Abstract
The white rot fungi Stereum ostrea displayed a wide diversity in their response to supplemented inducers, surfactants, and copper sulphate in solid state fermentation. Among the inducers tested, 0.02% veratryl alcohol increased the ligninolytic enzyme production to a significant extent. The addition of copper sulphate at 300 μM concentration has a positive effect on laccase production increasing its activity by 2 times compared to control. Among the surfactants, Tween 20, Tween 80, and Triton X 100, tested in the studies, Tween 80 stimulated the production of ligninolytic enzymes. Biosorption of dyes was carried out by using two lignocellulosic wastes, rice bran and wheat bran, in 50 ppm of remazol brilliant blue and remazol brilliant violet 5R dyes. These dye adsorbed lignocelluloses were then utilized for the production of ligninolytic enzymes in solid state mode. The two dye adsorbed lignocelluloses enhanced the production of laccase and manganese peroxidase but not lignin peroxidase.
1. Introduction
Most textile industries produced a large amount of wastewater with the excessive colour. Most of textile waste water contained approximately 20–200 mg/L of dye that could degrade the water quality [1]. White rot fungi are well known for their outstanding decolourization ability of synthetic dyes mediated by their oxidative ligninolytic complex [2], lignin peroxidase (LiP), manganese dependent peroxidase (MnP), and a family of multicopper oxidases, namely, laccases (Lcc).
Ligninolytic enzymes have a potential in several industrial and biotechnological processes [3, 4] including delignification of lignocellulosic biomass for fuel (ethanol) production; food, brewery, and wine; animal feed; denim stone washing; laundry detergents; paper and pulp industries; and bioremediation of chemical pollutants [5, 6]. Due to the potential applications of these enzymes, research in this area is oriented towards the search for efficient production systems. Reducing the cost of enzyme production by using cheaper raw materials and optimizing the fermentation process for industrial purposes is the ultimate target of basic research [7, 8]. A good strategy for this purpose is the production of these enzymes by solid state fermentation (ssf) technique using agroindustrial wastes as a support substrate. Most of such wastes are rich in soluble carbohydrates and also contain inducers of laccase synthesis, ensuring an efficient production of these enzymes [9]. SSF processes have shown to be particularly suitable for the production of enzymes by filamentous fungi, since they reproduce the natural living conditions of such fungi due to which they may be more capable of producing certain enzymes with high productivity in comparison to submerged fermentation [10].
Because of the diverse applications of ligninolytic enzymes in industrial processes, there is a wide interest in the induction, enhancement, and stabilization of these enzymes. The production of ligninolytic enzymes can be stimulated by the presence of a wide variety of inducing substrates mainly aromatic or phenolic compounds related to lignin or lignin derivatives such as ferulic acid, 2,5-xylidine, and veratryl alcohol [11]. Copper as a micronutrient has a key role as a metal activator, induces both laccase transcription, and plays an important role in laccase production [12]. Surfactants can stimulate the growth of spores and increase the bioavailability of less soluble substrates for the fungus thereby increasing the production of enzymes [13]. Hence the effect of these three types of compounds on enzymes was studied to evaluate their importance in getting maximum yields of ligninolytic enzymes.
As stated earlier, these enzymes are capable of decolorizing a variety of synthetic dyes. Effective decolorization of dyes is achieved by the integration of two methods—biosorption and biodecolorization. Biosorption by lignocelluloses may be an alternative method for removing dyes from effluents. Lignocellulosic biomass has potential of biosorption of various textile dyes. These dye adsorbed lignocelluloses could be utilized for the production of ligninolytic enzymes in solid state mode. The combination of biosorption and ssf of dye adsorbed lignocelluloses creates an effective method for dye removal and enzyme production.
2. Materials and Methods
The fungus was kindly supplied by Professor M. A. Singaracharya, Department of Microbiology, Kakatiya University, Andhra Pradesh, India, and was isolated from wood logs. The isolate was maintained at 4°C on 2% Koroljova-Skorobogat'ko medium [14] because of good growth. The maintenance medium was prepared according to Koroljova-Skorobogat'ko et al., (1998) containing the following composition (g/L): 3.0 peptone, 10.0 glucose, 0.6 KH2PO4, 0.001 ZnSO4, 0.4 K2HPO4, 0.0005 FeSO4, 0.05 MnSO4, 0.5 MgSO4, and 20.0 agar (pH 5.5).
2.1. Influence of Different Compounds on Enzyme Production
Duplicate flasks containing 5 g of wheat bran moistened with Koroljova medium (70% w/v) were used as production medium to carry out the following experiments.
(i) Different inducers were screened to get higher enzyme production from the culture of S. ostrea. Inducers like guaiacol (0.02%), veratryl alcohol (0.02%), lignin (0.1%), lignosulfonic acid (0.1%), gallic acid (0.02%), and tannic acid (0.05%) were amended in the production medium and sterilized. (ii) To find out the suitable concentration of copper sulphate for the maximum production of laccase, different concentrations of CuSO4, 30, 50, 100, 300, 500, and 1000 μM, were added to the production medium and sterilized. (iii) To study the influence of surfactants, 1 mL of different surfactants like Tween 20, Tween 80, and Triton X-100 was added at 1% conc. to the sterilized medium at the time of inoculation.
All the above flasks were aseptically inoculated with 12 mycelial plugs (7 mm) of 7-day-old culture and incubated at 30°C for a period of 12 days. Enzymes were extracted by adding 25 mL of phosphate buffer (pH 7.0; 100 mM) to each culture flask and kept on a temperature controlled gyratory shaker (ORBITEK-Chennai, India) (180 rpm) at 30°C for 1 hour. The mixtures were filtered through a sterile cotton cloth and the filtrate obtained was centrifuged (REMI C-24 BL) at 10,000 rpm at 4°C for 20 min. The supernatant obtained was analyzed for enzyme activities and extracellular proteins.
2.2. Biosorption and Biodecolorization of Dyes
Remazol brilliant blue (RBB) and remazol brilliant violet 5R (RBV) purchased from Sigma were used in the present study. 5 g of wheat bran/rice bran (RB/WB) and 100 mL of each dye (50 ppm) were taken into separate flasks and incubated at 30°C and 150 rpm for 30 min. After incubation period the suspensions were centrifuged at 5000 rpm for 15 min and then the supernatant solutions were analyzed for adsorption of dyes by monitoring the absorbencies at their λ max and adsorption was expressed in terms of percentage compared with control. For solid state fermentation studies Erlenmeyer flasks containing 5 g of dye adsorbed lignocelluloses moistened with Koroljova broth (70%) were sterilized and inoculated with 15 mycelial plugs (7 mm) and incubated for 15 days at 30°C under static conditions. Extraction of enzymes was carried out as mentioned above.
2.3. Enzyme Assay
Laccase activity was assayed using 0.4 mL 10 mM guaiacol in 10% (V/V) acetone containing 1.2 mL 100 mM acetate buffer (pH 5.0) and 0.4 mL enzyme source with appropriate dilution and monitored at 470 nm (ε = 6740 M−1 cm−1) [15]. Lignin peroxidase activity was determined by oxidation of veratryl alcohol in tartrate buffer (pH 2.5) at 310 nm (ε = 9,300 M−1 cm−1) [16]. MnP activity was assayed using a reaction mixture containing 1 mM guaiacol, 10 mM citrate phosphate buffer (pH 5.5), 1 mM MnSO4, and 50 μM H2O2 at 465 nm [17]. Enzyme activities were expressed in International Units (IU) where one unit corresponded to the amount of enzyme that oxidized one micromole of substrate per minute.
2.4. Protein Estimation
An aliquot of culture filtrate of S. ostrea with appropriate dilution was used for estimation of soluble protein content according to the Lowry et al. [18]. Bovine serum albumin was used as protein standard.
2.5. Statistical Analysis
All the experimental data given in the results were means of triplicates and followed Duncan's new multiple range (DMR) test to find significant difference (P ≤ 0.05) between values of each sampling [19].
3. Results
All the flasks with growing cultures of S. ostrea were withdrawn on alternate days of incubation for measurement of extracellular protein content and enzyme activities in the culture filtrate. Different inducers were screened to get higher enzyme activity from the culture of S. ostrea. The inducers included in the present study had diverse effects on enzyme production. Veratryl alcohol at 0.02% exerted maximum inductive effect on the production of three enzymes. Laccase activity is increased by 1.9 times (32,675 U/g of dry substrate) compared to control (17,153 U/g of dry substrate) and an increase of 50% was noted in MnP and LiP production in veratryl alcohol provided flasks. Guaiacol also stimulated the Lcc production by 1.72 times (29,583 U/g of dry substrate) and MnP by 1.4 times (5984 U/g of dry substrate) and has no influence on LiP production. Gallic acid does not have much effect on the production of three enzymes. The remaining three inducers lignin, lignosulfonic acid, and tannic acid had a toxic effect on laccase production. This may be due to the high concentration used in the study. Lignin stimulated LiP by 1.49 times (318.7 U/g of dry substrate) and lignosulfonic acid stimulated MnP by 1.4 times (5,861 U/g of dry substrate) (Table 1).
Table 1.
Effect of different inducers on ligninolytic enzyme production.
| Inducers |
Enzymes U/g of dry substrate |
Incubation period in days | |||||
|---|---|---|---|---|---|---|---|
| II | IV | VI | VIII | X | XII | ||
| Gallic acid (0.02%) | Lcc | 492d | 957b | 15,655e | 18,365f | 6,182d | 3066c |
| MnP | 126b | 384b | 2,164d | 4,586f | 2,264e | 1,062c | |
| LiP | 15.3a | 36.3b | 49.5c | 159.0f | 118.8e | 52.3d | |
|
| |||||||
| Tannic acid (0.05%) | Lcc | 400b | 858b | 10,870e | 12,152f | 4,432d | 1,130c |
| MnP | 93a | 260b | 1,365d | 3,563f | 1,561e | 462c | |
| LiP | 10.8a | 20.9b | 143.2d | 205.4e | 55.3c | 21.1b | |
|
| |||||||
| Guaiacol (0.02%) | Lcc | 599e | 1,571b | 19,895e | 29,583f | 9,238d | 1,828c |
| MnP | 182d | 343b | 3,961e | 5,984f | 2,982d | 982c | |
| LiP | 11.8a | 46.2b | 65.1c | 189.1e | 223.9f | 119.8d | |
|
| |||||||
| Veratryl alcohol (0.02%) | Lcc | 562a | 2,578b | 21,565e | 32,675f | 12,190d | 1,212c |
| MnP | 133bc | 390b | 3,183e | 6,273f | 2,164d | 563c | |
| LiP | 12.0a | 40.5b | 179.5e | 309.7f | 155.8d | 49.8c | |
|
| |||||||
| Lignin (0.1%) | Lcc | 462c | 1,096b | 8,401e | 10,355f | 3,397d | 1,158c |
| MnP | 119ab | 315b | 1,292d | 3,180f | 1,385e | 655c | |
| LiP | 13.2a | 42.2c | 152.3d | 318.7e | 43.4c | 39.2b | |
|
| |||||||
| Lignosulfonic acid (0.1%) | Lcc | 400b | 984b | 8,930e | 10,186f | 3,954d | 1,074c |
| MnP | 109a | 297b | 2,256c | 5,861e | 2,952d | 287b | |
| LiP | 12.4a | 31.6b | 122.8e | 198.4f | 69.1d | 42.9c | |
|
| |||||||
| Control | Lcc | 287a | 1,138b | 10,152d | 13,735e | 17,153f | 3,941c |
| MnP | 99a | 287b | 1,961c | 2,986d | 4,161f | 2,162e | |
| LiP | 12.5a | 37.2b | 56.7c | 95.3d | 211.2e | 212.8f | |
Values are the means of duplicates.
Means, in each column, followed by same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
Gallic acid caused maximum secretion of protein by S. ostrea followed by veratryl alcohol, guaiacol, and lignosulfonic acid. Low secretion of protein was observed in lignin and tannic acid amended medium compared to control (Figure 1).
Figure 1.
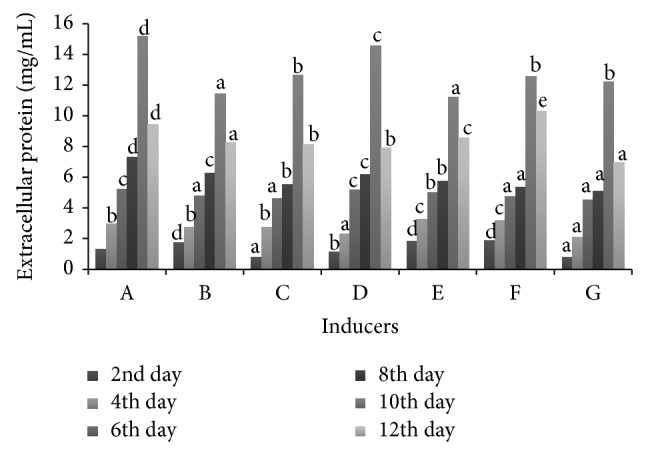
Effect of inducers on secretion of extracellular proteins. A: gallic acid, B: tannic acid, C: guaiacol, D: veratryl alcohol, E: lignin, F: lignosulfonic acid, and G: control. Values are the means of duplicates. Means, in each column, followed by same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
The effect of copper on Lcc production was determined by growing culture on wheat bran amended with copper sulphate at different concentration, namely, 30, 50, 100, 300, 500, and 1000 μM. The time course of solid state cultures supplemented with different amounts of copper is shown in Figure 2.
Figure 2.
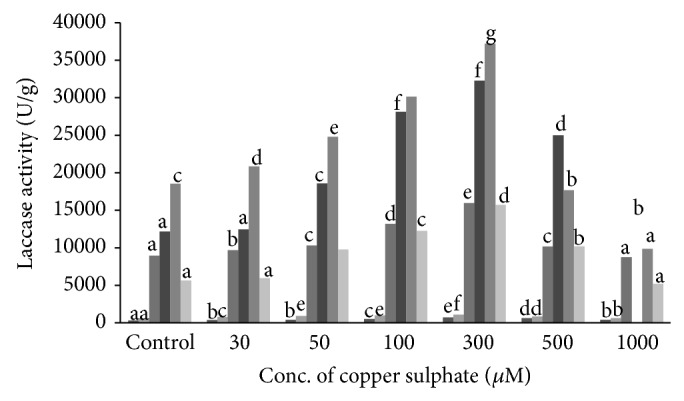
Influence of copper sulphate on laccase production. Values are the means of duplicates. Means, in each column, followed by same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
The increasing concentration of CuSO4 from 30 to 300 μM increased Lcc production. Maximum Lcc activity of 37,182 U/g of dry substrate was obtained on 10th day of incubation. At the same time, control flask (without CuSO4) showed Lcc activity of 18,535 U/g of dry substrate which was about 2 times lower than the flask supplemented with 300 μM CuSO4. The results clearly show the positive effect of copper sulphate on Lcc production. However when the concentration of copper was increased from 300 to 1000 μM significant decrease in Lcc production was observed. This may be attributed to the inhibitory effect of copper at higher concentrations. Whatever trend observed on Lcc production was also noticed on the influence of CuSO4 on secretion of extracellular protein. Maximum extracellular protein of 18.3 mg/mL was released by S. ostrea into the medium containing 300 μM CuSO4 on 10th day of incubation (Figure 3).
Figure 3.
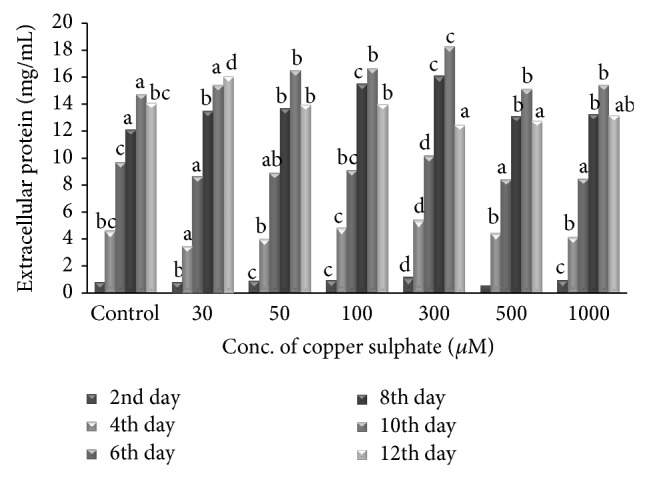
Effect of copper sulphate on secretion of extracellular proteins. Values are the means of duplicates. Means, in each column, followed by same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
The present study determined the effect of different surfactants on enzyme production. Provision of 1 mL of 1% Tween 80 favored maximum production of ligninolytic enzymes by S. ostrea. It exhibited maximum Lcc and MnP activities of 25,109 U/g and 6,303 U/g, respectively, on 10th day of incubation and LiP 252.5 U/g on 12th day of incubation. All the surfactants that are provided in the medium stimulated the production of Lcc and MnP in the order of Tween 80, Tween 20, and Triton X-100. But LiP activity was not enhanced with the addition of Tween 20 and Triton X-100 (Table 2).
Table 2.
Effect of surfactants on ligninolytic enzyme production.
| Surfactant (1%, 1 mL) |
Enzymes U/g of dry substrate |
Incubation period in days | |||||
|---|---|---|---|---|---|---|---|
| II | IV | VI | VIII | X | XII | ||
| Tween 80 | Lcc | 574a | 2,285c | 9,183c | 17,561d | 25,109c | 20,185d |
| MnP | 316c | 1,231c | 2,080bc | 4,084d | 6,303c | 3,868b | |
| LiP | 34.6c | 56.3b | 92.6c | 113.5d | 244.9d | 252.5d | |
|
| |||||||
| Tween 20 | Lcc | 690c | 2,773d | 9,859d | 14,805c | 19,667b | 16,985b |
| MnP | 297b | 1,505d | 2,235c | 3,865c | 5,692bc | 5,115c | |
| LiP | 77.3d | 101.5c | 132.6d | 180.0f | 67.9a | 63.7b | |
|
| |||||||
| Triton X- 100 | Lcc | 634b | 1,325a | 7,694b | 10,685a | 20,786b | 18,993c |
| MnP | 315c | 621a | 1,985a | 3,331b | 5,115b | 3,986b | |
| LiP | 20.5b | 35.3ab | 62.3b | 92.6d | 96.4b | 154.0c | |
|
| |||||||
| Control | Lcc | 571a | 1,524b | 6,377a | 12,661b | 17,189a | 12,159a |
| MnP | 266a | 958b | 1,962a | 2,834a | 3,631a | 3,205a | |
| LiP | 10.5a | 23.5a | 34.3a | 126.5e | 144.7c | 40.3a | |
Values are the means of duplicates.
Means, in each column, followed by the same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
Maximum extracellular protein with 21.06 mg/mL was recovered from S. ostrea grown on Triton X-100 amended medium on 8th day of incubation followed by Tween 80 (19.36 mg/mL) and Tween 20 (18.96 mg/mL) on 10th day of incubation (Figure 4).
Figure 4.
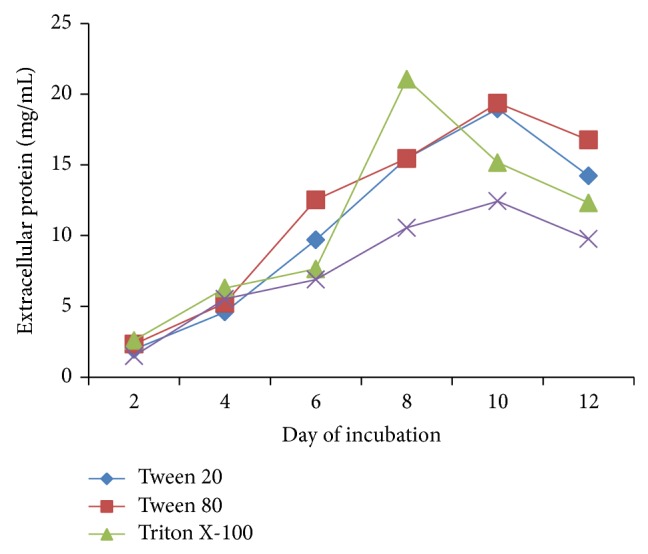
Effect of surfactants on secretion of extracellular protein. Values are the means of duplicates. Means, in each column, followed by same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
To evaluate optimum volume of Tween 80 required for maximum production of these enzymes different volumes, that is, 0.25, 0.5, 0.75, 1.0, 1.5, and 2.0 mL of Tween 80, were added to the production medium. Enhanced production of Lcc and MnP was observed in the flasks supplemented with 1 mL of Tween 80. 27,055 U/g of Lcc activity and 5,646 U/g of MnP activity were recorded on 10th day of incubation. Maximum production of LiP 302.9 U/g was noted in 1.5 mL Tween 80 supplemented flasks on the same day of incubation Table 3.
Table 3.
Effect of different volumes of Tween 80 on ligninolytic enzyme production.
| Volume of 1% Tween 80 |
Enzymes U/g of dry substrate |
Incubation period in days | |||||
|---|---|---|---|---|---|---|---|
| II | IV | VI | VIII | X | XII | ||
| 0.25 mL | Lcc | 300a | 701e | 10,275b | 12,907b | 20,462b | 19,338b |
| MnP | 98a | 273b | 3,227ab | 3,936a | 4,126a | 3,165a | |
| LiP | 28.9b | 63.4d | 138.3d | 162.8e | 240.8d | 57.6c | |
|
| |||||||
| 0.5 mL | Lcc | 301a | 920f | 10,653b | 14,571c | 23,228f | 20,636c |
| MnP | 112ab | 356c | 3,433b | 4,160ab | 5,351b | 3,563b | |
| LiP | 19.5ab | 50.7c | 70.0b | 233.6e | 155.8bc | 110.4d | |
|
| |||||||
| 0.75 mL | Lcc | 361c | 715e | 12,834d | 14,632c | 26,361d | 21,162c |
| MnP | 121b | 274b | 3,861bc | 4,360bc | 5,620c | 3,628b | |
| LiP | 18.4ab | 40.6b | 54.8a | 86.1b | 141.2b | 52.0c | |
|
| |||||||
| 1 mL | Lcc | 350c | 618d | 12,261d | 14,930c | 27,055e | 21,362cd |
| MnP | 132b | 228ab | 3,636bc | 4,937c | 5,646c | 2,650a | |
| LiP | 22.6b | 39.6b | 184.7e | 255.5f | 162.1c | 42.4b | |
|
| |||||||
| 1.5 mL | Lcc | 325b | 574c | 13,191e | 13,335bc | 25,943d | 20,652c |
| MnP | 122b | 209a | 4,124c | 4,824c | 5,602bc | 3,632b | |
| LiP | 11.2a | 20.5a | 94.5c | 105.2c | 302.9e | 150.1e | |
|
| |||||||
| 2 mL | Lcc | 365c | 524b | 11,196c | 12,461b | 23,532c | 20,009bc |
| MnP | 105a | 196a | 3,915c | 4,735b | 5,272b | 3,133ab | |
| LiP | 56.8c | 130.3e | 265.4f | 55.3a | 26.9a | 27.5a | |
|
| |||||||
| Control | Lcc | 293a | 496a | 8,641a | 10,336a | 18,334a | 15,414a |
| MnP | 93a | 189a | 2,855a | 3,583a | 4,654ab | 2,935ab | |
| LiP | 11.3a | 27.2a | 55.4a | 109.5c | 125.2b | 180.6f | |
Values are the means of duplicates.
Means, in each column, followed by the same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
Maximum extracellular protein with 18.86 mg/mL was recovered from the 1.5 mL amended medium by Stereum ostrea on 10th day of incubation followed by 18.63 mg/mL protein content in the 1 mL Tween 80 supplied medium on the same day of incubation (Table 4).
Table 4.
Effect of different volumes of Tween 80 on secretion of extracellular proteins.
| Volume of Tween 80 (mL) | Extracellular protein (mg/mL) | |||||
|---|---|---|---|---|---|---|
| II | IV | VI | VIII | X | XII | |
| 0.25 | 1.83e | 2.86b | 6.93b | 12.70a | 16.60a | 13.86b |
| 0.5 | 1.62d | 2.90b | 6.96b | 13.93b | 17.20ab | 14.10bc |
| 0.75 | 0.86a | 3.64c | 7.13c | 14.00b | 18.50c | 13.42b |
| 1.0 | 0.94a | 3.6c | 6.9b | 14.52c | 18.63c | 12.56a |
| 1.5 | 1.10b | 3.73c | 7.50d | 13.20ab | 18.86d | 15.26d |
| 2 | 1.40c | 2.60a | 6.66a | 12.60a | 16.26a | 12.80a |
Values are the means of duplicates.
Means, in each column, followed by the same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
Dye adsorption abilities of two lignocelluloses, rice bran and wheat bran, were tested by incubating them with two dyes RBB and RBV-5R. The RBB dye adsorbed after 30 min contact time was 80% (40 mg) on wheat bran and 73% (36.5 g) on rice bran whereas 77% (38.5 mg) and 69% (34.5 mg) of RBV-5R were adsorbed onto wheat and rice bran, respectively. The dye adsorbed lignocelluloses were used as growth substrates for enzyme production by S. ostrea in solid state fermentation. Both of the dye adsorbed lignocelluloses stimulated the production of laccase. RBV-5R dye adsorbed wheat bran produced the highest laccase of 24,962 U/g on 8th day of incubation compared to control of 13,796 U/g followed by RBV adsorbed rice bran—24,258 U/g (Table 5).
Table 5.
Effect of dye adsorbed lignocelluloses on ligninolytic enzyme production.
| Lignocelluloses |
Enzymes U/g of dry substrate |
Incubation period in days | |||
|---|---|---|---|---|---|
| IV | VIII | XII | XV | ||
| RBV-RB | Laccase | 1669c | 18,653d | 24,258f | 11,765d |
| MnP | 819d | 2952d | 3,653c | 1,345bc | |
| LiP | 23.2a | 86.5b | 120.6a | 118b | |
|
| |||||
| RBV-WB | Laccase | 1,906d | 24,962f | 19,545e | 13,531e |
| MnP | 1,104e | 3,895e | 3,793c | 1,843d | |
| LiP | 25.8a | 72.8a | 143.9c | 270.9e | |
|
| |||||
| RBB-RB | Laccase | 573a | 7,253b | 10,162b | 6,238b |
| MnP | 325b | 1,362a | 1,956a | 1,063b | |
| LiP | ND | 75.3a | 110.6a | 118.8b | |
|
| |||||
| RBB-WB | Laccase | 2,237e | 23,096e | 17,368d | 10,632c |
| MnP | 1,235e | 4,652f | 3,056b | 1562c | |
| LiP | 128.1b | 180.6d | 126.4ab | 36.5a | |
|
| |||||
| Rice bran | Laccase | 653ab | 6,036a | 8,635a | 5,185a |
| MnP | 125a | 1,814b | 2,092a | 876a | |
| LiP | 12.8a | 85.4b | 115.5a | 160.0d | |
|
| |||||
| Wheat bran | Laccase | 1,263b | 12,550c | 13,796c | 10,534c |
| MnP | 552c | 2,272c | 2,982b | 1,292b | |
| LiP | 21.6a | 112.6c | 120.3a | 144.4c | |
RBB-RB: remazol brilliant blue adsorbed rice bran, RBB-WB: remazol brilliant blue adsorbed wheat bran, RBV-WB: remazol brilliant violet 5R adsorbed wheat bran, and RBV-RB: remazol brilliant violet 5R adsorbed rice bran.
Values are the means of duplicates.
Means, in each column, followed by the same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
The laccase and MnP activities obtained in RBV-5R adsorbed rice and wheat bran were higher than the activities obtained in RBB adsorbed lignocelluloses. In both the cases not much influence was observed on LiP production. It was reported that addition of inducers and copper induces the laccase production. In the current study, laccase activity values were obtained without any additional mediators or inducers. Among the two substrates tested, RBV-5R adsorbed rice bran was determined to be the best substrate for laccase production and RBV-5R adsorbed wheat bran was found to be the best for MnP production.
In the initial days of incubation dye adsorbed lignocelluloses secreted high amount of extracellular proteins up to 12 days; later extracellular protein content was found to be decreased. Rice bran and wheat bran alone secreted high amount of protein of 13.05 and 16.41 mg/mL on 15th day of incubation (Figure 5).
Figure 5.
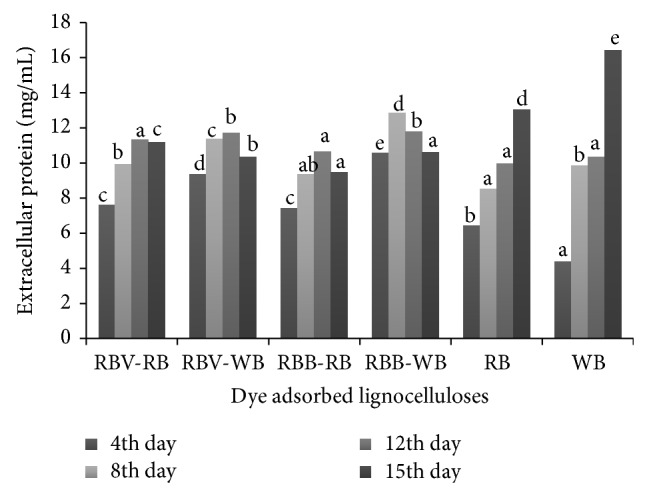
Secretion of extracellular proteins by dye adsorbed lignocelluloses. RBB-RB: remazol brilliant blue adsorbed rice bran, RBB-WB: remazol brilliant blue adsorbed wheat bran, RBV-WB: remazol brilliant violet 5R adsorbed wheat bran, and RBV-RB: remazol brilliant violet 5R adsorbed rice bran. Values are the means of duplicates. Means, in each column, followed by same letter are not significantly different (P ≤ 0.05) from each other according to DMR test.
4. Discussion
SSF has been considered as an efficient method for enzyme production in biotechnological process due to its potential advantages and high yield. In this study we selected ssf using wheat bran, an agro-byproduct containing arabinoxylans and phenolic acids, as a supporting substrate. The white rot fungi, S. ostrea, secreted low range of ligninolytic enzymes in submerged fermentation [20] compared to ssf. The ligninolytic activity of white rot fungi depends on many factors, and each strain responds in a particular way to each of these factors. The production of ligninolytic enzymes in ssf was further enhanced by the addition of various inducers, copper sulphate [21], and different surfactants. Phenolic and aromatic compounds such as guaiacol, veratryl alcohol, and ABTS have been widely employed to improve the production of ligninolytic enzymes by several fungal species [22–24]. Results revealed that veratryl alcohol and guaiacol stimulated higher enzyme production compared to other added inducers. The inductive effect of veratryl alcohol and guaiacol on laccase production has been reported by [25]. Guaiacol at 1 mM evidently enhanced the level of laccase production by Armillariella tabescens [26]. In this study a stimulating effect of these two compounds on MnP and LiP was observed. With lignosulfonic acid, lignin, and tannic acid, there is a toxicity effect on fungi and process optimization must be performed to consider the utilization of these compounds.
Copper is an essential micronutrient for most living organisms and copper requirements by microorganisms are usually satisfied by low concentrations of metal. However copper present in higher concentration is extremely toxic to microbial cells [27]. In the ascomycete Podospora anserina, in which laccase mRNA, amongst others, increased in response to copper and aromatic compounds, it was postulated that laccase acts as a defence mechanism against oxidative stress [28]. This protective function was partly attributed to the chelation of copper ions during synthesis of the laccase enzyme [29]. In Pleurotus ostreatus cultures, the presence of copper decreased the activity of an extracellular protease [12]. This might explain the positive effect of copper on enzyme stabilization. Reference [30, 31] reported that the copper at various concentrations stimulates laccase production in T. pubescens, P. eryngii, and P. ostreatus. Our findings are in accordance with those results. Reference [32] observed that laccase activity producing Phlebia radiata was increased in media with 1.5 mmol/L of Cu2+ while [33] found that optimal concentration of copper ions for laccase production by Trametes trogii is 11 mmol/L.
Some studies have demonstrated that the use of surfactants can stimulate fungal growth and enhance enzyme production. Nonionic surfactants such as Tween 80, Tween 20, and Triton X-100 are often considered to be nontoxic and, therefore, do not affect the fungal growth of S. ostrea. Several studies of chemical surfactants have shown that charge has an impact on toxicity; cationic surfactants are the most toxic and have been used as antimicrobials. Reference [34] found no negative effect of Tween 80 on P. chrysosporium growth. Reference [35] evaluated the toxicity of SDS, Triton X-100, and Tween 80 on fungal strains. The results showed growth inhibition by SDS (anionic surfactant), whereas Triton X-100 and Tween 80 (nonionic surfactants) were well tolerated at the doses evaluated in most of the tested fungi. Several authors have shown an improvement in enzyme excretion in the presence of certain surfactants such as Tween 80 in immobilized and submerged cultures of P. chrysosporium [34, 36]. Tween 80 at 0.3 mM greatly enhanced the activities of all the three enzymes by Ganoderma lucidum in solid state fermentation of pineapple leaf [37]. Moreover, Tween 80 is known to facilitate the secretion of ligninolytic enzymes [38] and its effect also enhanced LiP production by this selected strain. It was suggested that the surfactants enhance the extracellular enzyme production in filamentous fungi by promoting both the uptake and exit of compounds from the cells through the modification of plasma membrane permeability [34].
Dye adsorption abilities of two types of low cost eco-friendly lignocelluloses, rice bran and wheat bran, were tested. There are studies on biosorption potential of various species of lignocelluloses [39, 40]. Rice bran is a cheap adsorbent for the removal of textile dyes [41]. Kadam et al. [42] used rice bran as a cheap adsorbent for removal of reactive navy blue and reported 90% removal. It was previously reported that dye adsorbed lignocelluloses could be used for the production of ligninolytic enzymes [43]. The result obtained in this study showed that it was possible to use dye adsorbed lignocelluloses for production of ligninolytic enzymes in solid state fermentation. However enzyme production efficiency is strain and substrate dependent [44]. Among the two substrates tested, dye adsorbed wheat bran was determined to be the efficient substrate for the production of ligninolytic enzymes during solid state fermentation. Because wheat bran contains high amount of carbohydrates that can be used as a carbon source, it is a good substrate for fungal growth [45]. It was reported that malachite green adsorbed wheat bran could be used as a solid substrate to produce LiP with Fomes sclerodermeus [45].
Acknowledgments
The authors are very grateful to the Department of Science and Technology in India for financial support and also thankful to the Sri Krishnadevaraya University authorities for providing necessary facilities throughout their research work.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Pandey A., Singh P., Iyengar L. Bacterial decolorization and degradation of azo dyes. International Biodeterioration & Biodegradation. 2007;59(2):73–84. doi: 10.1016/j.ibiod.2006.08.006. [DOI] [Google Scholar]
- 2.Xu F. Applications of oxidoreductases: recent progress. Industrial Biotechnology. 2005;1:38–50. [Google Scholar]
- 3.Maciel M. J. M., Castro e Silva A., Ribeiro H. C. T. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Electronic Journal of Biotechnology. 2010;13(6):14–15. doi: 10.2225/vol13-issue6-fulltext-2. [DOI] [Google Scholar]
- 4.Nigam P. S. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules. 2013;3(3):597–611. doi: 10.3390/biom3030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai S. S., Nityanand C. Microbial laccases and their applications—a review. Asian Journal of Biotechnology. 2011;3:98–124. [Google Scholar]
- 6.Shraddha, Shekher R., Sehgal S., Kamthania M., Kumar A. Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzyme Research. 2011;2011:11. doi: 10.4061/2011/217861.217861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C. K., Darah I., Ibrahim C. O. Production and optimization of cellulase enzyme using Aspergillus niger USM AI 1 and comparison with Trichoderma reesei via solid state fermentation system. Biotechnology Research International. 2011;2011:6. doi: 10.4061/2011/658493.658493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soni S. K., Goyal N., Gupta J. K., Soni R. Enhanced production of α-amylase from Bacillus subtilis subsp. spizizenii in solid state fermentation by response surface methodology and its evaluation in the hydrolysis of raw potato starch. Starch. 2012;64(1):64–77. doi: 10.1002/star.201100119. [DOI] [Google Scholar]
- 9.Giese E. C., Dekker R. F. H., Barbosa A. M. Orange bagasse as substrate for the production of pectinase and laccase by Botryosphaeria rhodina MAMB-05 in submerged and solid state fermentation. BioResources. 2008;3(2):335–345. [Google Scholar]
- 10.Pandey A., Azmi W., Singh J., Banerjee U. C. Types of fermentation and factors affecting it. In: Joshi V. K., Pandey A., editors. Biotechnology: Food Fermentation. Educational Publishers; 1999. pp. 383–426. [Google Scholar]
- 11.Barbosa A. M., Dekker R. F. H., St. Hardy G. E. Veratryl alcohol as an inducer of laccase by an ascomycete, Botryosphaeria sp., when screened on the polymeric dye Poly R-478. Letters in Applied Microbiology. 1996;23(2):93–96. doi: 10.1111/j.1472-765x.1996.tb00038.x. [DOI] [Google Scholar]
- 12.Palmieri G., Giardina P., Bianco C., Fontanella B., Sannia G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus . Applied and Environmental Microbiology. 2000;66(3):920–924. doi: 10.1128/aem.66.3.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Z., Obbard J. P. Effect of non-ionic surfactants on elimination of polycyclic aromatic hydrocarbons (PAHs) in soil-slurry by Phanerochaete chrysosporium . Journal of Chemical Technology and Biotechnology. 2001;76(4):423–429. doi: 10.1002/jctb.396. [DOI] [Google Scholar]
- 14.Koroljova-Skorobogat'ko O. V., Stepanova E. V., Gavrilova V. P., et al. Purification and characterization of the constitutive form of laccase from the basidiomycete Coriolus hirsutus and effect of inducers on laccase synthesis. Biotechnology and Applied Biochemistry. 1998;28(1):47–54. [PubMed] [Google Scholar]
- 15.Das N., Sengupta S., Mukherjee M. Importance of laccase in vegetative growth of Pleurotus florida . Applied and Environmental Microbiology. 1997;63(10):4120–4122. doi: 10.1128/aem.63.10.4120-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tien M., Kirk T. K. Wood. In: Willis A., Scott T., editors. Methods in Enzymology: Biomass, Part B : Lignin, Pectin, and Chitin. Vol. 161. San Diego, Calif, USA: Academic Press; 1988. pp. 238–249. [Google Scholar]
- 17.Bonnen A. M., Anton L. H., Orth A. B. Lignin-degrading enzymes of the commercial button mushroom, Agaricus bisporus . Applied and Environmental Microbiology. 1994;60(3):960–965. doi: 10.1128/aem.60.3.960-965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 19.Duncan D. B. Multiple range and multiple F tests. Biometrics. 1955;11(1):1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 20.Praveen K., Viswanath B., Usha K. Y., et al. Lignolytic enzymes of a mushroom Stereum ostrea isolated from wood logs. Enzyme Research. 2011;2011:6. doi: 10.4061/2011/749518.749518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoud M. G., Rifaat H. M., El Sayed O. H., El Beih F. M., Selim M. S. Effect of inducers and process parameters on laccase production by locally isolated marine Streptomyces lydicus from Red Sea, Egypt. International Journal of ChemTech Research. 2013;5(1):15–23. [Google Scholar]
- 22.Farnet A. M., Criquet S., Cigna M., Gil G., Ferré E. Purification of a laccase from Marasmius quercophilus induced with ferulic acid: reactivity towards natural and xenobiotic aromatic compounds. Enzyme and Microbial Technology. 2004;34(6):549–554. doi: 10.1016/j.enzmictec.2003.11.021. [DOI] [Google Scholar]
- 23.Pazarlioğlu N. K., Sariişik M., Telefoncu A. Laccase: production by Trametes versicolor and application to denim washing. Process Biochemistry. 2005;40(5):1673–1678. doi: 10.1016/j.procbio.2004.06.052. [DOI] [Google Scholar]
- 24.Jaouani A., Tabka M. G., Penninckx M. J. Lignin modifying enzymes of Coriolopsis polyzona and their role in olive oil mill wastewaters decolourisation. Chemosphere. 2006;62(9):1421–1430. doi: 10.1016/j.chemosphere.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 25.Patel H., Gupte A., Gupte S. Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate pleurotus ostreatus HP-1 under solid state fermentation. BioResources. 2009;4(1):268–284. [Google Scholar]
- 26.He J., Ye X., Ling Q., Dong L. Enhanced production of an acid-tolerant laccase by cultivation of Armillariella tabescens . Journal of Chemical and Pharmaceutical Research. 2014;6(1):240–245. [Google Scholar]
- 27.Labbe S., Thiele D. J. Pipes and wiring: the regulation of copper uptake and distribution in yeast. Trends in Microbiology. 1999;7(12):500–505. doi: 10.1016/s0966-842x(99)01638-8. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Larrea J., Stahl U. Isolation and characterization of a laccase gene of Podospora anserina . Molecular Genetics and Genomics. 1996;149:65–70. doi: 10.1007/BF02172400. [DOI] [PubMed] [Google Scholar]
- 29.Trupkin S., Levin L., Forchiassin F., Viale A. Optimization of a culture medium for ligninolytic enzyme production and synthetic dye decolorization using response surface methodology. Journal of Industrial Microbiology and Biotechnology. 2003;30(12):682–690. doi: 10.1007/s10295-003-0099-0. [DOI] [PubMed] [Google Scholar]
- 30.Galhaup C., Goller S., Peterbauer C. K., Strauss J., Haltrich D. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology. 2002;148(7):2159–2169. doi: 10.1099/00221287-148-7-2159. [DOI] [PubMed] [Google Scholar]
- 31.Stajić M., Persky L., Hadar Y., et al. Effect of copper and manganese ions on activities of laccase and peroxidases in three Pleurotus species grown on agricultural wastes. Applied Biochemistry and Biotechnology. 2006;128(1):87–96. doi: 10.1385/abab:128:1:087. [DOI] [PubMed] [Google Scholar]
- 32.Mäkelä M. R., Lundell T., Hatakka A., Hildén K. Effect of copper, nutrient nitrogen, and wood-supplement on the production of lignin-modifying enzymes by the white-rot fungus Phlebia radiata . Fungal Biology. 2013;117(1):62–70. doi: 10.1016/j.funbio.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Levin L., Herrmann C., Papinutti V. L. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochemical Engineering Journal. 2008;39(1):207–214. doi: 10.1016/j.bej.2007.09.004. [DOI] [Google Scholar]
- 34.Ding J., Cong J., Zhou J., Gao S. Polycyclic aromatic hydrocarbon biodegradation and extracellular enzyme secretion in agitated and stationary cultures of Phanerochaete chrysosporium . Journal of Environmental Sciences. 2008;20(1):88–93. doi: 10.1016/s1001-0742(08)60013-3. [DOI] [PubMed] [Google Scholar]
- 35.Garon D., Krivobok S., Wouessidjewe D., Seigle-Murandi F. Influence of surfactants on solubilization and fungal degradation of fluorene. Chemosphere. 2002;47(3):303–309. doi: 10.1016/S0045-6535(01)00299-5. [DOI] [PubMed] [Google Scholar]
- 36.Jager A., Croan S., Kirk T. K. Production of ligninases and degradation of lignin in agitated submerged cultures of Phanerochaete chrysosporium . Applied and Environmental Microbiology. 1985;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariharan S., Nambisan P. Optimization of lignin peroxidase, manganese peroxidase, and lac production from Ganoderma lucidum under solid state fermentation of pineapple leaf. BioResources. 2013;8(1):250–271. [Google Scholar]
- 38.Couto S. R., Rosales E., Gundín M., Sanromán M. Á. Exploitation of a waste from the brewing industry for laccase production by two Trametes species. Journal of Food Engineering. 2004;64(4):423–428. doi: 10.1016/j.jfoodeng.2003.11.009. [DOI] [Google Scholar]
- 39.Wang X. S., Chen J. P. Biosorption of Congo red from aqueous solution using wheat bran and rice bran—batch studies. Separation Science and Technology. 2009;44(6):1452–1466. doi: 10.1080/01496390902766132. [DOI] [Google Scholar]
- 40.Sen T. K., Afroze S., Ang H. M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water, Air, and Soil Pollution. 2011;218(1–4):499–515. doi: 10.1007/s11270-010-0663-y. [DOI] [Google Scholar]
- 41.Hashemian S., Dadfarnia S., Nateghi M. R., Gafoori F. Sorption of acid red 138 from aqueous solutions onto rice bran. African Journal of Biotechnology. 2008;7(5):600–605. [Google Scholar]
- 42.Kadam A. A., Telke A. A., Jagtap S. S., Govindwar S. P. Decolorization of adsorbed textile dyes by developed consortium of Pseudomonas sp. SUK1 and Aspergillus ochraceus NCIM-1146 under solid state fermentation. Journal of Hazardous Materials. 2011;189(1-2):486–494. doi: 10.1016/j.jhazmat.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Couto S., Osma J. F., Toca-Herrera J. L. Removal of synthetic dyes by an eco-friendly strategy. Engineering in Life Sciences. 2009;9(2):116–123. doi: 10.1002/elsc.200800088. [DOI] [Google Scholar]
- 44.Tychanowicz G. K., Zilly A., de Souza C. G. M., Peralta R. M. Decolourisation of industrial dyes by solid-state cultures of Pleurotus pulmonarius . Process Biochemistry. 2004;39(7):855–859. doi: 10.1016/s0032-9592(03)00194-8. [DOI] [Google Scholar]
- 45.Papinutti L., Mouso N., Forchiassin F. Removal and degradation of the fungicide dye malachite green from aqueous solution using the system wheat bran—Fomes sclerodermeus . Enzyme and Microbial Technology. 2006;39(4):848–853. doi: 10.1016/j.enzmictec.2006.01.013. [DOI] [Google Scholar]


