Abstract
Mature maize leaves have defined cell types along the proximal distal and medial lateral axes. The patterning events that establish these axes take place early in leaf initiation. We have identified a new dominant mutation, Wavy auricle in blade1 (Wab1), which affects patterning in both axes in a dose-dependent manner. Wab1 leaves are narrower than normal leaves and displace proximal tissues distally. We show that the proximal distal patterning defects are not due to misexpression of knox genes. Genetic analyses suggest that the action of dominant Wab1 alleles is localized to a lateral domain of the leaf, located between the midvein and the marginal domain that is determined by narrow sheath function. Thus, Wab1 defines a knox-independent pathway that affects specification of the proximal distal axis of the maize leaf. We suggest that failure to elaborate a normal lateral domain in the Wab1 leaf is responsible for disrupting patterning of the proximal distal axis.
Leaves arise on the flanks of the shoot apical meristem (SAM) at the growing tip of higher plants. Maize (Zea mays) leaves initiate in a distichous pattern in which the midvein of a new leaf is opposite the midvein of the previous leaf (Sharman, 1942). The group of cells in the meristem that will form the next leaf is referred to as founder cells and are in plastochron 0 (P0), where a plastochron is the time interval between initiating leaves. Although P0 cells are morphologically indistinguishable from other SAM cells, they are distinct in their cell fate and gene expression patterns. For example, P0 cells do not express the homeobox gene knotted1 (kn1), which is required to maintain meristem cell fate (Smith et al., 1992; Jackson et al., 1994; Vollbrecht et al., 2000). This down-regulation of kn1 and other kn1-like homeobox (knox) genes in founder cells is thought to be required for leaf initiation (Tsiantis and Hay, 2003).
Patterning of the leaf is polarized along three axes of growth: the medial lateral, the adaxial abaxial, and the proximal distal. The appropriate specification of pattern in all three axes is essential for the elaboration of normal leaf morphology. The incipient maize leaf encircles the SAM with a distinct midvein on one SAM flank and margins recruited from the opposite flank, thus defining the medial lateral axis. The midvein is positioned asymmetrically along the adaxial abaxial axis of the primordium and cell types in this axis become morphologically distinct as the leaf primordium divides to form a visible ring. As the leaf primordium grows away from the shoot, proximal distal patterning becomes apparent with the differentiation of four distinct tissues. The proximal sheath wraps around the stem and the distal blade lies flat to optimize photosynthesis. Separating the blade and sheath is a linear fringe of epidermal tissue termed the ligule and two wedges of auricle tissue (Fig. 1A). Developmental progression of the different tissues has been well characterized histologically and by scanning electron microscopy (Sharman, 1942; Becraft et al., 1990; Sylvester et al., 1990; Walsh et al., 1998).
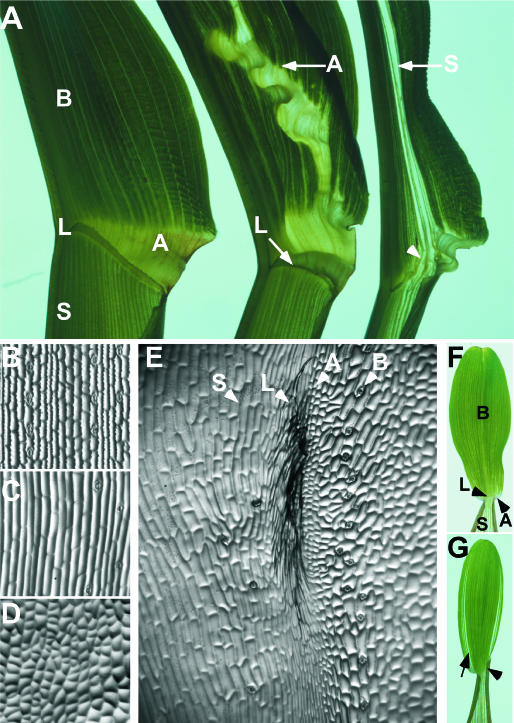
Figure 1.
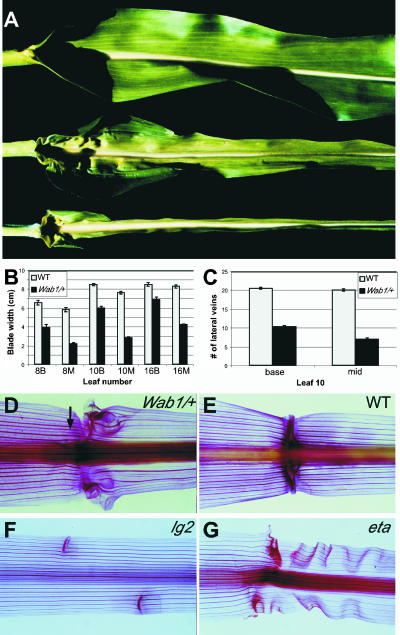
The dominant Wab1 leaf phenotype shows dose-dependent proximal distal tissue transformations. A, Wild-type leaf indicating sheath (S), blade (B), auricle (A), and ligule (L). Wab1/+ leaf indicating ectopic auricle in blade and normal ligule (arrow). Wab1/Wab1 leaf indicating ectopic sheath in blade and absence of ligule at a point midway between the midrib and margin (arrowhead). Wild-type adaxial epidermis of blade (B), sheath (C), and auricle (D). E, Adaxial epidermis of Wab1 blade showing mixture of four tissue identities from left to right: sheath (S), ligule (L), auricle (A), and blade (B). F, Wild-type first leaf indicating sheath, blade, auricle, and ligule. G, Wab1 first leaf indicating cleared tissue at the margin (arrow) and displaced ligule (arrowhead). Adaxial surface of only one-half of the leaf is presented in section A.
Within each axis, specific domains have been defined genetically. For example, mutations in the unlinked narrow sheath1 (ns1) and ns2 genes cause the deletion of a lateral domain in the maize leaf that includes the margin (Scanlon et al., 1996; Scanlon and Freeling, 1997). Plants doubly homozygous for ns1 and ns2 (referred to as ns) fail to down-regulate knox genes in a founder cell domain that gives rise to a wild-type margin and as a consequence, mutant leaves are narrow (Scanlon et al., 1996). Mosaic analysis has shown that NS1 acts noncell autonomously from two foci in the SAM to direct recruitment of marginal founder cells (Scanlon, 2000). Cloning of ns1 and ns2 supports the mosaic analysis; both genes are expressed in two foci of the SAM (Nardmann et al., 2004).
Along the proximal distal axis, recessive mutations at two unlinked loci, liguleless1 (lg1) and lg2, result in the loss of ligule and auricle tissue but do not affect the specification of blade and sheath (Becraft et al., 1990; Sylvester et al., 1990; Harper and Freeling, 1996; Moreno et al., 1997; Walsh et al., 1998). lg1 encodes a putative DNA-binding protein that functions in a cell-autonomous fashion in either the propagation or reception of a make ligule/auricle signal (Becraft and Freeling, 1991; Moreno et al., 1997). lg2 encodes a bZIP transcription factor that functions noncell autonomously to induce this signal (Harper and Freeling, 1996; Walsh et al., 1998).
Correct differentiation of tissues along the proximal distal axis of the maize leaf requires precise regulation of knox gene expression (Smith et al., 1992; Schneeberger et al., 1995, 1998; Muehlbauer et al., 1997; Foster et al., 1999; Timmermans et al., 1999; Tsiantis et al., 1999). When knox genes are ectopically expressed in the developing leaf primordia of the recessive mutant roughsheath2, or dominant Knox mutants, the blade is transformed to a more proximal cell identity such as sheath, ligule, or auricle. The acropetal shift in proximal distal pattern of the leaf occurs in dominant Knox mutants without affecting the medial lateral axis.
Here we describe a dominant mutation, Wavy auricle in blade1 (Wab1), which affects pattern specification in both the proximal distal and medial lateral axes of the maize leaf. Our analysis demonstrates that Wab1 is not a knox gene and that the phenotype is independent of ectopic knox expression. Genetic analyses suggest that the action of dominant Wab1 alleles is localized to a lateral domain, immediately internal to the margin domain defined by NS. Wab1 activity inhibits lateral growth in this domain, thereby disrupting establishment of proximal distal pattern.
RESULTS
Wab1 Is a Dominant Mutation Defined by Three Alleles
Wab1-R is a dominant mutation that arose in androgenesis tissue culture and was recovered by James Wassom (Hake et al., 1999). Wab1 was mapped to chromosome 2 L using waxy translocations and further mapped to bin 2.06 using restriction fragment length polymorphisms. Wab1 is 4 cM from umc5a (3/72 recombinants).
Two additional dominant mutations that showed linkage to Wab1-R were isolated from populations containing Mutator transposons. To determine allelism between Wab1-DLC and Wab1-R, the double heterozygote was crossed to wild-type plants and the progeny scored. All 122 progeny examined were mutant in appearance indicating that the 2 mutations map to within 0.8 cM of each other and are likely to be alleles of the same gene. Wab1-RM showed <4 centimorgans linkage with the RFLP marker umc5a, indicating that it maps to bin 2.06 and is also likely to be allelic to Wab1-R. All subsequent analyses were performed using Wab1-R or Wab1-DLC introgressed into B73 seven times.
Wab1 Leaves Show Proximal Distal Tissue Transformations in a Dosage-Dependent Manner
Normal maize leaves have four distinct tissues with characteristic epidermal features (Sylvester et al., 1990) arranged along the proximal distal axis; blade is positioned distally, separated from sheath by ligule and auricle (Fig. 1, A, left, and B–D). Wab1 mutant leaves show proximal to distal shifts, such that proximal tissue types including sheath, auricle, and ligule are found in the blade. The sheath is relatively unaffected. Auricle tissue extends distally from the normal position at the boundary between blade and sheath (Fig. 1A, middle), and also initiates at ectopic positions in the blade. Most heterozygotes have a normally-placed ligule that extends across the leaf between the sheath and auricle (Fig. 1A, middle). Homozygotes have a disrupted ligule in a B73 background (arrowhead, Fig. 1A, right), indicating that the presence or absence of ligule is affected by the Wab1 mutation in a dose-dependent manner.
At the position of the missing ligule, sheath tissue extends up the entire length of the leaf (Fig. 1A, right). This sheath tissue is accompanied by ectopic ligule, seen as a fringe of adaxial epidermal cells, and ectopic auricle, characterized by unexpanded dovetailed cells, running perpendicular to the normal ligule. The ectopic ligule and auricle separate the smooth-walled cells of the ectopic sheath from the crenulated blade cells (Fig. 1E). A normal ligule forms over the midrib and initiates again on the margin side of the sheath extension (Fig. 1A, right). The mutant phenotype is visible in all leaves and is manifest in the first leaf as cleared tissue near the margin and disrupted ligule formation (Fig. 1, F and G).
Both the Wab1-R and Wab1-DLC alleles were introgressed seven times into a number of different inbred backgrounds and the penetrance of phenotypes quantified in heterozygous families (Fig. 2E). No significant differences were seen between the phenotypes of Wab1-R and Wab1-DLC. The extension of auricle from its normal position up into the blade (Fig. 2A) is completely penetrant in all inbred backgrounds with the exception of W22 in which all aspects of the Wab1 phenotype are suppressed. Initiation of auricle in ectopic positions in the blade (Fig. 2A) occurs in all inbred backgrounds but at a lower penetrance in juvenile leaves. A patchwork pattern of narrow files of sheath cells alternating with blade tissue (Fig. 2B) is found near the margin in all leaves in all backgrounds. Extra flaps of blade develop at the margin both at the base of the blade and at a position midway up the blade (Fig. 2C) at low penetrance in all backgrounds but occurs most frequently in late adult leaves.
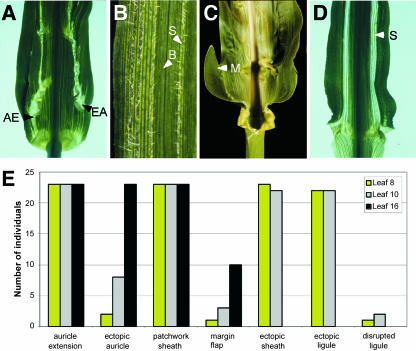
Figure 2.
Penetrance of leaf phenotypes in Wab1/+. A, Abaxial view showing auricle tissue extending from the normal position (AE) and ectopic auricle tissue (EA) in the blade. B, Narrow sheath sectors (S) identified by abaxial epidermal hairs interspersed with blade tissue (B) identified by glabrous abaxial epidermis. C, Flap of tissue at the margin of the blade (M). D, Abaxial view showing ectopic sheath tissue (S) extending throughout the blade. E, Penetrance of above phenotypes in Wab1/+ introgressed seven times into B73 (n = 25). None of the above phenotypes were present in wild-type siblings (n = 25).
Ectopic sheath tissue is found in the blade without disruption of the ligule (Fig. 2D) only in a B73 background, where this phenotype is completely penetrant in all but late adult leaves. Ectopic ligule occurs wherever ectopic sheath is juxtaposed with blade tissue. Disrupted ligule was seen at low penetrance in juvenile leaves of Wab1 heterozygotes only in a B73 background. Thus, the only aspects of the mutant phenotype that display significant differences in penetrance, dependent on dosage and background, are the disrupted ligule and ectopic sheath sectors.
Proximal Distal Tissue Transformations Occur Early in Leaf Development
To determine when the tissue transformations occur, ligule and auricle development were analyzed in 3-week-old wild-type and Wab1 mutant seedlings. Figure 3A shows a region of active cell divisions across the adaxial surface of wild-type P7 primordia forming the preligule band (Walsh et al., 1998). Periclinal divisions in a subset of these cells in P8 primordia cause a ridge of epidermal cells to protrude that will eventually form the mature ligule (Fig. 3B). Packets of cells dividing in both transverse and longitudinal directions can be seen distal to the ridge (arrow, Fig. 3B, and arrowhead, 3F). These cells will differentiate as auricle tissue and will contribute width to the base of the blade.
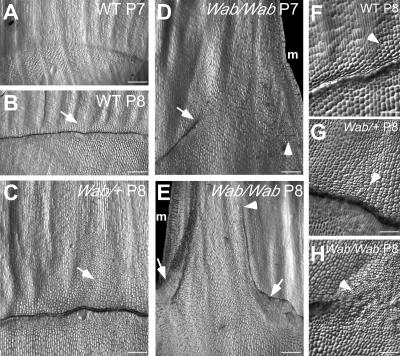
Figure 3.
Proximal distal defects in Wab1 leaf development. Nail polish replicas of the adaxial epidermis showing development of the ligule/auricle region. A, Wild-type P7, preligule band. B, Wild-type P8, ligular ridge; arrow points to auricle region. C, Wab1/+ P8; arrow points to extended region of auricle proliferation. D, Wab1/Wab1 P7; ligule ridge initates from the midrib at a 45° angle but stops at a point halfway between midrib and margin indicated by the arrow. Cell divisions indicative of preligule band formation at the margin are indicated by the arrowhead. E, Wab1/Wab1 P8; arrows indicate the position at which the ligule ridge reorients parallel to the proximal distal axis of the leaf. Arrowhead indicates where the ligule ridge on the midrib side stops. F, Close-up view of wild-type P8 ligular region; arrowhead indicates a few auricle cells dividing in transverse and longitudinal orientations. G, Close-up view of Wab1/+ P8 ligular region; arrowhead indicates that all auricle cells are rapidly dividing in a predominantly longitudinal orientation. H, Close-up view of Wab1/Wab1 P8 where the ligule ridge is reorienting; arrowhead indicates very small cells that are rapidly dividing in all orientations. m, Margin. Bars represent 500 μm in sections A to E, 100 μm in sections F to H.
Wab1 heterozygotes form a normal ligular ridge on the adaxial surface of P8 primordia (Fig. 3C). However, a greatly extended region of dividing cells is visible distal to the ridge (arrow, Fig. 3C, and arrowhead, 3G). The division activity extends most distally at a point midway between the midrib and margin.
Wab1 homozygotes show defective formation of the preligule band on the adaxial surface of P7 primordia. A developed ligule ridge initiates at the normal position at the midrib, but follows a steep distal trajectory before terminating at a position midway between midrib and margin (arrow, Fig. 3D). Preligule band divisions also initiate near the margin, and sheath tissue extends through this gap in the developing ligule. Adjacent to this sheath extension, the ligule is reoriented parallel to the proximal distal axis of the leaf such that it separates sheath cells from blade (arrows in Fig. 3E).
Cell division appears to be very rapid and disorganized in the altered auricle and ligule regions of Wab1 heterozygotes and homozygotes. In normal P8 primordia, cells that will differentiate as auricle divide in both transverse and longitudinal orientations (arrowhead, Fig. 3F). In Wab1/+ P8 primordia, these cells are much smaller and divide in a less organized manner in a predominantly longitudinal orientation (arrowhead, Fig. 3G). In Wab1/Wab1 P8 primordia, cells at the position where the ligule ridge changes orientation are extremely small and divide in all planes in a very disorganized manner (arrowhead, Fig. 3H). Thus, despite the narrowness of Wab1 leaves, cell divisions are abundant at the blade-sheath boundary.
Proximal Distal Patterning Defects in the Wab1 Leaf Are KNOX-Independent
The map position of Wab1 does not correspond to a known class I knox gene (Kerstetter et al., 1994). Regardless, phenotypic similarities with dominant Knox mutations, which show proximal to distal shifts in the maize leaf pattern (Freeling, 1992), led us to analyze Wab1 leaves for the presence of ectopic knox gene expression. Immunolocalization of KN1 protein in Wab1 shoot apices showed that expression is indistinguishable from wild type (Fig. 4, A and B). Furthermore, reverse transcription (RT)-PCR gel-blot analysis failed to detect any difference in expression of nine class I knox genes (kn1, knox4, lg3, lg4b, and rs1 shown) between Wab1 and normal leaves (Fig. 4C).
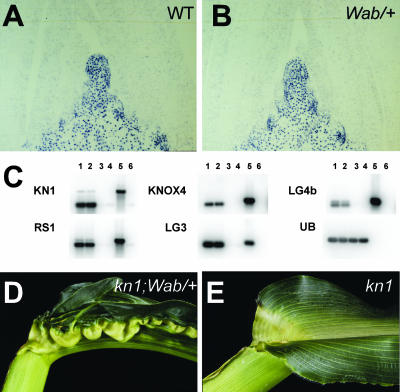
Figure 4.
Proximal distal patterning defects in the Wab1 blade are KNOX-independent. Immunolocalization of KN1 protein in the shoot apex of wild type (A) and Wab1/+ (B). C, RT-PCR gel-blot analysis of knox gene expression in wild-type shoot apex (lane 1), Wab1/+ shoot apex (lane 2), wild-type leaves (lane 3), Wab1/+ leaves (lane 4), genomic DNA (lane 5), and no cDNA included in PCR (lane 6). Control amplification of ubiquitin (UB) indicates equal amounts of cDNA present in each sample. D, Wab1;kn1-e1 leaf. E, kn1-e1 sibling leaf.
To assess whether loss of kn1 function would affect the Wab1 phenotype, double mutants between Wab1 and kn1-E1 were constructed in a B73 background. Reduced inflorescence branching and extra carpels were used to identify kn1-E1 mutants (Kerstetter et al., 1997; Vollbrecht et al., 2000). Double mutants showed a fully expressive Wab1 phenotype, indicating that the Wab1 mutation is unaffected by the absence of kn1 function (Fig. 4, D and E). Therefore Wab1 defines a novel, dominant mutation that conditions proximal distal tissue transformations in the leaf in a manner independent of knox function.
The Wab1 Leaf Blade Is Very Narrow
A consistent feature of the Wab1 mutant phenotype is a narrowing of the leaf blade, particularly at the distal end (Fig. 5, A and B). This gives the Wab1 leaf a different shape than wild type, characterized by hips at the blade-sheath boundary that taper into a narrow blade less than one-half the width of wild type. The leaf blade of homozygotes has the same shape as heterozygotes but with an extremely narrow blade, indicating that blade width is affected by the Wab1 mutation in a dose-dependent manner (Fig. 5A). A reduction in the number of lateral veins in Wab1/+ blades correlates with the reduction in blade width (Fig. 5, B and C). Approximately one-half the numbers of lateral veins are present at the base of a Wab1 blade compared with wild type, and less than one-half are present at a point midway up the blade (Fig. 5C). Cleared leaves stained with safranin show that a normal number of regularly spaced lateral veins are present in the Wab1 sheath but few traverse the aberrant auricle region into the blade and regular spacing is lost (Fig. 5, D and E). This phenotype is specific to Wab1 and not a secondary effect due to an aberrant ligule/auricle region because lateral veins are continuous though sheath and blade in liguleless2 leaves where auricle is incomplete (Fig. 5F; Walsh et al., 1998), and extended auricle-R leaves (Fig. 5G) where the auricle region is extended (Osmont et al., 2003).
Figure 5.
The Wab1 leaf blade is very narrow. A, From top to bottom: wild-type leaf, Wab1/+ leaf, and Wab1/Wab1 leaf. B, Leaf width of wild-type blade (gray bar) versus Wab1/+ blade (black bar) measured at the base of the blade (B) and the midpoint of the length of the blade (M) for leaves 8, 10, and 16 (leaf 8 being the eighth leaf initiated). C, Number of lateral veins in wild-type blade (gray bar) versus Wab1/+ blade (black bar) counted at the base of the blade and the midpoint of the length of the blade for leaf 10. Cleared leaves stained in safranin to show lateral veins of Wab1/+ (D), wild type (E), lg2-R (F), and eta-R (G). Arrow in D points to lateral vein in the sheath that does not continue into the blade.
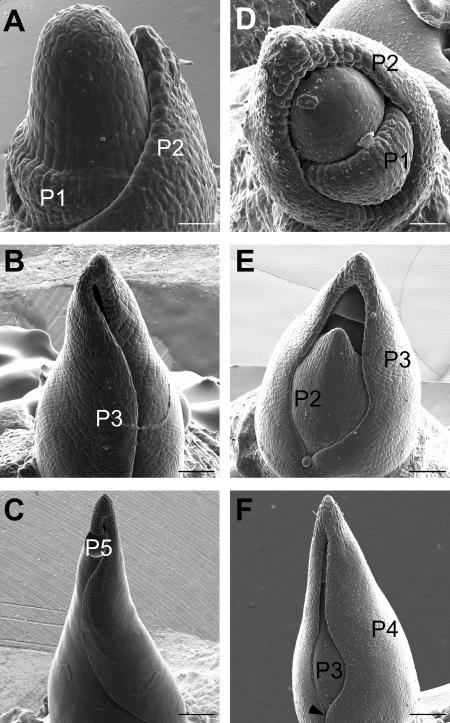
The correlation between a reduction of blade width and lateral vein number suggests that the leaf is narrower than wild type from an early stage of development. A defect in blade cell expansion, on the other hand, would correlate with a normal number of closely spaced lateral veins and a reduction in cell size, neither of which we see in the Wab1 leaf blade (data not shown). To investigate this hypothesis further, we examined Wab1 and normal leaf primordia by scanning electron microscopy. In wild type, the P1 primordium is first observed as a bulge on the flanks of the SAM encircling the apex (Fig. 6A). The P2 primordium encircles the base of the apex and the tip of the primordium approaches the top of the SAM. The P3 primordium encloses the shoot apex (Fig. 6B) and P4 and P5 primordia wrap around the shoot apex such that the edges of the primordia overlap (Fig. 6C).
Figure 6.
Defect in medial lateral patterning occurs early in Wab1 leaf development. Scanning electron micrographs using serial replica molds of a wild-type apex showing SAM, P1, and P2 leaf primordia (A), P3 leaf primordium (B), and P5 leaf primordium (C). Wab1/+ apex showing SAM, P1, and P2 primordia (D), P3 primordium not enclosing the apex such that P2 and the SAM are visible (E), and P4 primordium with a window exposing P3 primordium (F). Arrow points to where the base of the primordium encloses the apex. Bars represent 50 μm in sections A and D, 150 μm in sections B and E, and 250 μm in sections C and F.
Early defects are apparent in leaf development of both Wab1 heterozygotes and homozygotes. Wab1 mutant primordia at stages P3 through P5 fail to enclose the shoot apex such that the SAM and early leaf primordia are exposed (Fig. 6, E and F). The defect is more apparent in the upper part of the primordium that later forms the blade. Thus, the defect that makes Wab1 leaves narrower than wild type occurs as early as P1 to P2.
Ectopic Auricle Initiates at the NS Domain Boundary in Wab1 Leaves
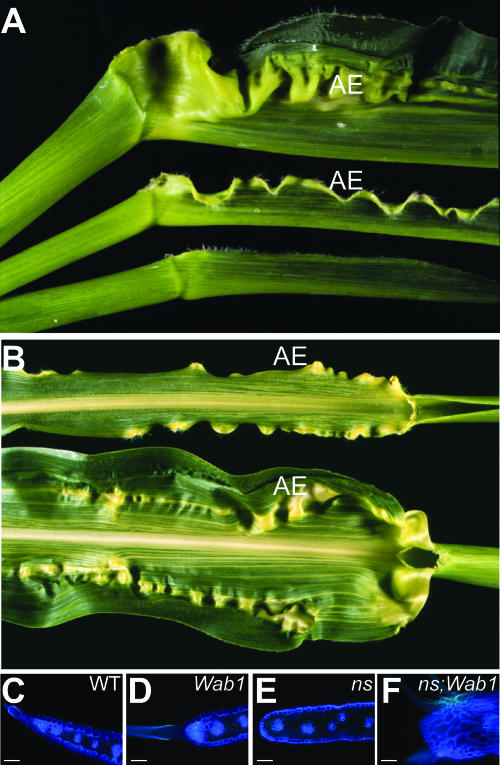
Both dominant Wab1 mutants and doubly homozygous recessive ns mutants display narrow leaves. ns1 and ns2 gene products are required for the recruitment of leaf founder cells from the SAM into marginal domains of the leaf (Scanlon et al., 1996). Mutant leaves have narrow sheath and proximal blade that do not display the normal morphological markers of margin identity such as sawtooth hairs and a tapered region of chlorophyll-free tissue (Fig. 7, A and E). In contrast, specification of the leaf margin is unaffected in Wab1 as these markers of margin identity are evident in Wab1 leaf margins (Fig. 7, C and D).
Figure 7.
Defect in proximal distal patterning occurs at the NS domain boundary in Wab1 leaves. A, From top to bottom: Wab1/+ leaf, Wab1/+;ns1;ns2 leaf, and ns1;ns2 leaf. B, Ectopic auricle initiates at similar lateral position in Wab1/+;ns1;ns2 blade (top) and Wab1/+ blade (bottom). Autoflourescence of hand-sectioned blade showing the margin of wild type (C), Wab/+ (D), ns1;ns2 (E), and Wab1/+;ns1;ns2 (F). AE, Auricle extension. Bars represent 150 μm in sections C to F.
To identify the position of the domain affected in Wab1 relative to the NS domain, the ectopic auricle tissue was examined in leaves that were triply mutant for ns1, ns2, and Wab1. If Wab1 acts in the marginal domain, ectopic auricle would not be visible in triple mutants. On the other hand, if Wab1 acts in a domain inside of the NS domain, triple mutants would display ectopic auricle. Figure 7A shows the leaf phenotype of these triple mutants. Both sheath and blade are narrow (Fig. 7A) and the blade edge is blunt and lacks sawtooth hairs (Fig. 7F), indicating that margin identity is missing. The thick, nonchlorophyllous tissue and large hairs at the blade edge of the triple mutants are indicative of mature auricle identity (Fig. 7F). Thus, the blade edge of Wab1;ns triple mutants is consistently transformed to auricle tissue. If the Wab1 and Wab1;ns leaves are superimposed upon each other, the position of the ectopic auricle tissue relative to the midrib aligns (Fig. 7B), suggesting that the defect in proximal distal patterning in Wab1 leaves occurs at the boundary of the ns margin domain.
We asked whether Wab1;ns triple mutants are narrower than ns double mutants. Leaf blade width was determined in a family that was homozygous for ns1 and ns2 but segregating for Wab1. Leaf width was measured at the ligule (L) and at a midpoint of the blade (M) for the sixth leaf counting down from the tassel (Table I). The ratio of M to L showed that the Wab1;ns triple mutants were consistently narrower in the midblade position than the ns double mutants (Student's t test 0.002). Thus Wab1 affects a domain distinct from that affected by ns.
Table I.
Wab1;ns mutants are narrower than ns mutants
| Blade Length | Width at Ligule (L) | Width at Midblade (M) | M/L | Phenotype |
|---|---|---|---|---|
| 69 | 5 | 7.3 | 1.46 | ns |
| 65 | 4.5 | 6.9 | 1.53 | ns |
| 65 | 4.5 | 7 | 1.55 | ns |
| 64 | 3.5 | 3.2 | 0.91 | Wab;ns |
| 54 | 1.8 | 2 | 1.11 | Wab;ns |
| 66 | 2.7 | 3.2 | 1.18 | Wab;ns |
| 59 | 2.5 | 2.4 | 0.96 | Wab;ns |
Measurements are in mm. The sixth leaf from the tassel was measured. Similar results were also found for the seventh leaf from the tassel (data not shown). The length of the blade was included for consideration of overall leaf size.
DISCUSSION
The dominant Wab1 mutation disrupts patterning within a lateral domain of the maize leaf. This domain is narrower than in wild type, resulting in thin leaves with inappropriate differentiation of distal cells into tissues normally found in more proximal positions. This shift in proximal distal patterning occurs independently of knox expression. The Wab1 mutation shows a dosage effect in both medial lateral and proximal distal phenotypes. Analysis of the Wab1 phenotype in an ns mutant background suggests that the lateral domain affected by the Wab1 mutation lies immediately internal to the margin domain defined by NS.
Lateral Domains of the Leaf
Mutations have been used to define different domains of the maize leaf. ns mutants produce narrow leaves that lack specific tissues of the margin, suggesting that the adult phenotype is due to loss of a marginal domain (Scanlon et al., 1996). Wab1 leaves are also narrower than wild type but are narrow in a different manner to ns leaves. Each single mutant has a distinct leaf shape; the ns mutant is narrowest in the sheath and proximal blade, while Wab1 mutants are narrowest in the middle region of the blade.
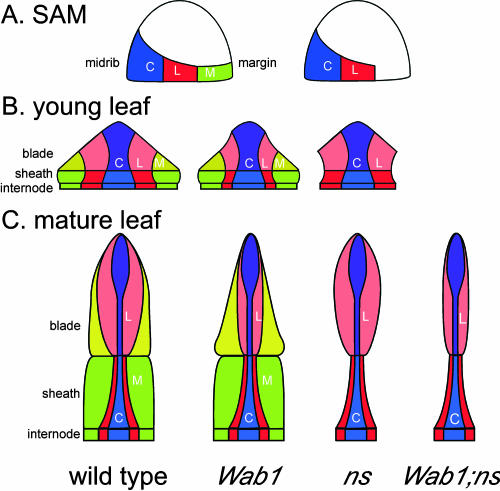
KNOX immunolocalizations and mosaic analyses in ns and nonmutant plants indicate that NS function is required in the meristem to recruit cells for the marginal domain (Fig. 8A, right; Scanlon et al., 1996; Scanlon and Freeling, 1997). This hypothesis is supported by the finding that the ns duplicate genes are expressed in the meristem in two foci located in the presumptive lateral domain of the initiating leaf (Nardmann et al., 2004). Consistent with a function in cell recruitment, ns1 and ns2 encode maize orthologs of the WUSCHEL-like homeodomain protein, PRESSED FLOWER (Matsumoto and Okada, 2001). Wab1 mutants, in contrast, demonstrate normal down-regulated accumulation of KNOX proteins in incipient leaf primordia, suggesting that the recruitment of founder cells in the medial lateral domains of the Wab1 leaf is normal (Fig. 8A, left). After leaf initiation, lateral growth occurs within each domain along the medial lateral axis (Fig. 8B). The lateral domain is narrower than normal in young Wab1 leaf primordia, resulting in a narrow P3 primordium that fails to enclose the shoot apex, similar to ns.
Figure 8.
Wab1 acts in a lateral domain in the maize leaf. A, Leaf founder cells are initially recruited into central (C, blue), lateral (L, red), and marginal (M, green) domains in the SAM; the marginal domain is not recruited in ns mutants. B, During P1 to P2, growth of the lateral domain is inhibited in Wab1 mutants, resulting in a narrow primordium. C, Cells in the lateral domain contribute the majority of the distal blade tissue in the mature leaf and this tissue is reduced in Wab1 leaves. Cells in the margin domain contribute the majority of the sheath and proximal blade tissue in the mature leaf and this tissue is absent in ns leaves. Wab1;ns mutants reveal both defects.
As the leaf grows, it differentiates into sheath and blade tissues along the proximal distal axis (Fig. 8C). Notably, the lateral domain makes a greater contribution to the blade than the sheath, while the margin contributes substantially to both blade and sheath (Poethig and Szymkowiak, 1995). Wab1 mutants display reduced leaf width predominantly in the distal part of the blade, whereas ns mutants are reduced in both blade and sheath width. We suggest that dominant Wab1 alleles act locally within the lateral domain to inhibit lateral growth.
One prediction of this hypothesis is that the shape of Wab1;ns leaves should reflect the loss of contribution from both margin and lateral domains to blade width. Wab1;ns leaves were shown to be significantly narrower than those of ns (Fig. 8C, right), supporting the idea that Wab1 specifically inhibits growth of a lateral domain of the leaf. A cell lineage analysis would help elucidate the exact contribution of the lateral domain to the mature leaf shape in Wab1 and wild-type plants.
Proximal Distal Axis Establishment
In addition to being narrower than wild-type leaves, Wab1 mutants have proximal distal patterning defects. Heterozygotes have a normal ligule in its proper position but have ectopic auricle or extended auricle distal to the normally placed ligule. Homozygotes have gaps in the ligule and sheath extends up into the blade in this position. Inside of the lateral domain of the blade, the tissue identities of Wab1 leaves are normal and on the margin side they are relatively normal. Given that proximal distal patterning is likely to occur after establishment of the medial lateral domains, the results suggest that the proximal distal defect in Wab1 mutants results from failure to fully elaborate the lateral domain in P1 to P2 primordia. The alternative hypothesis that Wab1 causes blade cells to adopt sheath identity, which in turns makes the mutant blade more narrow, is not supported by the fact that sheaths and blades are the same width in wild-type plants. We propose that a signal that is required to establish proximal distal pattern progresses from the midrib to the margin and that reception of this signal is disrupted when the lateral domain is incorrectly specified in Wab1 leaves. The consequence of this disruption is erratic cell divisions in the developing ligule/auricle region and inappropriate tissue differentiation in the adult Wab1 leaf.
Interdependence of proximal distal patterning on medial lateral specification has previously been suggested on the basis of LG1 gene action. Mosaic analysis of lg1 mutants suggests that an inductive signal for ligule/auricle initiation originates near the midvein and requires LG1 function to be transmitted through the lateral domain to the leaf margin (Becraft and Freeling, 1991). We propose that this signal induces lateral blade growth in addition to ligule/auricle initiation. In Wab1 leaves, the lateral domain is not fully or correctly elaborated, interfering with reception of the signal in this domain. As a consequence, lateral blade growth and ligule/auricle initiation are disrupted in this domain. Propagation and reception of this signal resumes in the margin. The patchwork phenotype of alternating blade and sheath cell files at the margin (Fig. 2B) may indicate a failure to completely recover outside of the lateral domain.
The exact relationship between wab+ and lg1 is not known; however, expression levels of lg1 are increased in Wab1 mutants and Wab;lg1 double mutants are narrower than Wab1 single mutants (T. Foster, A. Hay, R. Johnston, and S. Hake, unpublished data). The results suggest that Wab1 leaves recover some of their width via precocious and prolonged lg1 expression. These results support the idea of a role for LG1 in perceiving and propagating a medial/lateral signal that regulates leaf width in addition to positioning the blade sheath boundary. Given the dominant nature of the Wab1 mutation, the role of the wild-type gene product is unknown. The ectopic expression of lg1 suggests that wab+ may normally regulate lg1, thus playing a role in longitudinal cell divisions and establishment of the blade sheath boundary. Alternatively, the close linkage to ns1 does not exclude the possibility that Wab1 is a novel antimorphic allele of ns1 that has expanded the normal domain and function of ns1. Further experiments will explore the exact role of wab1+.
Although the defects in medial lateral and proximal distal pattern specification could be independent, other examples exist where polarity in one axis serves to specify pattern formation in a second axis (Waites and Hudson, 1995; Timmermans et al., 1998). For example, in leaves and petals of the phantastica mutant in Antirrhinum, novel boundaries between adaxial and abaxial cell types form ectopic axes of growth, suggesting that adaxial abaxial polarity is required for growth in the lateral axis in the wild-type leaf and petal (Waites and Hudson, 1995). Mosaic analysis carried out on Wab1 in the presence and absence of lg1 (T. Foster, A. Hay, R. Johnston, and S. Hake, unpublished data) shows that wab1+ sectors recover normal width and normal blade cell identity, thus supporting the link between proximal distal and medial lateral axes.
MATERIALS AND METHODS
Genetic Analysis
Wab1-R and Wab1-DLC alleles were introgressed into the following inbred backgrounds: B73, Mo17, W22, W23, A188, and A619. The phenotype was penetrant in all backgrounds except W22; therefore, introgressions were discontinued in W22 and performed seven times into the other backgrounds. Wab1 was crossed to ns1/ns1; ns2/ns2 plants and back-crossed again to ns1/ns1; ns2/ns2. A putative triple mutant was identified and sib-crossed to an ns1/ns1; ns2/ns2 individual to generate a 1:1 stock for phenotypic analysis. Wab1 was crossed to kn1-E1/kn1-E1, the F1 was self-pollinated, and the F2 were sib-crossed to generate a 1:1 stock for phenotypic analysis.
RT-PCR Gel-Blot Analysis
One microgram total RNA extracted using an RNeasy kit (Qiagen, Valencia, CA) from leaf and SAM-enriched tissues of 3-week-old seedlings segregating 1:1 for Wab1-DLC/+ and wild type, were used for cDNA synthesis with an oligo(dT) primer and SuperscriptII reverse transcriptase (Invitrogen, Carlsbad, CA).
knox Gene Expression
knox gene-specific PCR primers are as follows: kn1-5′, agctcgctcaagcaagaactgtc; kn1-B2, cataggcgcatatagatagagtagcaac; Gn1-B1, tacgcagaaacactccgacacggtcg; Gn1-F2, ggaagacgacgacatggatccgag; Rs1-11464, ttctgaagatgacatggacccgaatggtc; rs1\PBO-7extended, gagaactacaagccatgcatagacgctac; lg3/4-l, gtggaacacgcactaccgctg; Lg3-D2, tgagctggccagttgtcatccc; knox11-B1, ccagtatgctgagtgtacctaccgacac; knox5-B1, cgacaatacacgttgtcgcccatgc; ubiquitin 3, taagctgccgatgtgcctgcgtcg; ubiquitin-4, ctgaaagacagaacataatgagcacaggc. Twenty-four PCR cycles were performed to amplify cDNA products that were discriminated from genomic products by size when detected by Southern hybridization with gene-specific probes.
Phenotypic Analysis
Fifty individuals of Wab1-R and Wab1-DLC families, introgressed into B73, Mo17, and A188, were scored for leaf phenotypes on leaves 8, 10, and 16. Measurements of leaf width and lateral vein number were taken at the base and halfway point of the blade.
Immunolocalization
Apices of 3-week-old seedlings segregating 1:1 for Wab1-R/+ and wild type were fixed in 4% paraformaldehyde, processed, and paraffin-embedded as described (Jackson, 1992). Protein immunolocalization was performed using an affinity purified KN1 polyclonal rabbit antibody as described (Smith et al., 1992).
Epidermal Impressions
Leaf primordia (P6–9) were removed from 3-week-old seedlings of a selfed Wab1-DLC/+ family, and Cinch-Light dental impression media (Parkell, Farmingdale, NY) was applied to the adaxial surface to make an impression. Transparent nail polish was applied to the impression, removed, and placed upside down on a glass slide and viewed using differential interference contrast optics on a Zeiss Axiophot (Jena, Germany) or Nikon SMZ800 dissecting scope (Tokyo).
Leaf Clearings
Leaves of 3-week-old seedlings segregating 1:1 Wab1-R/+ to wild type were boiled for 15 min in 85% ethanol, cleared in lactic acid at 70°C for 3 h, stained with 1% safranin O in 70% ethanol, and viewed on a light box.
Replica Scanning Electron Microscopy
Leaf primordia (P5 through P2) were sequentially removed from 3-week-old seedlings of a selfed Wab1-DLC/+ family and molds were taken of the shoot using Cinch-Light dental impression media. Two-ton epoxy resin was used to cast the molds that were gold sputter-coated and observed using a Hitachi SV3400 scanning electron microscope operating at an accelerating voltage of 10 kV.
Hand Sections
Transverse sections were made through the leaf blade, mounted in 50% glycerol, and viewed under UV light using a Zeiss Axiophot.
Acknowledgments
We thank T. Rocheford, D. Laudencia-Chingquanco, and the Maize Gene Discovery project for the gift of Wab1 alleles. We thank H. Hester and A. Seah for valuable lab and field assistance, D. Hantz and UC Berkeley field staff for greenhouse and field assistance, the Freeling lab for seed stocks and helpful discussions, H. Candela-Anton for assistance with mapping, T. Foster for valuable discussions, and J. Langdale for helpful comments on the manuscript.
This work was supported by the U.S. Department of Agriculture-Agricultural Research Service and the National Science Foundation (IBN–0131431).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036707.
References
- Becraft PW, Bongard-Pierce DK, Sylvester AW, Poethig RS, Freeling M (1990) The liguleless-1 gene acts tissue specifically in maize leaf development. Dev Biol 141: 220–232 [DOI] [PubMed] [Google Scholar]
- Becraft PW, Freeling M (1991) Sectors of liguleless-1 tissue interrupt an inductive signal duirng maize leaf development. Plant Cell 3: 801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T, Veit B, Hake S (1999) Gnarley is a dominant mutation in the knox4 homeobox gene affecting cell shape and identity. Plant Cell 11: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M (1992) A conceptual framework for maize leaf development. Dev Biol 153: 44–58 [DOI] [PubMed] [Google Scholar]
- Hake S, Hester H, Wassom J, Widholm J, Rocheford T (1999) Wab (Wavy auricles in blades), a dominant leaf mutation located on chromosome 2L. Maize Genet Coop News Lett 73: 3 [Google Scholar]
- Harper L, Freeling M (1996) Interactions of liguleless1 and liguleless2 function during ligule induction in maize. Genetics 144: 1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D (1992) In situ hybridization in plants. In SJ Gurr, MJ McPherson, DJ Bowles, eds, Plant Molecular Pathology: A Practical Approach, Vol 1. Oxford University Press, Oxford, pp 163–174
- Jackson D, Veit B, Hake S (1994) Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413 [Google Scholar]
- Kerstetter R, Vollbrecht E, Lowe B, Veit B, Yamaguchi J, Hake S (1994) Sequence analysis and expression patterns divide the maize knotted1-like homeobox genes into two classes. Plant Cell 6: 1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S (1997) Loss of function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124: 3045–3054 [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Okada K (2001) A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev 15: 3355–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MA, Harper LC, Krueger RW, Dellaporta SL, Freeling M (1997) liguleless1 encodes a nuclear-localized protein required for induction of ligules and auricles during maize leaf organogenesis. Genes Dev 11: 616–628 [DOI] [PubMed] [Google Scholar]
- Muehlbauer GJ, Fowler JE, Freeling M (1997) Sectors expressing the homeobox gene liguleless3 implicate a time-dependent mechanism for cell fate acquisition along the proximal-distal axis of the maize leaf. Development 124: 5097–5106 [DOI] [PubMed] [Google Scholar]
- Nardmann J, Ji J, Werr W, Scanlon MJ (2004) The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development (in press) [DOI] [PubMed]
- Osmont KS, Jesaitis LA, Freeling M (2003) The extended auricle1 (eta1) gene is essential for the genetic network controlling postinitiation maize leaf development. Genetics 165: 1507–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS, Szymkowiak EJ (1995) Clonal analysis of leaf development in maize. Maydica 40: 67–76 [Google Scholar]
- Scanlon MJ (2000) NARROW SHEATH1 functions from two meristematic foci during founder-cell recruitment in maize leaf development. Development 127: 4573–4585 [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Freeling M (1997) Clonal sectors reveal that a specific meristematic domain is not utilized in the maize mutant narrow sheath. Dev Biol 182: 52–66 [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Schneeberger RG, Freeling M (1996) The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122: 1683–1691 [DOI] [PubMed] [Google Scholar]
- Schneeberger R, Tsiantis M, Freeling M, Langdale JA (1998) The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development 125: 2857–2865 [DOI] [PubMed] [Google Scholar]
- Schneeberger RG, Becraft PW, Hake S, Freeling M (1995) Ectopic expression of the knox homeo box gene rough sheath1 alters cell fate in the maize leaf. Genes Dev 9: 2292–2304 [DOI] [PubMed] [Google Scholar]
- Sharman BC (1942) Developmental anatomy of the shoot of Zea mays L. Ann Bot (Lond) 6: 245–282 [Google Scholar]
- Smith LG, Greene B, Veit B, Hake S (1992) A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development 116: 21–30 [DOI] [PubMed] [Google Scholar]
- Sylvester AW, Cande WZ, Freeling M (1990) Division and differentiation during normal and liguleless-1 maize leaf development. Development 110: 985–1000 [DOI] [PubMed] [Google Scholar]
- Timmermans MC, Hudson A, Becraft PW, Nelson T (1999) ROUGH SHEATH2: a Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 284: 151–153 [DOI] [PubMed] [Google Scholar]
- Timmermans MCP, Schultes NP, Jankovsky JP, Nelson T (1998) Leafbladeless1 is required for dorsoventrality of lateral organs in maize. Development 125: 2813–2823 [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Hay A (2003) Comparative plant development: the time of the leaf? Nat. Rev Genet 4: 169–180 [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA (1999) The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science 284: 154–156 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Reiser L, Hake S (2000) Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127: 3161–3172 [DOI] [PubMed] [Google Scholar]
- Waites R, Hudson A (1995) phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121: 2143–2154 [Google Scholar]
- Walsh J, Waters CA, Freeling M (1998) The maize gene liguleless 2 encodes a basic leucine zipper protein involved in the establishment of the leaf blade-sheath boundary. Genes Dev 12: 208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]