000g, 15 min), and the supernatants were pooled and saved for analysis of soluble sugars. The pellets were dried at room temperature, and 1 mL of amyloglucosidase (28 unit mL−1, in 100 mm of sodium acetate buffer, pH 4.8) was added, followed by incubation at 37°C for 3 h. The Glc released was measured by mixing 0.1 mL of sample with 1.5 mL of the complex GOD/POD/ABTS [Glc oxidase/peroxidase at 1.5 unit mL−1 and 2,2′-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) at 0.5 mg mL−1, all from Sigma (St. Louis) and diluted in 100 mm phosphate buffer, pH 7.0] and incubating at 37°C for 15 min. This reaction was read at 540 nm, and using a standard curve with Glc, the proportion of starch present in the cotyledons was calculated. Glc released was adjusted (−10%) to the mass of linked Glc that is present in starch.
To measure the soluble sugars (Suc, Glc, and Fru) the alcohol supernatants were dried, suspended in water (1 mL), filtered (Millipore 0.25; Bedford, MA), and analyzed by high performance anion-exchange chromatography on a CarboPak PA-1 column (Dionex, Sunnyvale, CA) using a gradient elution from 0 (water) to 200 mm NaOH in water (20 min). Sugars were detected by a pulsed amperometric detector (PAD; Dionex). Detector responses were compared with the standards of Glc, Fru, and Suc at 25, 50, 75, 100, 150, and 200 μm. The standard curve for each sugar was used to calculate carbohydrate contents in the cotyledons.
Determination of Enzyme Activities
Samples of cotyledons were weighed, cut into small pieces, and pooled to compose three subsamples (0.5 g each). These subsamples were homogenized for 15 s (Ultra-Turrax T25, IKA- Labortechnik, Staufen, Germany ) with 5 mL of sodium acetate buffer (500 mm, pH 5.0). The homogenized subsamples were kept at 5°C for 20 min, and after centrifugation (10,000g, 10 min) the supernatants were separated. Protein concentration was estimated according to Bradford (1976), and the activities of β-galactosidase, β-glucosidase, α-xylosidase (only in the second experiment), and XEH were performed. All enzyme activity measurements were adapted from Tiné et al. (2000).
The determination of β-galactosidase and β-glucosidase activities were performed by addition of 10 μL of protein extract (in 500 mm sodium acetate buffer, pH 5.0), 40 μL of distilled water, and 50 μL of 40 mm ρ-nitrophenil-β-galactopyranoside (ρNP-β-Gal) or 40 mm ρ-nitrophenyl-β-glucopyranoside (ρNP-β-Glc), respectively, per microplate well. Reactions were incubated at 40°C for 10 min and stopped by addition of 200 μL of 0.1 n Na2CO3, and the absorbance was read at 405 nm.
The α-xylosidase activity was measured by addition of 50 μL of protein extract (in 500 mm sodium acetate buffer, pH 5.0), 50 μL of xyloglucan oligosaccharides (XGO; 1%, w/v; a mixture of limit digest oligosaccharides produced by hydrolysis with Trichoderma cellulase for 24 h), and 50 μL of water per microplate well. The plates were incubated at 30°C (24 h). After incubation, 200 μL of fresh ρ-bromoaneline (0.5 g of ρ-bromoaneline diluted in 25 mL of acetic acid with 1 g of thiourea) was added, followed by incubation at 70°C for 10 min and 1 h at room temperature in the darkness. The plates were read at 520 nm. To avoid the interference of free endogenous pentoses, each analysis had a second plate that was not incubated in both 30°C (24 h) and 70°C (10 min), and the absorbance differences were subtracted. Xylose (5–30 μg) was used as a standard.
For determination of XEH, we adapted the method described by Sulová et al. (1995). The mixture of incubation was composed of 5 μL of XGOs (1%, w/v), 20 μL of protein extract (in 500 mm sodium acetate buffer, pH 5.0), and 20 μL of xyloglucan (0.1%, w/v) per well, and incubation was performed for 2 h at 30°C. Enzyme reaction was stopped by addition of 20 μL of 1 n HCl. This was followed by addition of 150 μL of distilled water, 50 μL of 20% (w/v) Na2SO4, and 40 μL of KI/I2:water (1:10, v/v), and after incubation for 10 min at room temperature, the mixture was read at 630 nm. As a control, we used the same extracts without incubation to avoid endogenous xyloglucan interference and the reagents were added, at the same volumes, but in the following order: HCl (to stop XTH enzyme activity), protein extract, XGO, xyloglucan, water, Na2SO4, and KI/I2. All plates (incubated and not) were read together, and the relative reduction (%) in absorbances of incubated samples in relation to nonincubated (control) were calculated. Since the reduction in A630 is proportional to reduction in the interaction between xyloglucan and iodine, the percentage of reduction in absorbance was considered as the XEH activity expressed as percentage of hydrolysis of xyloglucan per time (h) and per protein mass (mg) of one cotyledon. All enzymatic measurements were made in two replicates, and the specific activities were calculated in relation to the total protein present per cotyledon.
Measurements of Endogenous IAA
The levels of free endogenous IAA were determined by two distinct techniques. In the first experiment, an ELISA technique was employed in which the IAA was detected by a specific antibody (Peres et al., 1997). In the second experiment, a gas chromatography-mass spectrometer with a selected ion monitoring (gas chromatography-selected ion monitoring-mass spectrometry [GC-SIM-MS]) technique was employed to confirm ELISA data.
For IAA determination by ELISA, the same samples as for enzyme activity were used. These samples were from: (1) attached cotyledons in control (34, 41, and 48 d); (2) in darkness (34, 41, and 48 d); (3) in shoot excised seedlings (41, 54, 61, and 68 d); and (4) detached cotyledons (34 and 41 d). Each previously ground sample was divided into three aliquots comprising subsamples of 1 g that were individually powdered using liquid nitrogen and submitted to an alcohol extraction. This was performed by addition of 3 mL of methanol per subsample and stirring for 60 h in the darkness at 4°C. During this extraction step 100 μL of [3H]IAA (0.5 μCi mL−1) was added to each sample as an internal standard in order to determine the extraction and purification yield of IAA. The extracts were filtered in nitrocellulose (0.45-mm Millex-HV and 0.22-mm Millex-GS [Millipore]), followed by Sep-Pak C-18 column, previously preconditioned with 80% (v/v) methanol. The filtered sample was dried, suspended in 0.2 mL L−1 formic acid, pH 3.0, and submitted to IAA purification by HPLC on a C-18 column using a gradient elution with 0.2 mL L−1 formic acid, pH 3.0, and methanol (45 min). The radioactive fractions were pooled, dried, methylated with diazomethane, resuspended in water, and distributed onto a microplate of ELISA spectrophotometer preconditioned with the specific antibody to methylated-IAA, according to Peres et al. (1997). The IAA concentration was calculated by the use of a standard curve of methylated IAA and the relative optical density.
The GC-SIM-MS analyses of free endogenous IAA were performed according to Chen et al. (1988). Five fresh cotyledons (per date and treatment) were chopped into small pieces, weighed, and divided into three subsamples (0.5 g). Each subsample was ground (Ultra-Turrax T25) adding 4 mL of 65% (v/v) isopropanol with 0.2 m imidazole buffer, pH 7.0. As an internal standard, [13C6]IAA was added (0.5 μg subsample−1). After homogenization for 1 h at 5°C, the extracts were centrifuged at 5,000g for 5 min, and the supernatants were diluted six times with water to reduce the isopropanol concentration. The diluted extracts were applied to a preconditioned aminopropyl column (Sep-Pak NH2, washed sequentially with hexane, acetonitrile, water, 200 mm, pH 7.0 imidazole buffer, 2 mL each, and 10 mL of water) and sequentially washed with hexane, ethyl acetate, acetonitrile, and methanol (2 mL of each). The IAA was eluted from the aminopropyl column by addition of 3 mL of 2% (v/v) acetic acid in methanol. The eluted samples were neutralized with 20% (v/v) NH4OH (20 μL mL−1), freeze-dried, resuspended in 100 μL of methanol, and purified by HPLC using a C-18 column, which was eluted with a gradient elution of acetonitrile and 1% (v/v) acetic acid. The fractions corresponding to IAA were neutralized by 20% (v/v) NH4OH, dried, and resuspended in 100 μL of methanol. Samples were then methylated by addition of 1 mL of ethereal diazomethane (prepared according to Peres et al., 1997). The methylated samples were nitrogen dried, resuspended in ethyl acetate (30 μL), and analyzed by a gas chromatography (6890 series)/mass spectrometer (5973 series, Agilent Technologies, Palo Alto, CA) using a column of medium polarity (HP1701; Hewlett-Packard, Palo Alto, CA) in the scan ion modulation mode. The detection area of endogenous IAA (130) and internal standard (136) ions and the initial concentration of the internal standard were used to calculate the IAA concentration in the cotyledons as described by Cohen et al. (1986).
Acknowledgments
We thank the FAPESP for granting a doctoral fellowship to H.P.S. (98/02775–8) and CNPq for a research fellowship to M.S.B. We thank Mrs. A.M. Baroni, L.I.V. Amaral, and V. Tamaki for technical assistance in the auxin procedures. We also thank the colleagues Jocelyn Rose and Yvan Krapiel for the useful suggestions.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; BIOTA-FAPESP 98/05124–8).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040220.
References
- Alcântara PHN (2000) Isolamento e caracterização das enzimas xiloglucano endotransglicosilase e β-galactosidase do catabolismo do xiloglucano de reserva dos cotilédones de Hymenaea courbaril L. (Leguminosae-Caesalpinioideae). PhD thesis. Federal University of São Paulo (UNIFESP), São Paulo, Brazil
- Alcântara PHN, Dietrich SMC, Buckeridge MS (1999) Xyloglucan mobilisation and purification of a (XLLG/XLXG) specific β-galactosidase from cotyledons of Copaifera langsdorffii. Plant Physiol Biochem 37: 653–663 [Google Scholar]
- Arêas JAG, Lajolo FM (1980) Determinação enzimática específica de amido, glicose, frutose e sacarose em bananas pré-climatéricas e climatéricas. An Farm Quím S Paulo 20: 307–31810844740 [Google Scholar]
- Bartel B (1997) Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 51–66 [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seeds. Physiology of Development and Germination. Ed 2. Plenum Press, New York
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Dietrich SMC (1996) Mobilisation of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth. (Leguminosae-Faboideae). Plant Sci 117: 33–43 [Google Scholar]
- Buckeridge MS, Rocha DC, Reid JSG, Dietrich SMC (1992) Xyloglucan structure and post-germinative metabolism in seeds of Copaifera langsdorffii from savannah and forest populations. Physiol Plant 86: 145–151 [Google Scholar]
- Buckeridge MS, Santos HP, Tiné MAS (2000) Mobilisation of storage cell wall polysaccharides in seeds. Plant Physiol Biochem 38: 141–156 [Google Scholar]
- Catalá C, Rose JKC, Bennett AB (1997) Auxin regulation and spatial localization of an endo-1,4-β-D-glucanase and xyloglucan endotransglycosylase in expanding tomato hypocotyls. Plant J 12: 417–426 [DOI] [PubMed] [Google Scholar]
- Catalá C, Rose JKC, Bennett AB (2000) Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiol 122: 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Miller AN, Patterson GW, Cohen JD (1988) A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol 86: 822–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J (1993) Out of darkness: Mutants reveal pathways controlling light-regulate development in plants. Trends Genet 9: 167–172 [DOI] [PubMed] [Google Scholar]
- Cohen JD, Baldi BG, Slovin JP (1986) 13C6-[benzene ring]-indole-3-acetic acid: a new internal standard for quantitative mass spectral analysis of indole-3acetic-acid in plants. Plant Physiol 80: 14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38: 109–124 [DOI] [PubMed] [Google Scholar]
- Crombie HJ, Chengappa S, Hellyer A, Reid JSG (1998) A xyloglucan oligosaccharide-active, transglycosilating β-D-glucosidase from the cotyledons of nasturtium (Tropaeolum majus L.) seedlings-purification, properties and characterization of a cDNA clone. Plant J 15: 27–38 [DOI] [PubMed] [Google Scholar]
- Edwards M, Bowman JL, Dea ICM, Reid JSG (1988) A β-D-galactosidase from nasturtium (Tropaeolum majus L.) cotyledons. J Biol Chem 263: 4333–4337 [PubMed] [Google Scholar]
- Edwards M, Dea ICM, Bulpin PV, Reid JSG (1985) Xyloglucan (amyloid) mobilisation in the cotyledons of Tropaeolum majus L. seeds following germination. Planta 163: 133–140 [DOI] [PubMed] [Google Scholar]
- Edwards M, Dea ICM, Bulpin PV, Reid JSG (1986) Purification and properties of a novel xyloglucan-specific endo-β-(1,4)-D-glucanase from germinating nasturtium seeds (Tropaeolum majus L.). J Biol Chem 261: 9489–9494 [PubMed] [Google Scholar]
- Fanutti C, Gidley MJ, Reid JSG (1991) A xyloglucan oligosaccharide specific α-D-xylosidase or exo-oligoxyloglucan-α-xylohydrolase from germinated nasturtium (Tropaeolum majus L.) seeds. Purification, properties and its interaction with a xyloglucan-specific endo-β-(1,4)-glucanase and other hydrolases during storage xyloglucan mobilisation. Planta 184: 137–147 [DOI] [PubMed] [Google Scholar]
- Fanutti C, Gidley MJ, Reid JSG (1993) Action of a pure xyloglucan endo-transglycosilase (formerly called xyloglucan-specific endo-(1,4)-β-D-glucanase) from the cotyledons of germinated nasturtium seeds. Plant J 3: 691–700 [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Ito M, Nishida I, Watanabe A (2000) Multiple signalling pathways in gene expression during sugar starvation: pharmacological analysis of din gene expression in suspension-cultured cells of Arabidopsis. Plant Physiol 124: 1139–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger DR, Servaites JC, Fuchs MA (2000) Role of starch in carbon translocation and partitioning at the plant level. Aust J Plant Physiol 27: 571–582 [Google Scholar]
- Hayashi T (1989) Xyloglucan in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol 40: 139–168 [Google Scholar]
- Heinricher E (1888) Zur Biologie der gattung Impatiens. Flora Jena 71
- Hensel A, Brummell DA, Hanna R, MacLachlan G (1991) Auxin-dependent breakdown of xyloglucan in cotyledons of germinating nasturtium seeds. Planta 183: 321–326 [DOI] [PubMed] [Google Scholar]
- Hoson T (1993) Regulation of polysaccharide breakdown during auxin-induced cell wall loosening. J Plant Res 106: 369–381 [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-growth but not dark-grown Arabidopsis. Plant Physiol 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouve L, Gaspar T, Kevers C, Greppin H, Agosti RD (1999) Involvement of indole-3-acetic acid in the circadian growth of the first internode of Arabidopsis. Planta 209: 136–142 [DOI] [PubMed] [Google Scholar]
- Kooiman P (1960) On the occurrence of amyloids in plant seeds. Acta Bot Neerl 9: 208–219 [Google Scholar]
- Kotake T, Nakagawa N, Takeda K, Sakurai N (2000) Auxin-induced elongation growth and expressions of cell wall-bound exo- and endo-β-glucanases in barley coleoptiles. Plant Cell Physiol 41: 1272–1278 [DOI] [PubMed] [Google Scholar]
- Kraepiel Y, Agnès C, Thiery L, Maldiney R, Miginiac E, Delarue M (2001) The growth of tomato (Lycopersicon esculentum Mill.) hypocotyls in the light and in darkness differentially involves auxin. Plant Sci 161: 1067–1074 [DOI] [PubMed] [Google Scholar]
- Ljung K, Östin A, Lioussanne L, Sandberg G (2001) Developmental regulation of indole-3-acetic acid turnover in scots pine seedlings. Plant Physiol 125: 464–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig F, Sonnewald U, Kauder F, Heineke D, Geiger M, Stitt M, Muller-Rober BT, Gillissen B, Kuhn C, Frommer WB (1998) The role of transient starch in acclimation to elevated atmospheric CO2. FEBS Lett 429: 147–151 [DOI] [PubMed] [Google Scholar]
- McCleary BV (1983) Enzymic interactions in the hydrolysis of galactomannan: the role of exo-mannanase. Phytochemistry 22: 649–658 [Google Scholar]
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6: 535–542 [DOI] [PubMed] [Google Scholar]
- Peres LEP, Mercier H, Kerbauy GB, Zaffari GR (1997) Endogenous levels of IAA, cytokinins and ABA in shootless orchid and a rootless bromeliad determined by means of HPLC and ELISA. Braz J Plant Physiol 9: 169–176 [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JSG (1971) Reserve carbohydrate metabolism in germinating seeds of Trigonella foenun-graecum L. (Legum.). Planta 100: 131–142 [DOI] [PubMed] [Google Scholar]
- Reis D, Vian B, Darzens D, Roland JC (1987) Sequential patterns of intramural digestion of galactoxyloglucan in tamarind seedlings. Planta 170: 60–73 [DOI] [PubMed] [Google Scholar]
- Reiss R (1889) Über die Natur der Reservecellulose und über ihre Auflösungweise bei der Keimung der Samen. Landwirtsch Jahrb Schweiz 18: 711–765 [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14 (suppl.): S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletschek H, Weber H, Borisjuk LN (2003) Energy status and its control on embryogenesis of legumes. Embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiol 132: 1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JKC, Janet Braam Fry SC, Nishitani K (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43: 1421–1435 [DOI] [PubMed] [Google Scholar]
- Shinkle JR, Kadakia R, Jones AM (1998) Dim-red-light induced increase in polar auxin transport in cucumber seedlings. Plant Physiol 116: 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE (1999) Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol 121: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RP, Válio IFM (1999) Carbon translocation as affected by shade in saplings of shade tolerant and intolerant species. Biol Plant 42: 631–636 [Google Scholar]
- Sulová Z, Lednická M, Farkas V (1995) A colorimetric assay for xyloglucan endotransglycosylase from germinating seeds. Anal Biochem 229: 80–85 [DOI] [PubMed] [Google Scholar]
- Taguchi T, Uraguchi A, Katsumi M (1999) Auxin- and acid-induced changes in the mechanical properties of the cell wall. Plant Cell Physiol 40: 743–749 [Google Scholar]
- Tiné MAS, Cortelazzo AL, Buckeridge MS (2000) Xyloglucan mobilisation in cotyledons of developing plantlets of Hymenaea courbaril L. (Leguminosae-Caesalpinoideae). Plant Sci 154: 117–126 [DOI] [PubMed] [Google Scholar]
- Tiné MAS, Lima DU, Buckeridge MS (2003) Galactose branching modulates the action of cellulase on seed storage xyloglucans. Carbohydr Polym 52: 135–141 [Google Scholar]
- Valero P, Labrador E (1995) Effect of auxin on cell wall glycanhydrolytic enzymes in epicotyls of Cicer arietinum. Physiol Plant 93: 764–770 [Google Scholar]
- Yu S-M (1999) Cellular and genetic responses of plants of sugar starvation. Plant Physiol 121: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]