Abstract
The NOD-like receptors are cytoplasmic proteins that sense microbial by-products released by invasive bacteria. Although NOD1 and NOD2 are functionally expressed in cells from oral tissues and play a role triggering immune responses, the role of NOD2 receptor in the bone resorption and in the modulation of osteoclastogenesis is still unclear. We show that in an experimental model of periodontitis with Porphyromonas gingivalis W83, NOD2-/- mice showed lower bone resorption when compared to wild type. Quantitative polymerase chain reaction analysis revealed that wild-type infected mice showed an elevated RANKL/OPG ratio when compared to NOD2-/- infected mice. Moreover, the expression of 2 osteoclast activity markers—cathepsin K and matrix metalloproteinase 9—was significantly lower in gingival tissue from NOD2-/- infected mice compared to WT infected ones. The in vitro study reported an increase in the expression of the NOD2 receptor 24 hr after stimulation of hematopoietic bone marrow cells with M-CSF and RANKL. We also evaluated the effect of direct activation of NOD2 receptor on osteoclastogenesis, by the activation of this receptor in preosteoclasts culture, with different concentrations of muramyl dipeptide. The results show no difference in the number of TRAP-positive cells. Although it did not alter the osteoclasts differentiation, the activation of NOD2 receptor led to a significant increase of cathepsin K expression. We confirm that this enzyme was active, since the osteoclasts resorption capacity was enhanced by muramyl dipeptide stimulation, evaluated in osteoassay plate. These results show that the lack of NOD2 receptor impairs the bone resorption, suggesting that NOD2 receptor could contribute to the progression of bone resorption in experimental model of periodontitis. The stimulation of NOD2 by its agonist, muramyl dipeptide, did not affect osteoclastogenesis, but it does favor the bone resorption capacity identified by increased osteoclast activity.
Keywords: NOD receptors, cathepsin K, osteoimmunology, osteoclasts, periodontitis, oral infection
Introduction
Periodontal disease is considered a major public health problem and is the most prevalent chronic infection that affects bone structure (Baker, 2000). Periodontophatic organisms that colonize subgingival area trigger an inflammatory response that can lead to destruction of periodontal tissues of the host (Page et al., 1997; Kornman, 2008). Several studies have demonstrated that the host persistent immune inflammatory response against these pathogens, mediated by the release of several cytokines, favors the aggravation of periodontitis (Liu et al., 2010).
Periodontal disease is induced by a limited number of bacterial species, mainly Gram-negative anaerobes (specific infection factor), relatively restricted to the subgingival plaque, which is able to invade cells of periodontal tissue, such as epithelial cells and fibroblasts (Socransky et al., 1998; Loesche and Grossman, 2001). The family of pattern recognition receptors present in the cytoplasm called nucleotide binding and oligomerization domain receptors—in short, NOD-like receptors—is a second line of defense against pathogenic bacteria that escape from the extracellular receptors’ detection (Inohara et al., 2005).
The NOD2 receptor was one of the first intracellular sensors belonging to the family of NOD-like receptors to be discovered (Inohara et al., 1999). It is involved in the recognition of portions of muramyl dipeptide (MDP; Girardin et al., 2003; Grimes et al., 2012) constituent of the cell wall of Gram-positive and Gram-negative bacteria (Strober et al., 2006).
The expression of NOD1 and NOD2 receptors has been evidenced in various cell types of the oral cavity, including periodontal ligament cells (Jeon et al., 2012), pulp fibroblasts (Hirao et al., 2009), gingival fibroblasts (Hosokawa et al., 2010), fibroblasts of periodontal ligament (Tang et al., 2011), and cells of gingival epithelium (Chung et al., 2010). Moreover, it is known that osteoblasts also express NOD1 and NOD2 and are able to recognize and respond to bacterial stimuli by the activation of these receptors (Marriott et al., 2005).
The knowledge concerning the role of NOD-like receptors activation by Porphyromonas gingivalis on osteoclastogenesis is still limited. It is known that incubation of osteoblasts with LPS, IL-1α, or TNF-α leads to the expression of RANKL, which is exacerbated after second stimulation with Salmonella or MDP, a ligand of NOD2. This data suggest that Toll-like receptors and NOD-like receptors can synergistically act, increasing the expression of RANKL in osteoblasts (Marriott et al., 2005), which in turn can activate osteoclastogenesis. However, it has been shown that the activation of NOD2, by bacterial stimulation, displays an alternative role, leading to a suppressed production of inflammatory mediators, but this function is not fully determined (Chauhan and Marriott, 2010). Lee et al. (2014) observed that human dental pulp cells stimulated by MDP increase the expression of cytokines (TNF-α, IL-1β, IL-6, IL-11, IL-17) that positively regulate osteoclasts differentiation. They also observed that MDP promoted increased RANKL and M-CSF and reduced OPG expression by dental pulp cells.
Gingival fibroblasts are the first cell line that come into contact with periodontal pathogens and have NOD2 receptor expression increased after stimulation with P. gingivalis W83 (Liu et al., 2014). Others studies have shown that supragingival and subgingival biofilms differently regulate the gene expressions of NLRP3 and AIM2 inflammasomes in human gingival fibroblasts (Bostanci et al., 2011), and the downregulation of NLRP3 and IL-1β expression is partly induced by P. gingivalis present in subgingival biofilms (Belibasakis et al., 2013). Although NOD1 and NOD2 are involved in the recognition of periodontal pathogens and in the immune responses modulation (Okugawa, 2010), the effect of its activation in osteoclast is still unknown. To investigate the role of the NOD2 receptor in osteoclastogenesis and bone resorption, we used an experimental periodontitis model with NOD2-deficient mice. Moreover, in vitro studies were performed to investigate the consequence of NOD2 direct activation on osteoclast differentiation and activation.
Materials & Methods
Experimental Animals
Five to 6-wk-old male NOD2 knockout (Kobayashi et al., 2005), NOD2-/-, background C57BL/6, and wild-type (WT) C57BL/6 mice were used in this study. The experimental protocol used in this study was approved by the Committee on Ethics in Animal Experimentation, Campus of Ribeirão Preto, University of São Paulo.
Infection with P. gingivalis
The periodontal disease model was performed according to the protocol previously described by Sasaki et al. (2004) with some modifications. Animals were orally inoculated with 100 µL of 109 colony forming units (CFU) of P. gingivalis diluted in carboxy-methylcellulose and phosphate buffered saline, 5 times from day 0 at 2-d intervals. Negative control animals received the same volume of carboxy-methylcellulose and phosphate buffered saline. Details are described in the appendix.
Quantification of Alveolar Bone Loss
To evaluate the extent of alveolar bone loss, the maxillae were hemisected, exposed overnight to 3% hydrogen peroxide, and mechanically defleshed. To distinguish the cement-enamel junction, mouse maxilla was stained with 0.3% methylene blue (Kawai et al., 2007). The palatal faces of the molars were photographed with 2.5 × magnification using a stereomicroscope (ZEISS CL1500 ECO) and a digital camera (Canon E0S 1000D). The images were analyzed by IMAGE J software (National Institutes of Health, Bethesda, MD). Quantitative analysis was used to measure the distomesial area (mm2) between the cement-enamel junction and the alveolar bone crest on the palatal side of the first molar.
Osteoclast Generation and Pit Resorption Assay
Bone marrow cells were flushed from tibias and femurs of WT and NOD2-/- mice with α-MEM (GIBCO, Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 100 units/mL of penicillin, and 100 mg/mL of streptomycin. Cells were plated in 10 mL of α-MEM with 30 ng/mL of M-CSF (R&D Systems) and cultured for 3 d. The adherent cells (osteoclast precursors) were plated in 96-well microtiter plates or osteoassay surface plate (Corning Life Science) at 2 × 104 cells/well and cultured in presence of M-CSF (30 ng/mL), RANKL (5 ng/mL; R&D Systems), and MDP (0.01-10 µg/mL; Invivogen), or P. gingivalis (multiplicity of infection at 0.1, 1, 10). Formation of mature multinucleated osteoclasts (more than 3 nuclei) was confirmed by TRAP staining or resorption pit area measured. For quantitative polymerase chain reaction, osteoclast precursors were plated in 24-well microtiter plates at 2 × 105 cells/well and incubated with M-CSF, RANKL, and MDP (0.1-10 µg/mL).
Internalization of P. gingivalis by Preosteoclasts
To check preosteoclast ability to internalize P. gingivalis W83, we used 2 methodologies: flow cytometry analysis and fluorescence microscopy. Details are described in the appendix.
RNA Extraction and Quantitative Polymerase Chain Reaction Reactions
Total RNA was extracted from gingival tissues or osteoclasts cells using the Promega SV Total Isolation System. The complementary DNA was synthesized by means of a reverse transcription reaction (Pre-Improm II; Promega, Madison, WI, USA). Real-time polymerase chain reaction was carried out on StepOnePlus sequence detection system (Life Technologies) using TaqMan. Details are described in the appendix.
Statistical Analyses
The data are expressed as mean ± SEM of 2 or 3 independent experiments. All the statistical analyses were performed by 1-way analysis of variance or t test when appropriate using the GraphPad Prism 5.01 (GraphPad Software, San Diego, CA, USA). The level of significance was set at 5%.
Results
Participation of NOD2 Receptor in Alveolar Bone Loss Induced by P. gingivalis
To examine whether deficiency of NOD2 receptor would interfere in the oral microflora and bone resorption, we induced the periodontal disease model by P. gingivalis W83 inoculation into oral cavity in WT and NOD2-/- mice. The presence of bacteria was confirmed in the oral microflora, and the average CFU determined in infected NOD2-/- mice was significantly higher—an average of 20.7 × 103 CFU/mL (n = 7)—compared 7.0 × 103 CFU/mL (n = 6) for WT.
WT infected mice presented greater extent of bone resorption (1.14 ± 0.28 mm2) compared to control (0.90 ± 0.11 mm2) evaluated by macromorphometric analysis (p < .05) (Figure 1A, C, D). No difference was observed between the bone resorption area in infected animals (1.20 ± 0.17 mm2) and the extent of physiologic bone loss (0.99 ± 0.11 mm2) in NOD2-/- mice (Figure 1A, E, F). Although it was not significantly different, the physiologic bone loss in NOD2-/- mice was higher under no P. gingivalis inoculation when compared to WT. Thus, the alveolar bone loss quantification was also performed by considering the difference between the area measured in infected and noninfected mice of each strain (Figure 1B). NOD2-/- mice exhibited 87% of WT bone loss, which means that NOD2-/- mice blocked 13% of alveolar bone loss compared to WT mice after P. gingivalis infection (Figure 1B).
Figure 1.
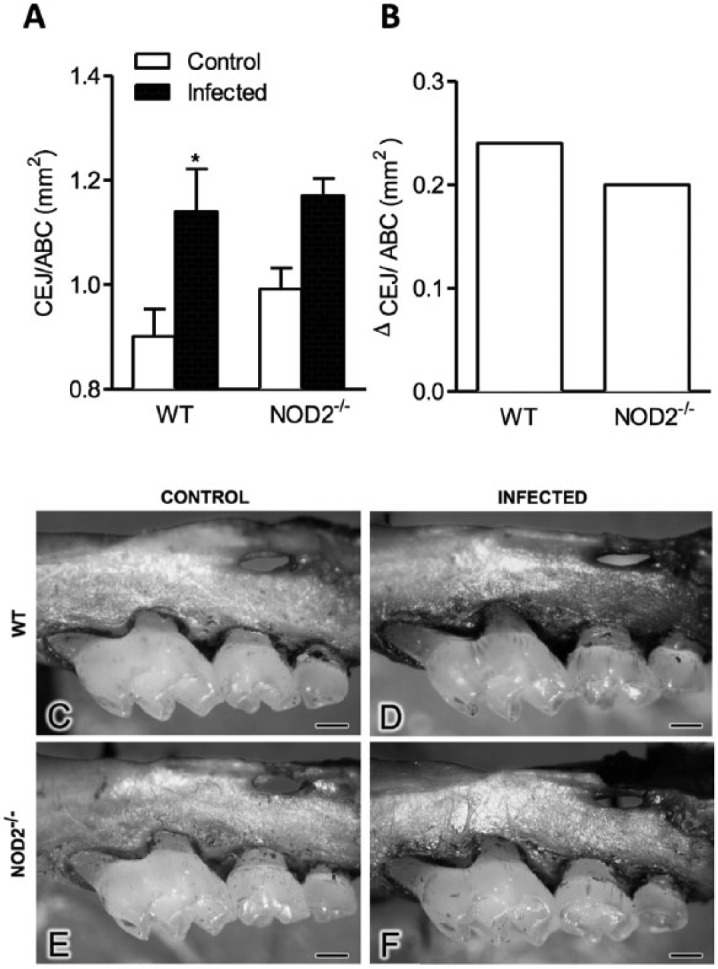
Evaluation of alveolar bone loss induced by Porphyromonas gingivalis W83 in wild-type (WT) and NOD2-/- mice. (A) Quantitative analysis of bone resorption area (mm2), (B) relative analysis of bone resorption by considering the difference between the areas in infected mice minus control mice (mm2) measured 4 wk after the last infection in WT and NOD2-/- mice. Bone loss evaluated in control (C, E) and infected (D, F) of WT and NOD2-/- mice. Bars = 1 mm. * p < .05, compared to respective control. One-way analysis of variance, followed by Bonferroni posttest. This experiment was repeated 2 times, and bars represent mean ± SEM (n = 6-8).
Osteoclastogenic Markers in Animals Infected by P. gingivalis
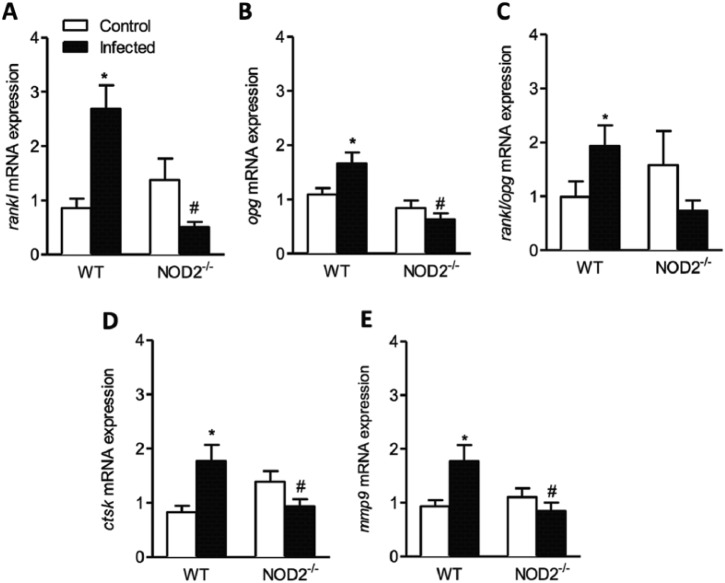
The RANKL/OPG system was identified as an important molecular component of the bone-remodeling process. We next evaluated the expression of factors related with osteoclastogenesis and osteoclast activity on gingival palatal and buccal tissue from WT and NOD2-/- infected animals. The gene expression of RANKL and OPG showed to be greater in WT infected animals when compared to their controls (p < .05) and lower in NOD2-/- infected samples when compared to WT infected ones (p < .05) (Figure 2A, B).
Figure 2.
Expression of mRNA for osteoclastogenic markers RANKL, OPG, RANK/OPG, Ctsk, and MMP-9. Expression of RANKL (A), OPG (B), RANKL/OPG ratio (C), CTSK (D), and MMP-9 (E) measured quantitatively by quantitative polymerase chain reaction in gingival tissue from wild-type (WT) and NOD2-/- mice infected by Porphyromonas gingivalis. * p < .05, compared to respective control. # p < .05, compared to WT infected. One-way analysis of variance, followed by Bonferroni posttest. This experiment was repeated 3 times, and bars represent mean ± SEM (n = 10-17).
Based on the expression of genes that encodes RANKL and OPG, the ratio of RANKL/OPG expression was evaluated. The RANKL/OPG ratio was higher in WT infected animals when compared to its control (p < .05) (Figure 2C).
The gene expression of 2 enzymes—cathepsin K and matrix metalloproteinase 9 (MMP-9), responsible for degradation of bone matrix mediated by osteoclasts—was evaluated. The infection of WT animals with P. gingivalis evoked higher gene expression of CTSK (Figure 2D) and MMP-9 (Figure 2E) when compared to their controls (p < .05) and to NOD2-/- infected mice (p < .05). The infection of NOD2-/- animals did not change Ctsk and MMP-9 mRNA expression level compared to their control.
MDP Enhance Osteoclasts Activity via NOD2 Activation
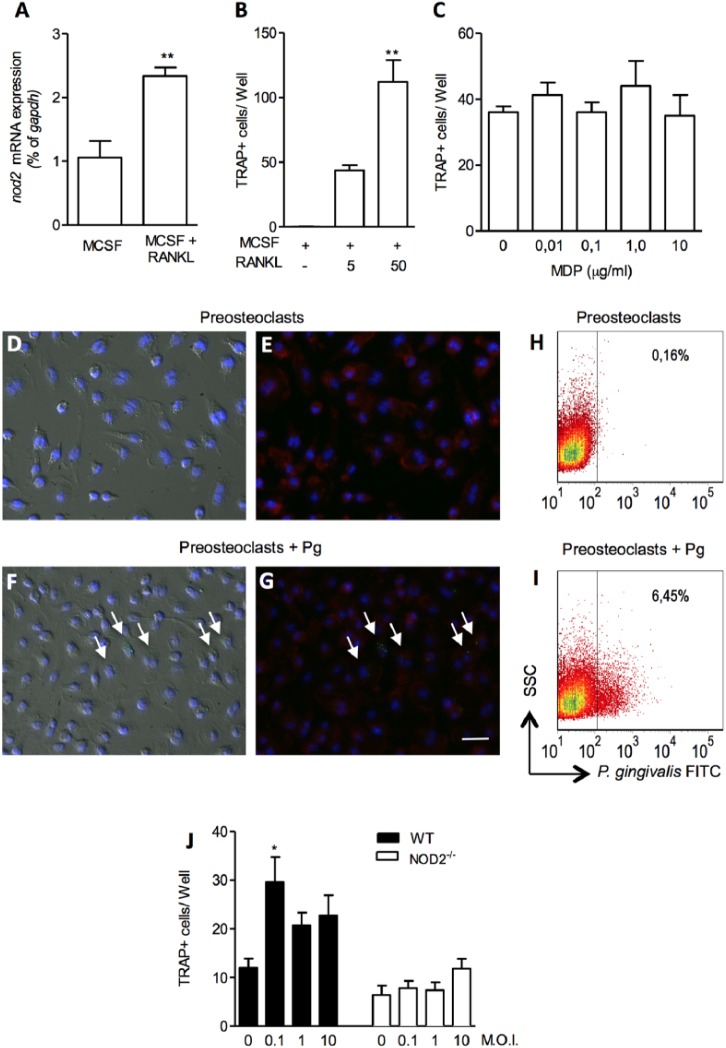
Next, we assessed in vitro if NOD2 would be directly involved in osteoclastogenesis and osteoclasts activation by stimulating osteoclasts precursor cells with MDP, a specific NOD2 ligand. An increased expression of NOD2 receptor was observed in bone marrow osteoclasts precursors cells 24 hr after stimulation with M-CSF and RANKL (2.9-fold increase; p < .01) (Figure 3A). To find a suboptimal concentration of RANKL that induces osteoclasts differentiation, we stimulated preosteoclast with 5 and 50 ng/mL of RANKL. The osetoclasts numbers were significantly higher when stimulated with 50 compared to 5 ng/mL of RANKL (p < .01) (Figure 3B).
Figure 3.
NOD2 expression and effect of its activation in osteoclasts differentiation. mRNA of NOD2 in osteoclast precursors stimulated with M-CSF (30 ng/mL) or M-CSF + RANKL (50 ng/mL) for 24 hr. ** p < .01, t test compared to M-CSF (A). Number of TRAP+ cells counted 96 hr after M-CSF + RANKL (5 or 50ng/mL). ** p < .01, t test compared to M-CSF (B). Number of TRAP+ cells counted 96 hr after M-CSF (30 ng/mL) + RANKL (5 ng/mL) and MDP (0.01-10 µg/mL) stimulation (C). Representative images of noninfected (D, E) and infected (F, G) preosteoclasts. The images were obtained by differential interference contrast microscopy (D, F) and fluorescence microscopy (E, G). Arrows indicate bacteria (green) inside the cells. Bars = 50 µm. Bacteria stained inside preosteoclasts by flow cytometry (H, I). Number of TRAP+ cells counted 96 hr after M-CSF + RANKL (5 ng/mL) and Porphyromonas gingivalis (multiplicity of infection [MOI] at 0.1, 1, 10) induced in wild-type (WT) and NOD2-/- cells (J). * p < .05, compared to noninfected cells.
To determine the effect of NOD2 activation on osteoclast formation, bone marrow preosteoclasts were stimulated with 5 ng/mL of RANKL and increasing concentrations of MDP (0.01-10 µg/mL). Figure 3C shows that the number of TRAP-positive multinucleated cells did not change with different concentration of MDP.
We next checked if P. gingivalis would have the same effect in osteoclastogenesis as the ligand MDP. First, we observed that P. gingivalis could enter preosteoclasts cells (Figure 3D-I). We performed a coculture of preosteoclasts with CFSE-stained P. gingivalis and could detect bacteria inside the cells by fluorescence microscopy. Representative images of noninfected (Figure 3D, E) and infected (Figure 3F, G) preosteoclasts show that P. gingivalis (green) is inside the cells. The images were obtained by differential interference contrast (Figure 3D, F) and fluorescence microscopy (Figure 3E, G). Arrows indicate cells with bacteria. We could detect by flow cytometry analysis that 6.45% of preosteoclasts had internalized bacteria and were positive for FITC within 1 hr of incubation (Figure 3I).
Since P. gingivalis can enter preosteoclasts cells and activate citoplasmatic NOD2 receptor, we cultivated preosteoclasts with P. gingivalis (multiplicity of infection at 0.1, 1, 10) and checked TRAP+ cells number after 96 hr. The osteoclasts numbers were significantly higher when stimulated with P. gingivalis (multiplicity of infection at 0.1) (Figure 3J). The infection of NOD2-/- preosteoclasts with P. gingivalis did not change the osteoclasts number (Figure 3J).
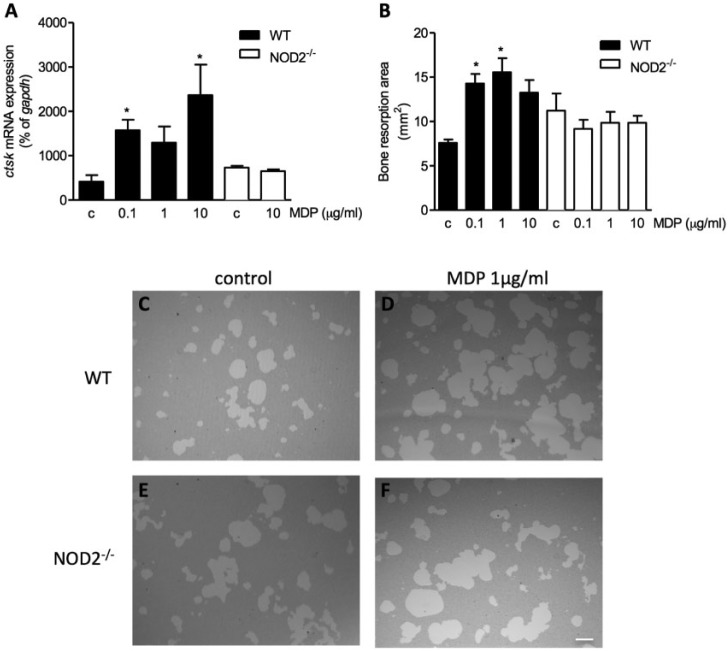
Although osteoclasts number was not altered by MDP, we next addressed if osteoclast activity could be altered by NOD2 receptor activation. We evaluated the expression of cathepsin K in osteoclasts incubated with MDP. Cathepsin K mRNA expression was significantly increased (dose-dependent manner) in preosteoclasts stimulated with increasing concentration of MDP (0.1-10 µg/mL) (Figure 4A). However, the level of cathepsin K did not alter with MDP stimulation in NOD2-/- mice–derived preosteoclasts, confirming that the increased cathepsin K expression by MDP is NOD2 activation dependent (Figure 4A).
Figure 4.
Ctsk expression and bone resorption activity after NOD2 receptor activation. mRNA of Ctsk in osteoclast precursors stimulated with M-CSF or M-CSF + RANKL for 96 hr in wild-type (WT) and NOD2-/- mice (A). * p < .05 compared to control (c). One-way analysis of variance, followed by Bonferroni posttest. This experiment was repeated 3 times, and bars represent mean ± SEM (n = 3-6). Effect of NOD2 ligand in osteoclast bone resorption capacity. Dose-response effect of MDP (0.1-10 µg/mL) on bone resorption area evaluated in osteoclast precursors cells from WT and NOD2-/- mice (B), cultivated with M-CSF or M-CSF + RANKL. * p < .05, compared to control (c). One-way analysis of variance, followed by Bonferroni posttest. Bars represent mean ± SEM (n = 6). Representative images (C-F) of bone resorption pit formation in osteoassay plate evaluated in osteoclasts precursors cells from WT and NOD2-/- mice cultivated with M-CSF + RANKL (control) and MDP 1 µg/mL. Bars = 100 µm
To test whether increasing cathepsin K expression by NOD2 receptor activation would increase osteoclasts function, we grew osteoclasts from WT and NOD2-/- mice in an osteoassay surface plate to measure the resorption activity. Osteoclasts are capable of bone resorption, not macrophages. Osteoclasts stimulated by MDP showed dose-dependent increase in resorption capacity as identified by the bigger resorption area (Figure 4B, C, D). MDP increased activity could not be seen in NOD2-/- mice–derived osteoclasts (Figure 4E, F), where bone resorption area was not altered by MDP (Figure 4B).
Discussion
Periodontal disease is a chronic inflammatory disease resulting from disruption of homeostasis between the subgingival periodontophatic organisms and host defense, which leads to periodontal bone resorption, a consequence of damage of supportive tissue surrounding the teeth. P. gingivalis causes an increase in ICAM-1, NOD1, and NOD2 expression in periodontal fibroblasts, suggesting that this receptor is important for periodontal disease development (Liu et al., 2014). It has been reported that gingival fibroblasts express NOD2 receptor and that MDP, the monomer of the peptidoglycan subunit, activates these cells to produce various inflammatory cytokines, such as IL-6, IL-8, and CCL2. The production of those cytokines is regulated by the signal transduction pathways MAPKs, PI3K, and NF-κB (Hosakawa et al., 2010; Okugawa et al., 2010). Another study shows that NOD2 receptor activation by MDP promoted production of proinflammatory cytokines, such as IL-6 and IL-8, in periodontal cells promoting an inflammatory response (Jeon et al., 2012).
In the present study, although the phenotype is mild, P. gingivalis induced significant alveolar bone resorption within 4 wk after infection in WT mice. On the other side, P. gingivalis did not induce significant bone resorption in NOD2-/- mice. This is observed essentially because of the elevated physiologic bone loss in NOD2-/- mice without infection. When we compared the bone loss of each strain, we observed a small difference between the bone resorption level of WT and NOD2-/- mice; however, NOD2-/- mice still exhibited approximately 87% of WT bone loss, which means that NOD2-/- mice blocked 13% of alveolar bone loss induced by P. gingivalis infection.
We observed that NOD2 receptor is crucial for oral bacteria detection and control, since NOD2-/- mice exhibited higher bacterial load compared to WT mice 4 wk after bacterial inoculation. Along these lines, the absence of this receptor could collapse the control of the indigenous oral flora and accelerate physiologic bone loss.
Contradictory, we have shown here that NOD2-/- mice present a lower alveolar bone loss induced by P. gingivalis when compared to WT mice. Although it needs deeper investigation, the deficience of NOD2 in P. gingivalis–infected mice could have avoided local inflammatory response, which in turn could be responsible for a lower level of cytokine to activate osteoclast differentiation and evoke a smaller bone loss.
Recent advances in osteoimmunology have identified the crucial role of RANKL and OPG in the pathogenesis of periodontal disease. The osteolytic activity is determined by the balance between RANKL and OPG expression. In fact, Takahashi et al. (2013) found an increased ratio of RANKL/OPG expression after induction of experimental periodontitis by ligation. Gingival fibroblasts also express RANKL after challenge with periodontopathogens such as Actinobacillus actinomycetemcomitans (Belibasakis et al., 2005) and P. gingivalis (Belibasakis et al., 2007). In the present study, we determined RANKL expression in samples of palatal gingival tissue of control and infected mice with P. gingivalis. Similar to Cantley et al. (2011), we observed a positive correlation between bone loss and the expression of RANKL in gingival tissue. We found that the NOD2-/- infected mice showed less alveolar bone loss and significantly lower expression of RANKL/OPG ratio when compared to WT infected animals. The present results agree with those of Garlet et al. (2006), who showed that the speed of alveolar bone loss and the progression of experimental disease are determined by a balanced expression of these factors (RANKL and OPG).
So far, we have shown that the absence of NOD2 receptor protected against bacterial-induced alveolar bone resorption in vivo. In the in vitro study, we found that NOD2 expression is increased in osteoclast precursor cells 24 hr after osteoclastogenesis stimulus with M-CSF and RANKL. This receptor may be activated by P. gingivalis since this bacteria can be internalized by preosteoclasts. Our results indicate, by 2 methodologies, that preosteoclasts could internalize P. gingivalis W83 within 1 hr. Flow cytometry analysis showed that 6.45% of preosteoclasts internalize P. gingivalis. This result was also confirmed by fluorescence microscopy.
Thus, to further address the role of NOD2 receptor in osteoclastogenesis, we performed in vitro experiments in the presence of P. gingivalis or MDP. Although the expression of RANKL/OPG is reduced in NOD2-/- mice–infected tissue, we have shown that the number of TRAP-positive cells was not altered by different concentrations of MDP in vitro. This can be justified because in osteoclasts culture, the concentration of RANKL is not variable by NOD2 activation, as in gingival tissue. However, the TRAP+ cell numbers were significantly higher when stimulated with P. gingivalis (multiplicity of infection at 0.1). This apparent discrepant result may be explained, since P. gingivalis can activate other receptors and stimulate osteoclastogenesis by coordinating the effects of TLR2, NOD1, NOD2, and TLR4 signaling (Kishimoto et al., 2012).
We could observe that the activation of NOD2 receptor is able to directly induce the increase of the expression of cathepsin K, the major bone-degrading enzyme. In this report, we have shown that not only does NOD2 activation stimulate this resorptive enzyme transcript but that NOD2 deficiency in mice also leads to a reduced expression of cathepsin K and MMP-9 in periodontal inflamed tissue in vivo. We further investigated whether increased expression of cathepsin K in osteoclasts stimulated by MDP, would reflect in enhanced resorption activity. Indeed, we have elucidated that osteoclast activity, on osteoassay plate, is increased by MDP in a dose-dependent manner. The resorption measurement in osteoassay plate gives functional support for the increased expression of transcripts for cathespin K and MMP-9.
Hie et al. (2007) found that high expression of cathepsin K resulted in an increased bone resorption. In fact, in vitro and in vivo studies suggest that the 2 types of proteases, MMP-9 and cathepsin K, are involved in bone resorption mediated by osteoclasts, performing the collagen degradation in bone (Delaisse et al., 2003; Everts et al., 1992). Votta et al. (1997) found reduced bone resorption, both in vitro and in vivo, using an inhibitor of cathepsin K. Moreover, cathepsin K–deficient mice showed significant osteopetrosis (Saftig et al., 1998; Gowen et al., 1999). In periodontal area, increased levels of cathepsin K (Mogi & Otogo, 2007; Garg et al., 2009) and matrix metalloproteinases (Leppilahti et al., 2014) were found in the gingival crevicular fluid of patients with periodontitis when compared to healthy patients, suggesting that the inhibition of cathepsin K may be a viable treatment for chronic diseases, such as periodontal disease (Stroup et al., 2001; Garg et al., 2009).
In summary, we have confirmed that NOD2 receptor contributes to the progression of alveolar bone resorption in an experimental model of periodontitis by increasing osteoclast resorptive enzyme. Furthermore, we confirmed by in vitro experiment that NOD2 receptor activation resulted in improved osteoclast activity without affecting the osteoclast differentiation process. Considering this, we suggest that this intracellular receptor is a potential therapeutic target to control and prevent bone resorption in periodontal disease.
Supplementary Material
Acknowledgments
We thank Dr. Richard Flavell for providing us with the NOD2-deficient mice, to Dr. Julio Scharfstein for having granted the strain of Porphyromonas gingivalis W83, and to Marina Del Arco for technical support during the microbiological procedures. We thank Dr. Daniele C. B. Nascimento for supporting us with the flow cytometry analysis.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by grants from Capes, NAP-DIN (11.1.21625.01.0), FAPESP: Projeto Regular (2011/20736-6) and Center for Research in Inflammatory Disease (2013/08216-2).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Baker PJ. (2000). The role of immune responses in bone loss during periodontal disease. Microbes Infect 2:1181-1192. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Johansson A, Wang Y, Chen C, Kalfas S, Lerner UH. (2005). The cytolethal distending toxin induces receptor activator of NF-kappaB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect Immun 73:342-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N, Hashim A, Johansson A, Aduse-Opoku J, Curtis MA, et al. (2007). Regulation of RANKL and OPG gene expression in human gingival fibroblasts and periodontal ligament cells by Porphyromonas gingivalis: a putative role of the Arg-gingipain. Microb Pathog 43:46-53. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Guggenheim B, Bostanci N. (2013). Down-regulation of NLRP3 inflammasome in gingival fibroblasts by subgingival biofilms: involvement of Porphyromonas gingivalis . Innate Immun 19:3-9. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Meier A, Guggenheim B, Belibasakis GN. (2011). Regulation of NLRP3 and AIM2 inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell Immunol 270:88-93. [DOI] [PubMed] [Google Scholar]
- Cantley MD, Haynes DR, Marino V, Bartold PM. (2011). Pre-existing periodontitis exacerbates experimental arthritis in a mouse model. J Clin Periodontol 38:532-541. [DOI] [PubMed] [Google Scholar]
- Chauhan VS, Marriott I. (2010). Differential roles for NOD2 in osteoblast inflammatory immune responses to bacterial pathogens of bone tissue. J Med Microbiol 59(Pt 7):755-62. [DOI] [PubMed] [Google Scholar]
- Chung WO, An JY, Yin L, Hacker BM, Rohani MG, Dommisch H, et al. (2010). Interplay of protease-activated receptors and NOD pattern recognition receptors in epithelial innate immune responses to bacteria. Immunol Lett 131:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaisse JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. (2003). Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech 61:504-513. [DOI] [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Niehof A, Vaes G, Beertsen W. (1992). Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol 150:221-231. [DOI] [PubMed] [Google Scholar]
- Garg G, Pradeep AR, Thorat MK. (2009). Effect of nonsurgical periodontal therapy on crevicular fluid levels of Cathepsin K in periodontitis. Arch Oral Biol 54:1046-1051. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. (2006). Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol 21:12-20. [DOI] [PubMed] [Google Scholar]
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. (2003). Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- Gowen M, Lazner F, Dodds R, Kapadia R, Feild J, Tavaria M, et al. (1999). Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 14:1654-1663. [DOI] [PubMed] [Google Scholar]
- Grimes CL, Ariyananda Lde Z, Melnyk JE, O’Shea EK. (2012). The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc 134:13535-13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hie M, Shimono M, Fujii K, Tsukamoto I. (2007). Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone 41:1045-1050. [DOI] [PubMed] [Google Scholar]
- Hirao K, Yumoto H, Takahashi K, Mukai K, Nakanishi T, Matsuo T. (2009). Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J Dent Res 88:762-767. [DOI] [PubMed] [Google Scholar]
- Hosokawa I, Hosokawa Y, Ozaki K, Yumoto H, Nakae H, Matsuo T. (2010). Proinflammatory effects of muramyldipeptide on human gingival fibroblasts. J Periodontal Res 45:193-199. [DOI] [PubMed] [Google Scholar]
- Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, et al. (1999). Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- Inohara N, Chamaillard M, McDonald C, Nunez G. (2005). NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355-383. [DOI] [PubMed] [Google Scholar]
- Jeon DI, Park SR, Ahn MY, Ahn SG, Park JH, Yoon JH. (2012). NOD1 and NOD2 stimulation triggers innate immune responses of human periodontal ligament cells. Int J Mol Med 29:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Paster BJ, Komatsuzawa H, Ernst CW, Gonçalves RB, Sasaki H, et al. (2007). Cross-reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor-kappaB ligand (RANKL)-dependent periodontal bone resorption in a mouse model. Oral Microbiol Immunol 22:208-215. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Kaneko T, Ukai T, Yokoyama M, Ayon Haro R, Yoshinaga Y, et al. (2012). Peptidoglycan and lipopolysaccharide synergistically enhance bone resorption and osteoclastogenesis. J Periodontal Res 47:446-454. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, et al. (2005). Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Sicence 307:731-734. [DOI] [PubMed] [Google Scholar]
- Kornman KS. (2008). Mapping the pathogenesis of periodontitis: a new look. J Periodontol 79(8):1560S-1568S. [DOI] [PubMed] [Google Scholar]
- Lee SI, Kim GT, Kim HJ, Park SH, Kim EC. (2014). NOD2 mediates odontoblast differentiation and RANKL expression. J Dent Res 93:678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppilahti JM, Hernandez-Rios PA, Gamonal JA, Tervahartiala T, Brignardello-Petersen R, Mantyla P, et al. (2014). Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J Clin Periodontol 41:348-356. [DOI] [PubMed] [Google Scholar]
- Liu J, Duan J, Wang Y, Ouyang X. (2014). Intracellular adhesion molecule-1 is regulated by porphyromonas gingivalis through nucleotide binding oligomerization domain-containing proteins 1 and 2 molecules in periodontal fibroblasts. J Periodontol 85:358-368. [DOI] [PubMed] [Google Scholar]
- Liu YC, Lerner UH, Teng YT. (2010). Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000. 52:163-206. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Grossman NS. (2001). Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev 14:727-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott I, Rati DM, McCall SH, Tranguch SL. (2005). Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun 73:2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Otogoto J. (2007). Expression of cathepsin-K in gingival crevicular fluid of patients with periodontitis. Arch Oral Biol 52:894-898. [DOI] [PubMed] [Google Scholar]
- Okugawa T, Kaneko T, Yoshimura A, Silverman N, Hara Y. (2010). NOD1 and NOD2 mediate sensing of periodontal pathogens. J Dental Res 89:186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. (1997). Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 14: 216-248. [DOI] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, et al. (1998). Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A 95:13453-13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. (2004). The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis–induced alveolar bone loss. J Periodontal Res 39:432-441. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr (1998). Microbial complexes in subgingival plaque. J Clin Periodontol 25:134-144. [DOI] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A, Watanabe T. (2006). Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 6:9-20. [DOI] [PubMed] [Google Scholar]
- Stroup GB, Lark MW, Veber DF, Bhattacharyya A, Blake S, Dare LC, et al. (2001). Potent and selective inhibition of human cathepsin K leads to inhibition of bone resorption in vivo in a nonhuman primate. J Bone Miner Res 16:1739-1746. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Fukuda M, Mitani A, Fujimura T, Iwamura Y, Sato S, et al. (2013). Follicular dendritic cell-secreted protein is decreased in experimental periodontitis concurrently with the increase of interleukin-17 expression and the Rankl/Opg mRNA ratio. J Periodontal Res 49:390-397. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhou XD, Wang Q, Zhang L, Wang Y, Li XY, et al. (2011). Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch Oral Biol 56:1064-1072. [DOI] [PubMed] [Google Scholar]
- Votta BJ, Levy MA, Badger A, Bradbeer J, Dodds RA, James IE, et al. (1997). Peptide aldehyde inhibitors of cathepsin K inhibit bone resorption both in vitro and in vivo. J Bone Miner Res 12:1396-1406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.