Abstract
The present work represents the biosorption of Cd(II) and Pb(II) from aqueous solution onto the biomass of the blue green alga Anabaena sphaerica as a function of pH, biosorbent dosage, contact time, and initial metal ion concentrations. Freundlich, Langmuir, and Dubinin–Radushkevich (D–R) models were applied to describe the biosorption isotherm of both metals by A. sphaerica biomass. The biosorption isotherms studies indicated that the biosorption of Cd(II) and Pb(II) follows the Langmuir and Freundlish models. The maximum biosorption capacities (qmax) were 111.1 and 121.95 mg/g, respectively, at the optimum conditions for each metal. From the D–R isotherm model, the mean free energy was calculated to be 11.7 and 14.3 kJ/mol indicating that the biosorption mechanism of Cd(II) and Pb(II) by A. sphaerica was chemisorption. The FTIR analysis for surface function group of algal biomass revealed the existence of amino, carboxyl, hydroxyl, and carbonyl groups, which are responsible for the biosorption of Cd(II) and Pb(II). The results suggested that the biomass of A. sphaerica is an extremely efficient biosorbent for the removal of Cd(II) and Pb(II) from aqueous solutions.
Keywords: Anabaena sphaerica, Alga, Biosorption, Heavy metals
Introduction
Water pollution is one of the most serious problems because inorganic and organic wastes are discharged to the aquatic environment either in water soluble or insoluble forms [1], [2]. Among the inorganic pollutants, heavy metals are the most serious because they are non-biodegradable and have the ability to accumulate in living organisms. Lead and cadmium are considered the most toxic and hazardous to the environment [3], [4]. Lead is currently implemented in a significant number of industries such as cables, batteries, pigments, paints, steels and alloys, metal, glass, and plastic industries [5]. The discharge of these industries causes the contamination of the aquatic environment by lead. On the other hand, the exposure to cadmium may cause hypertension, hepatic injury, renal dysfunction, teratogenic effects, and lung damage [3], [6].
The removal of heavy metals is considered an important issue with respect to the environment and economical considerations. There are several methods for the removal of heavy metals from aqueous solution including, adsorption on activated carbon, reverse osmosis, ion exchange, chemical precipitation, and membrane filtration [7], [8]. However, the feasibility of economical and technical factors may limit the implementation of these methods [3].
One of the emerging and attractive technologies to remove heavy metals from aqueous solution is the biosorption process. Various biomasses such as bacteria [9], yeast [10], fungi [11], [12], and algae [13], [14], [15], [16] were investigated as biosorbent for the removal of heavy metals. The aforementioned articles demonstrated that algae biosorbent might be effective, in particular, when they are existed in dead cells form. Microalgae biosorbent seem to be more promising than macroalgae (seaweeds) because of (a) the cultivation of microalgae is normally easier and has higher production yield and (b) they have higher performance and efficiency (due to their micron size) and in turn higher specific biosorption area.
Blue-green algae (Cyanobacteria) including Dunaliella, Spirulina (Arthrospira), Nostoc, Anabaena, and Synechococcus were the typical examples that showed the potential as biosorbents for efficient removal of heavy metals from wastewaters [17], [18], [19]. Cyanobacteria have some advantages over other microorganisms including their greater mucilage volume with high binding affinity, large surface area, and simple nutrient requirements [20]. Cyanobacteria are easily cultivated in a large scale in laboratory cultures providing a low cost biomass for the biosorption process. The present work was designed to investigate the biosorption behavior of Pb and Cd to the blue green alga (A. sphaerica).
Material and methods
Materials preparation
The blue green alga (A. sphaerica) was collected from the Nile River water in Ismailia canal in front of Port Said water plant intake, purified, and recultivated in BG11 medium containing the following macroelements: K2HPO4, MgSO4, CaCl2, citric acid, Na2CO3, Na2EDTA, and ferric ammonium citrate [21]. NaNO3 was excluded completely from the algal media. A. sphaerica was in the logarithmic phase of growth when introduced to the standard algal culture medium. The algal cultures were incubated at 24 ± 2 °C under continuous illumination (≈2500 lux). The cultures were swirled once daily to prevent clumping and adherence of the algal cells to the containers.
At maximum growth of A. sphaerica, the biomass was collected by centrifugation at 5000 rpm for 10 min. The algal biomass was washed with distilled water for five times to avoid any effect of salt and then dried in an oven at 40 °C to constant weight. Afterwards, the dried biomass was ground and sieved through a 0.2 mm size sieve and stored in polyethylene bottles. All chemicals used throughout the experimental works were provided by Merck (Darmstadt, Germany).
Metal solutions standards
Metal salts used in the preparation of the synthetic metal bearing solutions were CdCl2·5/2H2O and Pb(NO3)2. The synthetic wastewater solutions were then prepared by diluting the stock standards of concentration 1000 mg/L of each metal. Deionized water was used in all experiments.
Analytical methods
Determination of metals concentration
The concentrations of metals in all samples were determined according to the APHA method [22] using Atomic Absorption Spectrometer (Varian SpectrAA 220, USA) with graphite furnace accessory and equipped with deuterium arc background corrector. Precision of the metal measurement was determined by analyzing the metal concentration of all samples.
Quality control
For each series of measurements, absorption calibration curve was constructed composed of a blank and three or more standards. The accuracy and precision of the metals measurement were confirmed using external standard reference material 1643e for trace elements in water and quality control sample from National Institute Standards and Technology (NIST).
Batch biosorption studies
Each of the batch biosorption studies was carried out by contacting the A. sphaerica biomass with the metal ions in 250 ml stopper conical flask. The experiments were conducted at room temperature (25 ± 0.1 °C) to determine the effects of pH, biosorbent dosage, contact time, and initial ions concentration on the biosorption of Cd(II) and Pb(II) ions. Each experiment was conducted in a mechanical shaker at 120 rpm. The samples were filtered through Whatman filter paper (No. 41) and the metal ions concentration was determined in the filtrate. To distinguish between possible metal precipitation and actual metal sorption, controls (blank) were used without biosorbent materials.
All the experiments were carried out in triplicate and the mean of the quantitative results were used for further calculations. For the calculation of mean value, the percent relative standard deviation for results was calculated and if the value of standard deviation for a sample was greater than 5%, the data were discarded.
Effect of pH
The batch experiment was carried out by contacting 0.1 g of alga with 100 ml of 50 mg/L of metal solution in 250 ml stopper conical flask at different pH value, ranging from 2 to 6 (below 2, the high proton concentration minimizes the metal sorption and above 6 the metal precipitation is favored). The pH of the solutions was adjusted either by hydrochloric acid or sodium hydroxide. The mixture was shaken for 2 h at room temperature, filtered, and the final pH for each sample was determined.
Effect of contact time
The optimum time was carried out at optimum pH by conducting batch biosorption experiments with an initial metals ions concentration of 50 mg/L, 10 g/L biosorbent dosage and at different time periods (5, 15, 30, 60, 90, 120 min).
Effect of biosorbent dosage
The biosorbent dosage varied from 0.025 to 0.25 g using a fixed volume of 100 ml of 50 mg/L of metal solution at the optimum pH and the equilibration time for each metal.
Biosorption isotherms
Isotherms were measured by varying the initial metal ion concentrations at the optimum conditions for each metal. Different biosorption models were used for comparison with experimental data [23].
Results and discussion
Biosorbent
The biosorbent used in this study was blue green alga (A. sphaerica) collected from the Nile River water and was purified and recultivated in BG11 medium. Data presented in Fig. 1 shows that, there are different shapes of A. sphaerica such as filament solitary or free clusters, the cells were cylindrical barrel-shaped or spherical and the terminal cells were spherical or slightly elongated. The spherical heterocysts were elongated and slightly greater than vegetative cells due to nitrogen fixation (Fig. 1).
Fig. 1.

Photo of River Nile Alga Anabaena sphaerica.
Characterization of dried biosorbent
The results illustrated in Fig. 2 showed the FTIR spectra of the unloaded biomass and Pb(II)-loaded biomass. These results represented the information about the functional groups on the surface of the cell wall of the biomass and the possible interaction between metals and the functional groups. From these data, it is clear that the strong and broad band at 3393 cm−1 might be related to the overlapping between N—H and O—H stretching vibration. However, the band at 2924 cm−1 could be related to the —CH stretch and the band at 1646 cm−1 could be assigned to asymmetric stretching vibration of C O. On the other hand, the intense and strong band at 1053 cm−1 might be attributed to the stretching of C—O group on the surface of the biomass [3]. Meanwhile, some bands in the fingerprint region could be related to the phosphate groups. It could be observed that the bands at 3393, 2924, 1646, 1053, and 580 cm−1 were shifted to 3411, 2922, 1653, 1043, and 556 cm−1 after loading of Pb(II). The significant changes in the wave number of these peaks after loading of Pb(II) indicate that the functional groups (amido, hydroxyl, C O and C—O) were involved in the biosorption of Pb(II) on the surface of A. sphaerica. Similar results for the biosorption of heavy metals on different species of algae have been previously reported by others [3], [24], [25].
Fig. 2.
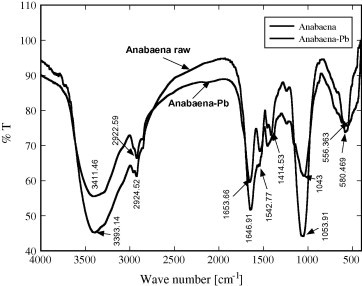
FTIR spectra of Anabaena sphaerica dry biomass unloaded and Pb-loaded biomass.
Optimum conditions
Effect of pH
It is well documented that the pH of the aqueous solution affects the metal solubility and the concentration of the counter ions on the functional group of the cell wall of the biosorbent, consequently, the pH is considered as the most important parameter that could affect the biosorption of metal ions from solutions [26], [27]. The effect of pH value on the biosorption of Cd(II) and Pb(II) ions onto A. sphaerica biomass was evaluated and the results were presented in Fig. 3. It is clear that the maximum biosorption for Cd (II) and Pb(II) reached 84.5% and 88.3% at pH 5.5 and 3, respectively. Therefore, all the experiments were carried out at pH 5.5 for Cd and pH 3 for Pb.
Fig. 3.
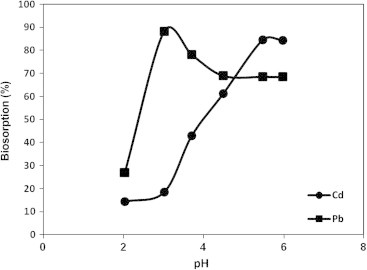
Effect of pH on the biosorption of Cd and Pb by Anabaena sphaerica.
The current results indicated that the biosorption of Cd(II) and Pb(II) was increased with increasing the pH value. This is because, at lower pH, the concentration of positive charge (protons) increased on the sites of biomass surface, which restricted the approach of metal cations to the surface of biomass (because of charge repulsion) [28]. As the pH increase, the proton concentration decreases and the biomass surface is more negatively charged. The biosorption of the positively charged metal ions increased till reaching their maximum biosorption around pH 5.5 and 3 for Cd(II) and Pb(II) respectively. The maximum biosorption efficiency of Cd(II) and Pb(II) was occurred at different pH values. This could probably correlate to the different characteristics between the metals (size, electronegativity), or the more available metal was better biosorbed on the adsorption sites [2]. For Pb(II), the maximum biosorption at lower pH is related to its higher electronegativity than Cd and hydronium ion, so Pb affinity to the surface functional groups of the cell wall is higher than Cd and hydronium ions at low pH value. While, the decrease in biosorption yield at higher pH not only related to the formation of soluble hydroxilated complexes of the metal ions (lead ions in the form of Pb(OH)2 [29], but also to the ionized nature of the cell wall surface of the biomass under the tested pH. Also, the main cadmium cation sequestration mechanism by the algal biomass was apparently chelation, while lead cations exhibit higher affinity to the algal biomass, and their binding mechanism include a combination of ion exchange, chelation, and reduction reactions, accompanied by metallic lead precipitation on the cell wall matrix [29].
Effect of biosorbent dosage
Different biomass dosage ranged from 0.025 to 0.25 g/100 ml was applied to study the effect of biomass dose on the biosorption of Cd(II) and Pb(II) ions Fig. 4. The data revealed that the biosorption efficiency of Cd(II) and Pb(II) ions on A. sphaerica was significantly affected by the dose of A. sphaerica in the solution. In other words, the biosorption of Cd(II) and Pb(II) ions was increased with subsequent increasing the biosorbent dose and almost became constant at higher dosage than 0.1 g/100 ml and 0.2 g/100 ml for Pb and Cd, respectively. This behavior could be explained by the formation of aggregates of the biomass at higher doses, which decreases the effective surface area for biosorption [3], [30]. Therefore; the doses 0.1 g/100 ml for Pb(II) and 0.2 g/100 ml for Cd(II) were selected as the optimum doses of the biosorbent for the rest of the study.
Fig. 4.
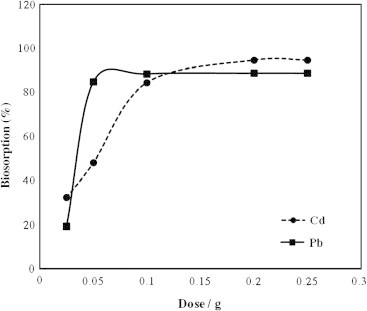
Effect of the dose of Anabaena sphaerica biomass on the biosorption of Cd and Pb.
Effect of contact time
The effect of contact time is highly influencing the biosorption process. Fig. 5 showed the effect of contact time on the biosorption of Cd(II) and Pb(II) ions using the A. sphaerica. These results indicated that the biosorption of both metals was rapid in the first 20 min then was gradually increased till the equilibrium attained at 60 and 90 min for Cd and Pb, respectively, and the biosorption became almost constant thereafter. Therefore, a contact time of 60 and 90 min was used as the optimum time for Cd and Pb for the rest of experiments. The rate of biosorption of Cd(II) and Pb(II) ions using the A. sphaerica seems to occur in two steps; a very rapid surface biosorption in the first step and a slow intracellular diffusion in the second step. In this concern, Chen et al., [2] reported similar behavior for the biosorption of Ni and Cu on treated alga Undaria pinnatifida and the adsorption of Cd by biomass fungal biosorbent [31].
Fig. 5.
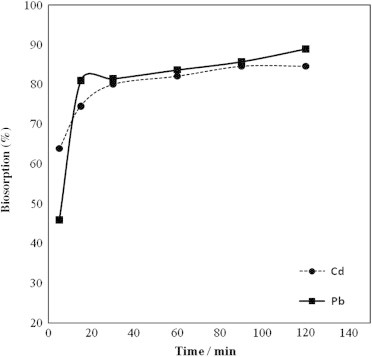
Effect of contact time on the biosorption of Cd and Pb by Anabaena sphaerica.
Effect of metal concentration
The data presented in Fig. 6 showed the effect of metal concentration on biosorption process. The concentration of Cd and Pb were varied between 50 and 300 mg/l at the optimum pH, contact time, and optimum dose for each metal, respectively. The results presented in Fig. indicated that the biosorption of Cd and Pb at the beginning was 94.3% and 88.6%, respectively. The biosorption was decreased with increasing the metal concentration. This behavior was attributed to the fact that, initially, all binding sites on the biomass surface were vacant resulting in high metal biosorption at the beginning. After that, with increasing metal concentration, the biosorption of metal was decreased because of a few active sites were available on the surface of the algal biomass.
Fig. 6.
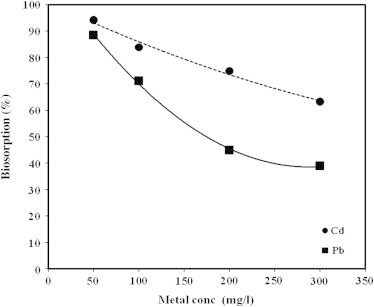
Biosorption of Cd and Pb on Anabaena sphaerica as function of initial concentration at the optimum removal conditions.
Equilibrium studies and isotherm modeling
The biosorption isotherm models described the biosorption data at equilibrium and showed the correlation between the mass of solute adsorbed per unit mass of sorbent at equilibrium. The biosorption isotherms were calculated using three different isotherms models including the Langmuir, Freundlich and Dubinin–Radushkevich (D–R) isotherms [32].
Freundlich and Langmuir models
Due to their simplicity, the Freundlich and Langmuir equations are the most widely used models to describe the relationship between equilibrium metal biosorption qe (mg/L) and final concentrations Ce (mg/L) at equilibrium.
The Freundlich equation is given by:
| (1) |
where Kf and n are the Freundlich constants and are related to the adsorption capacity of the sorbent and the adsorption intensity. To simplify the determination of Kf and 1/n, Eq. (1) can be linearized in logarithmic form, which allows the determination of the unknown parameters by plotting log qe versus log Ce:
| (2) |
The Langmuir isotherm relationship is given as:
Langmuir isotherm [33] presented by the following equation:
| (3) |
where Ce (mg/L) is the concentration of metal in solution at equilibrium, Cads (mg/g) is the amount of metal sorbed per unit mass of A. sphaerica, Q (mg/g) and b are Langmuir constants related to mono layer capacity sorption and sorption energy, respectively.
The selection between Freundlich and Langmuir isotherms is mainly controlled by the equilibrium data [34]. These isotherms are commonly describe the adsorption phenomena at the solid liquid interface and the isotherms data were used for the design of adsorption systems and to understand the relation between adsorbent and adsorbate [35].
The Freundlich isotherm plot for the biosorption of Cd and Pb onto A. sphaerica biomass (Fig. 7) indicated that Cd and Pb were fitted to Freundlich isotherm (R2 = 0.991 and 0.979). The isotherm data calculated from Freundlich isotherm (Table 1) revealed that the 1/n values for Cd and Pb were 0.390 and 0.263, respectively. The 1/n values were less than one, indicating that the biosorption process for Cd and Pb onto A. sphaerica biomass was favorable at the studied experimental conditions.
Fig. 7.
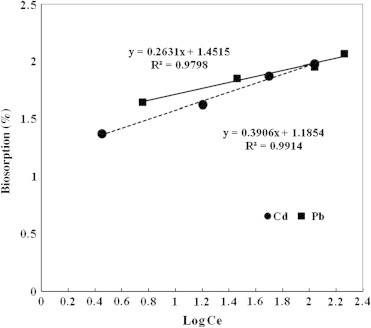
Freundlich isotherm for the biosorption of Cd and Pb on Anabaena sphaerica.
Table 1.
Summary of isotherm model parameters for Anabaena sphaerica biomass.
| Metals | Freundlich model |
Langmuir model |
||||
|---|---|---|---|---|---|---|
| Kf | 1/n | R2 | Qmax (mg/g) | 1/b | R2 | |
| Cd | 15.31 | 0.390 | 0.991 | 111.1 | 18.88 | 0.9801 |
| Pb | 28.28 | 0.2631 | 0.9798 | 121.95 | 19.19 | 0.9699 |
The current results (Fig. 8) showed the Langmuir isotherm model for the biosorption of Cd and Pb onto A. sphaerica biomass and indicated that the correlation coefficient (R2) was 0.98 and 0.969 for Cd and Pb, respectively. These results indicated that the biosorption of Cd is more fitted to the Langmuir isotherm model than the biosorption of Pb. In other words, the biosorption of Cd and Pb onto A. sphaerica was occurred on the functional groups binding sites as a monolayer biosorption. These results are in agreement with the FTIR spectra illustrated in Fig. 2. The maximum biosorption capacity calculated from Langmuir isotherm presented in Table 1 was 111.1 and 121.95 mg/g for the biosorption of Cd and Pb, respectively. These biosorption capacities are higher than those reported for the modified rice husk [36] and green alga (Cladophora hutchinsiae) biomass [37]. These results proved that A. sphaerica biomass could be used as potential biosorbent for the removal of Cd and Pb from aqueous solutions.
Fig. 8.
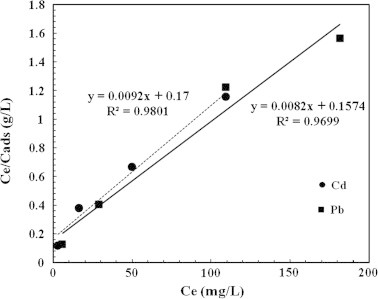
Langmuir isotherm for Cd and Pb biosorption onto Anabaena sphaerica biomass.
Dubinin–Radushkevich isotherms (D–R isotherms)
Freundlich and Langmuir isotherms could not provide any information about the biosorption mechanism. The D–R isotherm is an analog of Langmuir type but it is more general because it does not assume a homogeneous surface or constant sorption potential [38]. The Dubinin–Radushkevich isotherm model was used to predict the nature of adsorption processes as physical or chemical [39].
The linearized D–R isotherm equation can be written as shown:
| (4) |
where qe is the amount of metal ions adsorbed per unit mass of adsorbent (mol/g), Xm is the maximum sorption capacity, β is the activity coefficient related to mean sorption energy, and ε is the Polanyi potential, which is equal to:
| (5) |
where R is the gas constant (J/mol K) and T is the temperature (K). The saturation limit Xm may represent the total specific micropore volume of the sorbent. The sorption potential is independent of the temperature but varies according to the nature of sorbent and sorbate [40]. The sorption space in the vicinity of a solid surface is characterized by a series of equi-potential surfaces having the same sorption potential. The sorption energy can also be worked out using the following equation:
| (6) |
The data illustrated in Fig. 9 and Table 2 represent the D–R plot of the biosorption of Cd(II) and Pb(II) ions onto A. sphaerica biomass. It is well known that the mean free energy of biosorption gives information about biosorption mechanism, physical or chemical. If E value lies between 8 and 16 kJ/mol, the biosorption process occurs chemically and if E < 8 kJ/mol, the biosorption process takes place physically [3], [25]. In the current study, the mean biosorption energy was calculated as 11.7 and 14.3 kJ/mol for the biosorption of Cd(II) and Pb(II) ions, respectively (Table 2). These results indicated that the biosorption process of Cd(II) and Pb(II) onto A. sphaerica biomass may be carried out chemically via involving valence forces through sharing or exchange of electrons between sorbent and sorbate [41].
Fig. 9.
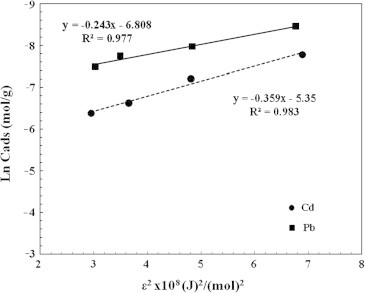
D–R biosorption isotherm of Cd and Pb ion on Anabaena sphaerica biomass.
Table 2.
Summary of DKR model parameters for (Anabaena sphaerica).
| Metals | Xm (mol/g) | β (mol2/j2) | Sorption energy (E, kJ/mol) |
|---|---|---|---|
| Cadmium | 2.374 × 10−3 | −0.3592 × 10−8 | 11.798 |
| Lead | 1.104 × 10−3 | −0.2433 × 10−8 | 14.335 |
Conclusion
The present work was designed to investigate the biosorption behavior of Pb and Cd to the blue green alga A. sphaerica. The maximum biosorption capacities were 111.1 mg/g and 121.9 mg/g for Cd and Pb at optimum operating conditions, respectively. The experimental data revealed that Cd and Pb biosorption were fitted to both Freundlish and Langmuir isotherms. The mean free energy values calculated from the D–R plot were 11.7 and 14.3 kJ/mol indicating that the biosorption type was chemisorption. The FTIR indicated that the amino, carboxyl, hydroxyl and carbonyl groups on the surface of the biomass are responsible for biosorption of Cd(II) and Pb(II). Based on these results, A. sphaerica biomass can be used as an efficient low cost biomass for the removal of heavy metals from wastewater.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Dahiya S., Tripathi R.M., Hegde A.G. Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. J Hazard Mater. 2008;150:376–386. doi: 10.1016/j.jhazmat.2007.04.134. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z., Ma W., Han M. Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): application of isotherm and kinetic models. J Hazard Mater. 2008;155:327–333. doi: 10.1016/j.jhazmat.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 3.Sari A., Tuzen M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J Hazard Mater. 2008;152:302–308. doi: 10.1016/j.jhazmat.2007.06.097. [DOI] [PubMed] [Google Scholar]
- 4.Barbier F, Duc G, Petit-Ramel M, Adsorption of lead and cadmium ions from aqueous solution to the montmorillonite: water interface, Colloids Surf., A: Physicochem. Eng. Aspects 2000;166:153–159.
- 5.Selatnia A., Boukazoula A., Kechid N., Bakhti M.Z., Chergui A., Kerchich Y. Biosorption of lead(II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem Eng J. 2004;19:127–135. [Google Scholar]
- 6.Hajialigol S., Taher M.A., Malekpour A. A new method for the selective removal of cadmium and zinc ions from aqueous solution by modified clinoptilolite. Adsorp Sci Technol. 2006;24:487–496. [Google Scholar]
- 7.Yu Q., Matheickal J.T., Yin P., Kaewsarn P. Heavy metal uptake capacities of common marine macro algal biomass. Water Res. 1999;33:1534–1537. [Google Scholar]
- 8.Turker A.R. Separation, preconcentration and speciation of metal ions by solid phase extraction. Sep Purif Rev. 2012;41:169–206. [Google Scholar]
- 9.Iyer A., Mody K., Jha B. Biosorption of heavy metals by a marine bacterium. Mar Pollut Bull. 2005;50(3):340–343. doi: 10.1016/j.marpolbul.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Goksungur Y., Uren S., Guvenc U. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol. 2005;96(1):103–109. doi: 10.1016/j.biortech.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Tunali S., Akar T., Ozcan A.S., Kiran I., Ozcan A. Equilibrium and kinetics of biosorption of lead (II) from aqueous solutions by cephalosporium aphidicola. Sep Purif Technol. 2006;47(3):105–112. [Google Scholar]
- 12.Anayurt R.A., Sari A., Tuzen M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem Eng J. 2009;151:255–261. [Google Scholar]
- 13.Gupta V.K., Rastogi A. Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp. – a comparative study. Colloids Surf B: Biointerf. 2008;64:170–178. doi: 10.1016/j.colsurfb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Gupta V.K., Rastogi A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater. 2008;152:407–414. doi: 10.1016/j.jhazmat.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Deng L., Su Y., Su H., Wang X., Zhu X. Biosorption of copper (II) and lead (II) from aqueous solutions by nonliving green algae Cladophora f ascicularis: equilibrium, kinetics and environmental effects. Adsorption. 2006;12:267–277. [Google Scholar]
- 16.Gupta V.K., Rastogi A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater. 2009;163:396–402. doi: 10.1016/j.jhazmat.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 17.DÖnmez G., Aksu Z. Removal of chromium (VI) from saline wastewaters by Dunaliella species. Process Biochem. 2002;38:751–762. [Google Scholar]
- 18.Chojnacka K., Chojnacki A., Górecka H. Trace element removal by Spirulina sp. from copper smelter and refinery effluents. Hydrometallurgy. 2004;73:147–153. [Google Scholar]
- 19.El-Sheekh M.M., El-Shouny W.A., Osman M.E.H., El-Gammal E.W.E. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ Toxicol Pharmacol. 2005;19:357–365. doi: 10.1016/j.etap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Roy D., Greenlaw P.N., Shane B.S. Adsorption of heavy metals by green algae and ground rice hulls. J Environ Sci Health. 1993;28:37–50. [Google Scholar]
- 21.Carmichael WW. Isolation, culture and toxicity testing of toxic fresh water cyanobacteria (blue-green algae). In: Shilo V, editor. Fundamental research in homogeneous catalysis, vol. 3. New York: Gordon& Breach Publ.; 1986. P. 1249.
- 22.APHA. Standard Methods for the Examination of Water and Wastewater, 21th ed. Washington, DC: American Public Health Association; 2005.
- 23.Volesky B. Biosorption process simulation tools. Hydrometallurgy. 2003;71:179–190. [Google Scholar]
- 24.Murphy V., Hughes H., McLoughlin P. Cu(II) binding by dried biomass of red, green and brown macroalgae. Water Res. 2007;41:731–740. doi: 10.1016/j.watres.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Lodeiro P., Barriada J.L., Herrero R., Sastre de Vicente M.E. The marine macroalga cystoseira baccataas biosorbent for cadmium(II) and lead(II) removal: kinetic and equilibrium studies. Environ Pollut. 2006;142:264–273. doi: 10.1016/j.envpol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Gaur N., Dhankhar R. Removal of Zn+2 ions from aqueous solution using Anabaena variabilis: equilibrium and kinetic studies. Int J Environ Res. 2009;3(4):605–616. [Google Scholar]
- 27.King P., Rakesh N., Beenalahri S., Kumar P., Prasad V.S.R.K. Removal of lead from aqueous solution using syzygium cumini L. equilibrium and kinetic studies. J Hazard Mater. 2007;142:340–347. doi: 10.1016/j.jhazmat.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Kaewsarn P. Cadmium biosorption of copper(II) from aqueous solutions bypre-treated biomass of marine algae Padina sp. Chemosphere. 2002;47:1081–1085. doi: 10.1016/s0045-6535(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 29.Amini M., Younesi H., Bahramifar N., Lorestani A.A.Z., Ghorbani F., Daneshi A., Sharifzadeh M. Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J Hazard Mater. 2008;154:694–702. doi: 10.1016/j.jhazmat.2007.10.114. [DOI] [PubMed] [Google Scholar]
- 30.Karthikeyan S., Balasubramanian R., Iyer C.S.P. Evaluation of the marine algae Ulvafasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol. 2007;98:452–455. doi: 10.1016/j.biortech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Yin P.H., Yu Q.M., Jin B., Ling Z. Biosorption removal of cadmium from aqueous solution by using pretreated fungal biomass cultured from starch wastewater. Water Res. 1999;33:1960–1963. [Google Scholar]
- 32.Su-Hsia L., Ruey-Shin J. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manage. 2009;90:1336–1349. doi: 10.1016/j.jenvman.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Langmuir I. Adsorption of gases on plain surface of glass mica platinum. J Am Chem Soc. 1918;40:1361–1403. [Google Scholar]
- 34.Schiewer S, Volesky B. In: Lovely DR, editor. Environmental microbe –metal interactions. Washington, DC: ASM Press; 2000 [chapter 14].
- 35.Saygideger S., Gulnaz O., Istifli E.S., Yucel N. Adsorption of Cd(II), Cu(II) and Ni(II) ions by Lemna minor L.: effect of physicochemical environment. J Hazard Mater. 2005;126:96–104. doi: 10.1016/j.jhazmat.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 36.El-Shafey E.I. Sorption of Cd(II) and Se(IV) from aqueous solution using modified rice husk. J Hazard Mater. 2007;147:546–555. doi: 10.1016/j.jhazmat.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Tuzen M., Sari A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: equilibrium, thermodynamic and kinetic studies. Chem Eng J. 2010;158:200–206. [Google Scholar]
- 38.Kilislioglu A., Bilgin B. Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl Radiat Isotopes. 2003;50:155–160. doi: 10.1016/s0969-8043(02)00316-0. [DOI] [PubMed] [Google Scholar]
- 39.Dubinin M.M., Zaverina E.D., Radushkevich L.V. Sorption and structure of active carbons I. Adsorption of organic vapors. Zhurnal Fizicheskoi Khimii. 1947;21:1351–1362. [Google Scholar]
- 40.Khan S.A., Rehman U.R., Khan M.A. Adsorption of chromium (III), chromium (VI) and silver (I) on bentonite. Waste Manage. 1995;15:271–282. [Google Scholar]
- 41.Smith JM. Chemical engineering kinetics, 3rd ed. New York: McGraw-Hill; 1981. p. 310–22.



