Abstract
Alveolar soft part sarcoma (ASPS) is a rare, aggressive malignancy of uncertain histological origin with propensity of vascular invasion and distant metastasis. ASPS demonstrates strong predilection for adolescents and young adults with a female preponderance. The head and neck region is the commonly affected region in the pediatric population with orbit and tongue being most common. The indolent clinical course and asymptomatic nature often leads to misdiagnosis and delayed treatment. Herein, we present a case of ASPS affecting the tongue in 14-year-old boy which clinically mimicked hemangioma, common benign vascular tumor of tongue.
Keywords: Alveolar soft part sarcoma, lingual, MyoD1 expression
INTRODUCTION
Alveolar soft part sarcoma (ASPS) is a rare, malignant neoplasm of uncertain histogenesis first coined by Christopherson et al. in 1952.[1] Its incidence have been reported to be <1% of all soft tissue neoplasms. ASPS may occur at any age, it has a strong predilection for adolescents and young adults between 15 and 35 years.[2,3] ASPS seen in infants and children younger than 15 years is considered rare with predilection for head and neck region, especially the orbit and tongue.[4] Herein, we present the clinical, histopathological and immunohistochemical (IHC) features of ASPS on the anterolateral aspect of the tongue in a 14-year-old boy.
CASE REPORT
A 14-year-old boy presented with a complaint of swelling on the dorsum of the tongue since 1 year. Gradual enlargement associated with discomfort in chewing and speaking made him consult the physician. It was not associated with any bleeding episodes. However, patient had noticed an increase in thickness of the tongue [Figure 1]. Clinical examination revealed a swelling on the dorsum of the tongue that had slight bluish appearance and on palpation was soft, nontender and immobile. The tumor measured approximately 4 × 3 cm. There was no palpable cervical lymph node enlargement. The routine hemogram and the preoperative profile were within normal limits. Clinical diagnosis of hemangioma was made.
Figure 1.
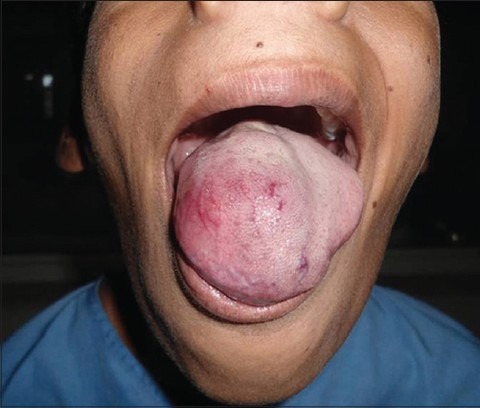
Nodular swelling on the dorsal aspect of tongue
The lesion was completely excised under general anesthesia, and cut margins were approximated with 4-0 vicryl and hemostasis was achieved [Figures 2 and 3]. Specimen was received in surgical pathology section. Grossly, tumor was nodular, firm mass and on cut section it appeared as solid grey white. Histopathological examination revealed hyperplastic mucosa with underlying circumscribed tumor composed of round to polygonal cells arranged in the alveolar pattern. Tumor cells displayed vesicular nuclei with prominent nucleoli and abundant eosinophilic granular cytoplasm [Figures 4 and 5]. Skeletal muscle was seen at the periphery of the tumor showing infiltration by the tumor cells. Few mitotic figures were also seen. Diagnosis of ASPS was made. IHC and special stains were carried out for confirmation of the diagnosis. On periodic acid Schiff (PAS) stain, characteristic PAS-positive, diastase-resistant granular material was found and was consistent with a diagnosis of ASPS [Figure 6]. IHC results were negative for S-100, and positive for cytoplasmic myoblast determination protein 1 (MyoD1) confirming the diagnosis of ASPS [Figure 7]. Patient had uneventful postoperative period and was advised adjuvant chemotherapy (injection epirubicin and injection adriamycin), however patient refused any further management.
Figure 2.
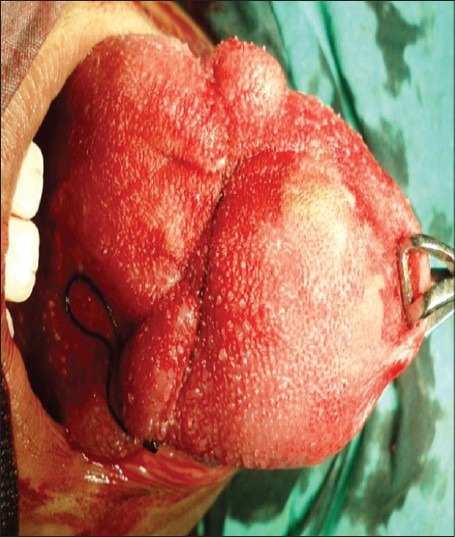
Circumferential continuous interlocking hemostatic suture around the mass
Figure 3.
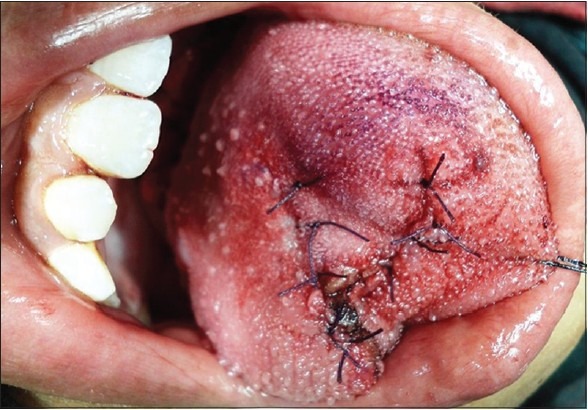
Post excision photograph showing the approximated edges of the tongue
Figure 4.
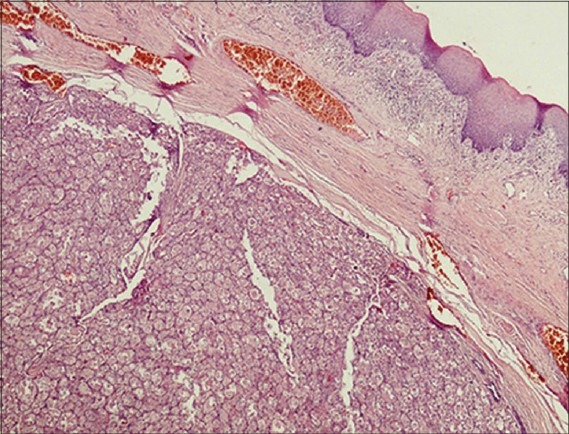
Scanner view showing circumscribed tumor cells arranged in alveolar pattern
Figure 5.
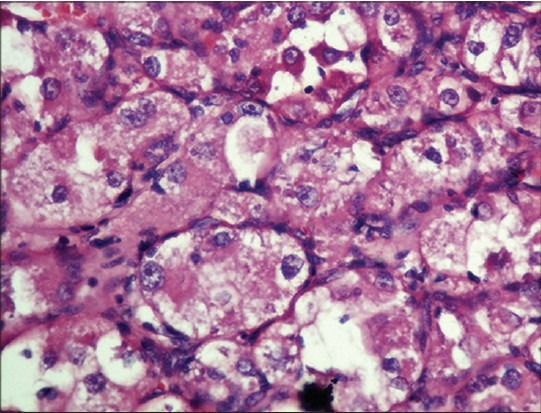
Tumor cells displaying vesicular nuclei with prominent nucleoli and eosinophilic granular cytoplasm (H and E, ×400)
Figure 6.
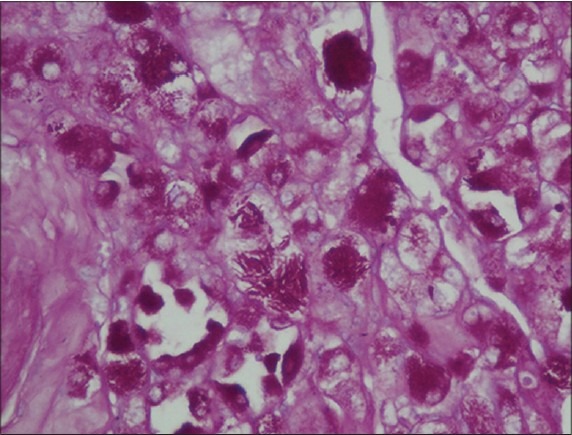
Periodic acid Schiff stain demonstrates cytoplasmic eosinophilic granules in tumor cells
Figure 7.
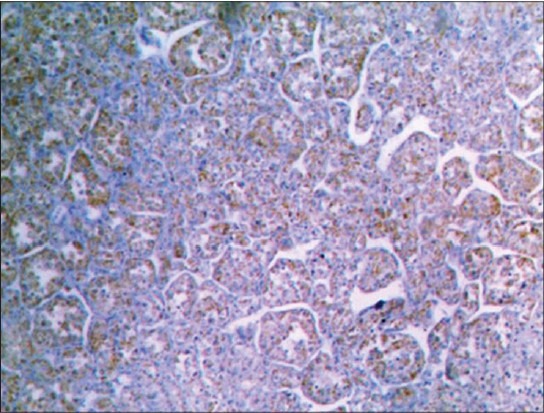
Immunohistochemical stain demonstrates myoblast determination protein 1 positivity in cytoplasm of tumor cells
DISCUSSION
Alveolar soft part sarcoma of tongue is a rare variety of Sarcoma affecting individuals in first two decades of life. Various terminologies such as malignant organoid granular cell myoblastoma, angioendothelioma, and liposarcoma were used to represent the same condition. ASPS was originally considered as malignant variant of granular cell myoblastoma and subsequently disproved by several ultrastructural and IHC studies by denoting the absence of neuroendocrine differentiation in ASPS.[5] Although the histogenesis remains controversial, IHC stains indicate a myogenic origin. The tumors common occurrence in skeletal muscle and desmin reactivity support myogenin origin. Although recent studies have been unable to confirm MyoD1 expression or myogenin reactivity, this may be attributed to its derivation from myoid precursors with lack of differentiation.[6] However, demonstration of PAS-positive diastase-resistant crystals with appropriate clinical history confirms the diagnosis.[5] Masson was first to describe and depict these crystals as the diagnostic features of ASPS. The typical crystalline material is present in at least 80% of the tumors, in the remainder they are PAS-positive granules, probably precursors of crystals.[7]
Alveolar soft part sarcoma has a close clinical and imaging resemblance to common benign vascular tumors such as hemangioma, which may lead to misdiagnosis and delayed treatment and tumor may metastasize to the lungs, brain or bones. The term hemangioma is restricted to the classic involuting vascular lesion of infancy. Vascular malformation is present at birth, grow in proportion to patient growth, and do not involute. Rarely, they may spontaneously enlarge through the development of arteriovenous fistulas, thrombosis, or ectasia. In contrast, ASPS is not present at birth, grows rapidly and occur in an older age group. ASPS has a blue coloration on gross examination, which suggest a vascular nature, and mimics venous malformation.[3] Patient with ASPS of tongue experience dysphagia, dysphonia, or mild discomfort. In the present case, patient had similar clinical symptoms. However there was no overlying ulceration or bleeding. Clinical examination also revealed pulsation with thrill. Computerized tomography (CT) and magnetic resonance imaging (MRI) mainly help to decide tumor-free resection margins. Aiken and Stone reported a case of ASPS on tongue, which mimicked features of hemangioma on both CT and MRIs.[3] They suggested that the presence of a hyperintense lesion with central necrosis and flow voids within the lesion on T1- and T2-weighted MRI will support the diagnosis of ASPS.
Alveolar soft part sarcoma can also be differentiated from other histologically similar malignant neoplasm in its cytological uniformity, lack of nuclear atypia, and paucity of mitotic figures and contain PAS-positive, diastase-resistant cytoplasmic inclusions that are thought to consist of actin. The differential diagnosis of ASPS includes granular cell tumor, paraganglioma, metastatic renal carcinoma (RCC), alveolar rhabdomyosarcoma (ARMS). Cells in granular cell tumor are less well defined, have a distinctly granular cytoplasm and show strong S-100 positivity. Primary or metastatic RCC has a striking similarity to ASPS. However absence of PAS-positive crystalline material and epithelial membrane antigen (EMA) positivity helps in confirming diagnosis of RCC. ARMS is sometime confused more because of the similarity in name than in microscopic picture. Paragangliomas show nested zellballen pattern and are positive for neuroendocrine markers whereas S-100 highlight the supporting S-100 supporting sustentacular cells.[7]
Numerous IHC studies have attempted to elucidate the histogenesis of this tumor, often results remain contradictory. The cells do not stain with cytokeratin, EMA, Neurofilament, glial fibrillary acidic protein, Serotonin or synaptophysin; occasionally express S-100 and neuron specific enolase.[7] There have been inconsistent reports on the detection of nuclear MyoD1 in ASPS, and many reports have shown positive cytoplasmic staining of MyoD1, a finding that has been rationalized as occurrence of cross reactivity with undetermined cytoplasmic antigen.[8] ASPS is, usually, negative for myogenin. In present case cytoplasmic MyoD1 reactivity was seen [Figure 7].
Aggressive surgical excision of the primary tumor is the recommended treatment option to prevent local recurrence.[9] The role of adjuvant chemotherapy is inconclusive. Radiotherapy remains to be established for primary tumor management but is recommended for residual lesions after surgical excision and for metastatic conditions.[10] Prognosis of ASPS depends mainly on clinical features such as location of the primary lesion, size of the tumor, and age of the patient. In contrast to ASPS in other parts of the body, lingual ASPS have a rather good prognosis, particularly in young children.
CONCLUSION
Alveolar soft part sarcoma of tongue is a rare tumor which occurs in infants and children, usually in head and neck region with orbital and lingual sites being commonest locations. To our best knowledge, very few cases of lingual ASPS have been reported from India. ASPS should be considered as a differential diagnosis of lingual soft tissue mass in young children due to their tendency for early metastasis. Histopathological examination and special stains help in confirming the diagnosis of ASPS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Christopherson WM, Foote FW, Jr, Stewart FW. Alveolar soft-part sarcomas; structurally characteristic tumors of uncertain histogenesis. Cancer. 1952;5:100–11. doi: 10.1002/1097-0142(195201)5:1<100::aid-cncr2820050112>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Ordóñez NG, Mackay B. Alveolar soft-part sarcoma: A review of the pathology and histogenesis. Ultrastruct Pathol. 1998;22:275–92. doi: 10.3109/01913129809103349. [DOI] [PubMed] [Google Scholar]
- 3.Aiken AH, Stone JA. Alveolar soft-part sarcoma of the tongue. AJNR Am J Neuroradiol. 2003;24:1156–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Velasco A, Fermán-Cano F, Cerecedo-Díaz F. Rare tumor of the tongue in a child: Alveolar soft part sarcoma. Pediatr Dev Pathol. 2009;12:147–51. doi: 10.2350/07-07-0317.1. [DOI] [PubMed] [Google Scholar]
- 5.Ordóñez NG. Alveolar soft part sarcoma: A review and update. Adv Anat Pathol. 1999;6:125–39. doi: 10.1097/00125480-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hunter BC, Devaney KO, Ferlito A, Rinaldo A. Alveolar soft part sarcoma of the head and neck region. Ann Otol Rhinol Laryngol. 1998;107:810–4. doi: 10.1177/000348949810700914. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tumor. 5th ed. Philadelphia, USA: Mosby Elsevier; 2008. pp. 1182–9. [Google Scholar]
- 8.Gómez JA, Amin MB, Ro JY, Linden MD, Lee MW, Zarbo RJ. Immunohistochemical profile of myogenin and MyoD1 does not support skeletal muscle lineage in alveolar soft part sarcoma. Arch Pathol Lab Med. 1999;123:503–7. doi: 10.5858/1999-123-0503-IPOMAM. [DOI] [PubMed] [Google Scholar]
- 9.Ryu J, Kwon Y, Park BK, Jung YS. Lingual alveolar soft part sarcoma treated only by conservative resection. Int J Pediatr Otorhinolaryngol Extra. 2006;1:243–8. [Google Scholar]
- 10.Donald PJ. Alveolar soft part sarcoma of the tongue. Head Neck Surg. 1987;9:172–8. doi: 10.1002/hed.2890090308. [DOI] [PubMed] [Google Scholar]


