Abstract
Aim:
The aim of this study was to evaluate the accuracy of 3D soft tissue predictions generated by a computer-aided maxillofacial planning system in patients undergoing orthognathic surgery.
Methods and Materials:
Twenty patients with dentofacial dysmorphosis were treated with orthognathic surgery after a preoperative orthodontic treatment. Fourteen patients had an Angle Class II malocclusion; three patients had an Angle class III malocclusion, and three patients had an Angle Class I malocclusion. Skeletal asymmetry was observed in six patient. The surgeries were planned using the Maxilim software. Computer assisted surgical planning was transferred to the patient by digitally generated splints. The validation procedures were performed in the following steps: (1) Standardized registration of the pre- and postoperative Cone Beam CT volumes; (2) Automated adjustment of the bone-related planning to the actual operative bony displacement; (3) Simulation of soft tissue changes; (4) Calculation of the soft tissue differences between the predicted and the postoperative results by distance mapping.
Statistical Analysis and Results:
Eighty four percent of the mapped distances between the predicted and actual postoperative results measured between -2 mm and +2 mm. The mean absolute linear measurements between the predicted and actual postoperative surface was 1.18. Our study shows the overall prediction was dependent on neither the surgical procedures nor the dentofacial deformity type.
Conclusion:
Despite some shortcomings in the prediction of the final position of the lower lip and cheek area, this software promises a clinically acceptable soft tissue prediction for orthognathic surgical procedures.
Keywords: 3D planning, orthognathic surgery, soft tissue prediction, virtual surgery
INTRODUCTION
Achieving optimal results in orthognathic surgery depends on the accuracy of the surgical planning and the surgical technique used. Three-dimensional (3D) data acquisition and creation of a virtual 3D head using the following procedures have made the 3D maxillofacial planning system an indispensable part of the modern orthognatic: Scanning patients in the natural head position (NHP) with cone-beam computed tomography (CBCT); accurate visualization of the interocclusal relationship in the 3D model of the patient's skull;[1] adding highly detailed texture information to the skin that is segmented out of the CBCT by using 3D photographs or mapping of traditional two-dimensional (2D) photographs; and virtual occlusion planning.[2] 3D virtual treatment planning allows a efficient preparation, which could improve the surgical outcomes and shorten the operation time.[3] The aim of this study was to quantitatively evaluate the accuracy of the 3D soft tissue predictions generated by a computer-aided maxillofacial planning system in patients undergoing orthognathic surgery.
MATERIALS AND METHODS
The study protocol was approved by the Local Ethics Committee (DIRSEC/EC/119, AZ Monica, Antwerp, Belgium). A total of 20 consecutive patients aged 13-42 years with dentofacial malformations, who underwent orthognathic surgery between mid-November 2008 and December 2009, were included in the study. All patients were white Caucasian man and women. Fourteen patients had an Angle Class II malocclusion (seven with vertical maxillary excess [VME], two with vertical asymmetry and one with an open bite); three patients had an Angle Class III malocclusion (two with horizontal asymmetry, one with horizontal and vertical asymmetry); and three patients had an Angle Class I malocclusion (one with bimaxillary sagittal hypoplasia and vertical asymmetry and one with horizontal asymmetry). Informed consent was obtained from the participating patients who met the study criteria [Table 1].
Table 1.
Study criteria

A full extended height CBCT (i-CATTM, Imaging Sciences International, Inc., Hatfield, USA) was used for the standard scanning protocol (field of view, 17 cm diameter/22 cm height; scan time, 2 s × 20 s; voxel size 0.4 mm). A multidimensional hard and soft tissue patient-specific virtual model was computed.[4] All procedures were planned in Maxilim (Medicim NV, Mechelen, Belgium). The bony surgery was virtually performed and transferred to the patients using computer-generated surgical splints.[5] A postoperative CBCT-scan was taken 4 months after surgery with the orthodontic brackets still in place. To minimize the effect of the head position on the intermaxillary relationship and the facial soft tissues, the following standard protocol was used during pre- and post-operative scanning procedures: The pre- and post-operative scans were taken with the patient in the NHP while gently biting on a very thin wax wafer in centric relation with the lips in a relaxed position. The NHP was obtained by asking the patient to stand up and look at his/her image in the mirror. The distance between the supra-sternal notch and the soft tissue pogonion was measured with a ruler. The possible cant of the head was corrected during the positioning of the patient in the CBCT device by using the provided laser lines. We called this distance the natural head distance (NHD) and made sure the NHD was respected during the pre- and post-operative scanning procedures [Figure 1].
Figure 1.

Patient positioned in natural head position (NHP). The NHP was respected during the pre- and post-operative scanning procedure
All the steps in the validation process were automated and incorporated in the software to eliminate human error. The entire validation process was executed by one single examiner (ED) and included the four steps described below.
Step 1: Standardized registration
To compare the preoperative simulation of the soft tissue changes and the postoperative data, both image data sets had to share the same spatial reference frame; therefore, a registration of the pre- and post-operative CBCT volumes was executed. This registration process was fully automated and incorporated in the software. It involved maximization of mutual information, a well-known registration algorithm.[3] The registration is applied on an unaltered sub-volume, manually defined as the region between the infraorbital rim and the rest of the scanned viscerocranium. After defining the registration parameters for this sub-volume, the transformation was applied to the whole postoperative dataset [Figure 2].
Figure 2.
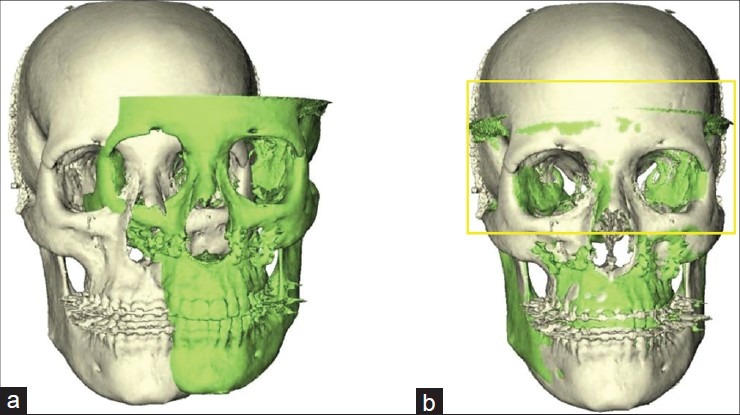
Three-dimensional reconstruction of the pre- (white) and post- (green) operative skull before (a) and after registration (b). The yellow frame indicates the selected registration area
Step 2: Automated adjustment of the bone-related planning
Because the validation of the accuracy of the soft tissue simulator was the aim of this study, the simulated planned procedure had to be the same that is performed by the surgeon. In the second stage of the validation procedure, the virtually osteotomised bone fragments were moved to the real postoperative position, using the rigid Iterative Closest Point algorithm to obtain the best alignment between the simulated and postoperative bone segments and to align the surfaces[5,6] [Figure 3].
Figure 3.
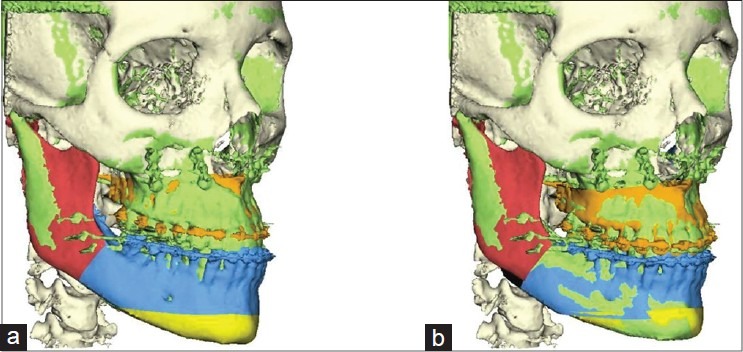
The rigid Iterative Closest Point algorithm was used to align the surfaces of osteotomised maxilla (brown) and the proximal segment of the mandible (blue) with the postoperative bony segments (green). (a) Before alignment and (b) after alignment. The white area shows the unaltered bony structures. The yellow colour shows the osteotomised chin
Step 3: Simulation of soft tissue changes
The soft tissue changes caused by the movement of the osteotomised bony segments, as derived in the previous step, were automatically simulated by the software [Figure 4]. The software uses a biomechanical simulation model to calculate these changes.[6] This biomechanical model uses no hard/soft tissue movement ratio, but directly mimics the 3D elastic deformation behavior of facial soft tissues.
Figure 4.
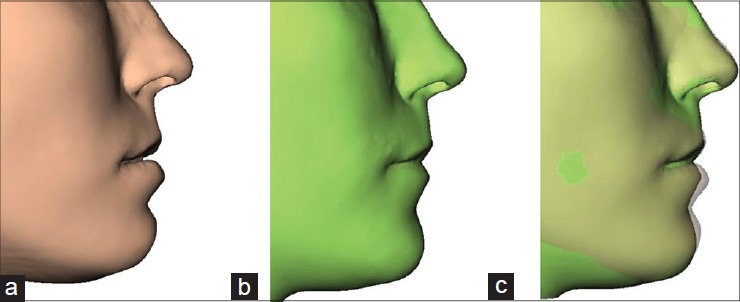
(a) Simulated soft tissue, (b) actual postoperative profile, and (c) overlap simulated-actual postoperative profile
Step 4: Calculation of differences
In the final stage of the validation process, the distances between the closest points of the simulated and the postoperative facial outlook were computed. In general, approximately 8000 points uniformly spread around the patient's face were used. The measured distances were visualized by projecting these distances onto the postoperative facial skin surface by means of a color code,[6] which resulted in an easily interpretable image of the error distribution over the facial skin surface [Figure 5]. The signed-distance was considered as positive value when the postoperative surface was in front of the predicted surface. The statistical properties for each distance map were calculated using MATLAB (MathWorks Inc., Natick, United States).
Figure 5.
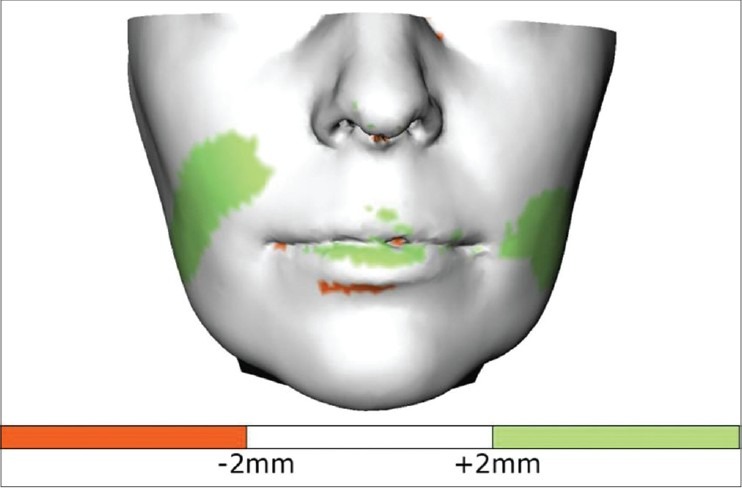
Distance map with the measured errors projected onto the postoperative facial skin surface. Green indicates under-simulated regions (postoperative in front of prediction)
To describe the distribution of the errors, the mean signed distance of the absolute value, the 25-75% distance range, and the 5-95% distance range were computed for each data set.
RESULTS
A total of 20 patients, of which 15 were females and 5 were males, between the age ranges between 13 and 42 years (mean age 23 years; SD 9) met the inclusion criteria. In 16 patients, a bimaxillary procedure was performed, in six patients, this was combined with genioplasty. Four patients underwent a bilateral sagittal osteotomy.
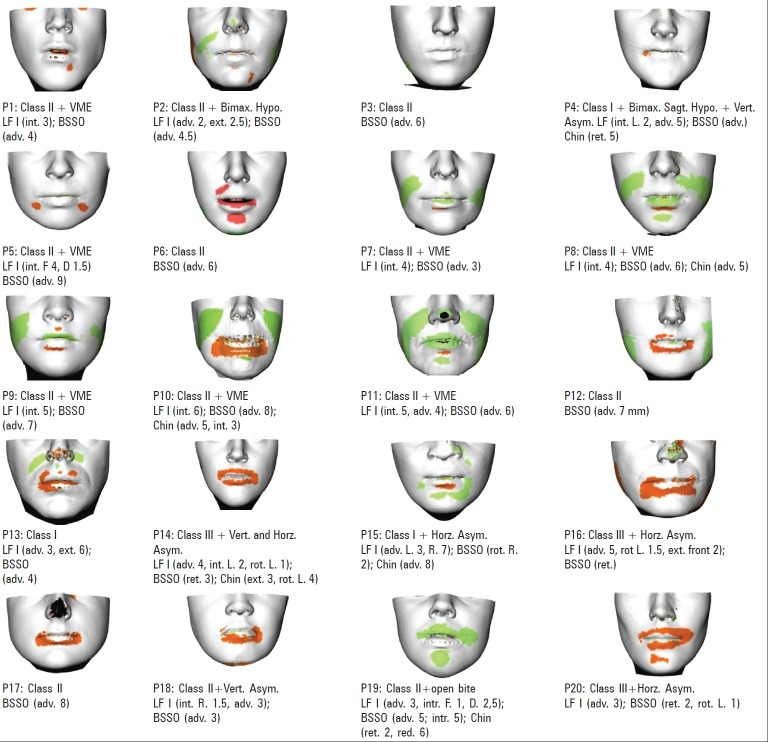
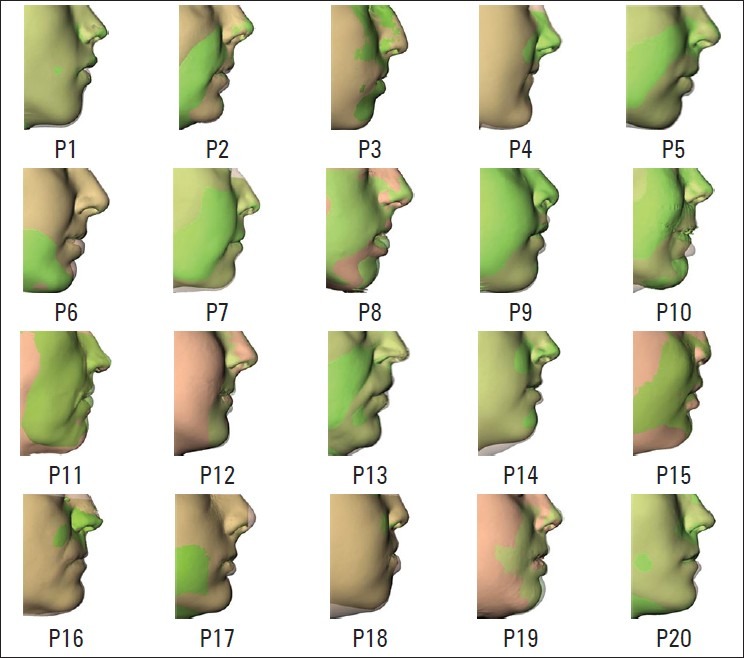
Averaging over all 20 patients, the mean absolute difference between the predicted and actual postoperative surface was 1.18 mm (minimum: 0, maximum: 2.67 mm). As shown in Figure 6, the boxplot presents the mean signed distance (red bar), the 25-75% distance range (blue box) and the 5-95% distance range (dotted lines) for each data set. A visual inspection of the predicted skin surfaces shows the areas of inaccuracy are concentrated around the lips and cheek [Figure 7]. The performed procedure and the amount of movements are also depicted. A visual analysis of the distance maps shows the inaccuracy in the prediction increased with the amount of bony displacement. The overlapped simulated and actual postoperative profiles are demonstrated in Figure 8.
Figure 6.
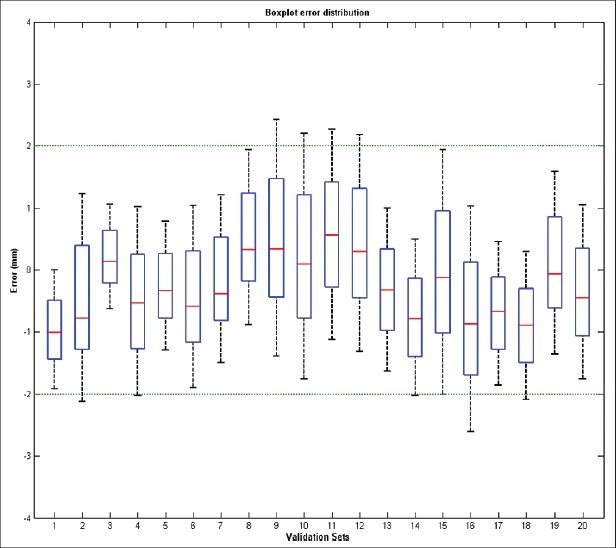
The boxplot presents the mean signed distance (red bar), the 25-75% distance range (blue box) and the 5-95% distance range (dotted lines) for each data set
Figure 7.
Color-coded distance maps for all patients. Green is −2 mm and red is +2 mm. The performed procedure is mentioned below each distance map. LF I: Le Fort I; BSSO: Bilateral sagittal split osteotomy; int.: Intrusion; ext.: Extrusion; adv.: Advancement; rot.: Rotation; L.: Left; R.: Right; VME: Vertical maxillary excess; Bimax.: Bimaxillary; Hypo.: Hypoplasia; Vert.: Vertical; Horz.: Horizontal; Asym.: Asymmetric
Figure 8.
The overlapped simulated (brown) and actualpostoperative (green) profiles
DISCUSSION
The success of a complex orthognathic surgical procedure is dependent on careful planning that can be reproduced in the operating room.[7] The common practice of 3D assisted orthognathic surgery involves planning for bony structures and predicting soft tissue changes. A few important questions arise from this practice: (1) How accurately can the computer assisted bone surgery be transferred to the patient? (2) How reliable is the soft tissue simulation? (3) Is this simulation reliable enough to enhance the original orthognathic planning? (4) Should we not proceed in a reverse manner: Making a patient specific “ideal” esthetic soft tissue contour and volume, then measure and predict the amount of bony movement we need to achieve the ideal?
The aim of this study was to answer the second and the third questions, while eliminating the first question by virtually ensuring the planned procedure is the same as that performed by the surgeon. The bone-related planning was adapted, so it resembled the performed surgery as much as possible. Smith et al. were the first to conduct a prospective study with 2D software's available at the time. They concluded that choosing a software is depending on many factors beside the simulation accuracy.[8]
The first study that systematically evaluated the accuracy of computer prediction programs was published recently by Kaipatur and Flores-Mir.[9] They concluded that the most significant source of error in prediction through the available computer prediction programs involved the lower lip area. The measurements in their selected studies were performed only in 2D, with horizontal and vertical mean measurements. The measurement on the same axis of two equal prediction errors in a different direction will neutralize each other and result in a 0 mean value that falsely presumes a perfect prediction result. In other words, the mean value should be taken from the absolute prediction error, as this would always be a positive value. In the study of Kaipatur and Flores-Mir,[9] some of the mean values of the prediction error were negative, which could indicate that this conceptual mistake has been made in several reviewed articles.
Xia et al.[10] have reported the accuracy of their computer-aided surgical simulation system in a pilot study, but failed to report the absolute mean value of the error in their results. The mean of the absolute error, as presented in our analysis, cannot be compared with that presented by Xia et al.
In this study, only areas that were altered during surgery were included in the error map. Including the complete skull would “falsely” improve our simulation results because nonaltered areas would closely match the “simulated” areas. A difference within 2 mm, between the predicted and actual postoperative soft tissue result, is considered clinically acceptable.[1] In our study, averaging over all 20 patients, the mean absolute difference between the predicted and actual postoperative surface was 1.18 mm, and 84% of the prediction errors were situated between −2 mm and +2 mm. The error smaller than −2 mm and higher than +2 mm (16% of the map distances) were mainly situated around the lips.
The visual evaluation of the color coded distance maps and the overlapped profile images for all patients showed the nasal and paranasal areas are better simulated than the areas around the lips (particularly the lower lip, P6, P8, P10, P11, P18). The problem of predicting the lip position has already been addressed in 2D studies.[8] They concluded that additional software tool was beneficial to provide the surgeon control of the lip posture. We suggest three main reasons why the lip movement is less accurately simulated. The first reason is due to the connection between lower and upper lip: The current soft tissue model cannot explicitly separate the lips from each other during simulation. This causes problems when the lips are in contact and not in a relaxed position during preoperative CT. Second, the lack of optimal definition of boundary conditions, such as sliding contact behavior between teeth and lips, may be another reason. Even when the lips are in a relaxed position during the preoperative CT, the upward movement, and inward rotation of the lower lip is not precisely simulated. Third, the lack of proper simulation of the adaptation of mental and orbicularis oris muscles after the harmonization of occlusion may negatively influence the results. In addition to the errors in the lower lip, another error region was observed in the cheek area. These were observed in intrusion cases in patients with moderate to severe anterior VME, where the impaction of the upper jaw exceeded 4 mm [the green bands on the distance maps of P7, P8, P9 and P10 in Figure 6]. Our hypothesis is that the soft tissues adjusted to the area of bony impaction are less cranially displaced as simulated by the biomechanical model used in this software. We hypothesize that what happens to the soft tissue surrounding the maxilla after impaction procedure depends on the suturing technique of the subcutaneous soft tissue and the elasticity of the overlaying cutaneous tissue. These factors cannot be incorporated in the current biomechanical model.
CONCLUSION
Despite the shortcomings explained above, the results of this study encourage the use of this software in all different combinations of orthognathic surgical procedures. However, we must realise that the prediction of soft tissue changes around the lips, is the fundamental shortcoming of the software that has been used in our study. Further intensive research is necessary to enhance the prediction results of the lower lip region and the cheek area where maxillary impaction of more than 4 mm is performed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Donatsky O, Bjørn-Jørgensen J, Holmqvist-Larsen M, Hillerup S. Computerized cephalometric evaluation of orthognathic surgical precision and stability in relation to maxillary superior repositioning combined with mandibular advancement or setback. J Oral Maxillofac Surg. 1997;55:1071–9. doi: 10.1016/s0278-2391(97)90282-2. [DOI] [PubMed] [Google Scholar]
- 2.Nadjmi N, Mollemans W, Daelemans A, Van Hemelen G, Schutyser F, Bergé S. Virtual occlusion in planning orthognathic surgical procedures. Int J Oral Maxillofac Surg. 2010;39:457–62. doi: 10.1016/j.ijom.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–98. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 4.Swennen GR, Mollemans W, De Clercq C, Abeloos J, Lamoral P, Lippens F, et al. A cone-beam computed tomography triple scan procedure to obtain a three-dimensional augmented virtual skull model appropriate for orthognathic surgery planning. J Craniofac Surg. 2009;20:297–307. doi: 10.1097/SCS.0b013e3181996803. [DOI] [PubMed] [Google Scholar]
- 5.Gateno J, Xia J, Teichgraeber JF, Rosen A, Hultgren B, Vadnais T. The precision of computer-generated surgical splints. J Oral Maxillofac Surg. 2003;61:814–7. doi: 10.1016/s0278-2391(03)00240-4. [DOI] [PubMed] [Google Scholar]
- 6.Mollemans W, Schutyser F, Nadjmi N, Maes F, Suetens P. Predicting soft tissue deformations for a maxillofacial surgery planning system: From computational strategies to a complete clinical validation. Med Image Anal. 2007;11:282–301. doi: 10.1016/j.media.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Xia J, Wang D, Samman N, Yeung RW, Tideman H. Computer-assisted three-dimensional surgical planning and simulation: 3D color facial model generation. Int J Oral Maxillofac Surg. 2000;29:2–10. [PubMed] [Google Scholar]
- 8.Smith JD, Thomas PM, Proffit WR. A comparison of current prediction imaging programs. Am J Orthod Dentofacial Orthop. 2004;125:527–36. doi: 10.1016/S0889540604001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaipatur NR, Flores-Mir C. Accuracy of computer programs in predicting orthognathic surgery soft tissue response. J Oral Maxillofac Surg. 2009;67:751–9. doi: 10.1016/j.joms.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Xia J, Samman N, Yeung RW, Wang D, Shen SG, Ip HH, et al. Computer-assisted three-dimensional surgical planing and simulation 3D soft tissue planning and prediction. Int J Oral Maxillofac Surg. 2000;29:250–8. [PubMed] [Google Scholar]