Abstract
Transgenic rice (Oryza sativa) plants were engineered to express a N-(hydroxycinnamoyl)transferase from pepper (Capsicum annuum), which has been shown to have hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase activity, a key enzyme in the synthesis of hydroxycinnamic acid amides, under the control of constitutive maize (Zea mays) ubiquitin promoter. The transgenic rice plants require foliar application of amines to support synthesis of hydroxycinnamic acid amides, suggestive of limiting amine substrates in rice shoots. In addition, when T2 homozygous transgenic rice plants were grown in the presence of amines or phenolic acids, two novel compounds were exclusively identified in the leaves of the transgenic plants. These compounds eluted earlier than p-coumaroyltyramine and feruloyltyramine during HPLC chromatography and were identified as p-coumaroylserotonin and feruloylserotonin by liquid chromatography/mass spectrometry and other methods. To test whether the unpredicted production of serotonin derivatives is associated with the pepper N-(hydroxycinnamoyl)transferase, the substrate specificity of the pepper enzyme was analyzed again. Purified recombinant pepper N-(hydroxycinnamoyl)transferase exhibited a serotonin N-hydroxycinnamoyltransferase (SHT) activity, synthesized p-coumaroylserotonin and feruloylserotonin in vitro, and demonstrated a low Km for serotonin. SHT activity was inhibited by 10 to 50 mm tyramine. In addition, SHT activity was predominantly found in the root tissues of wild-type rice in parallel with the synthesis of serotonin derivatives, suggesting that serotonin derivatives are synthesized in the root of rice. This is the first report of SHT activity and the first demonstration, to our knowledge, that serotonin derivatives can be overproduced in vivo in transgenic rice plants that express serotonin N-(hydroxycinnamoyl)transferase.
Hydroxycinnamic acid amides (HCAA) are thought to play a role in plant defense against pathogen attacks (Clarke, 1982; Hahlbrock and Scheel, 1989; Von Roepenack-Lahaye et al., 2003). Consistent with this idea, HCAA are synthesized in the cytosol and transported into the cell wall, where they undergo peroxidative polymerization (Negrel and Martin, 1984; Negrel and Jeandet, 1987; Negrel and Lherminier, 1987; Negrel et al., 1993). The accumulation of HCAA in the cell wall creates a resilient barrier against pathogens by reducing cell wall digestibility and/or by directly inhibiting the growth of fungal hyphae (Grandmaison et al., 1993). Accordingly, the synthesis of HCAA is induced in response to various stresses, including physical injury, pathogen infection, and elicitor treatment. These responses have been observed in Solanaceae plants such as potato (Solanum tuberosum), tobacco (Nicotiana tabacum), tomato (Lycopersicon esculentum), and grass plants such as maize (Zea mays; Clarke, 1982; Negrel and Martin, 1984; Miyagawa et al., 1998; Pearce et al., 1998; Ishihara et al., 2000).
Hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase (THT; EC 2.3.1.110) is a key enzyme for synthesis of HCAA, such as N-feruloyltyramine and p-coumaroyltyramine. THT homologs have been isolated from tobacco leaves infected by tobacco mosaic virus (Negrel and Martin, 1984), California poppy (Eschscholzia california) cell-suspension cultures elicitated by chitosan (Villegas and Brodelius, 1990), and wounded maize leaves (Ishihara et al., 2000). Four genes encoding THT were cloned recently from various plants and the recombinant enzymes characterized enzymatically (Farmer et al., 1999; Schmidt et al., 1999; Back et al., 2001; Von Roepenack-Lahaye et al., 2003). These THTs are active as homodimers and have molecular masses of 26 to 28 kD; they share approximately 67% amino acid identity (Back et al., 2001).
THT catalyzes the synthesis of HCAA with two different types of substrates: hydroxycinnamoyl-CoA thioesters and aromatic amines. The hydroxycinnamoyl-CoA thioesters (e.g. cinnamoyl-CoA, p-coumaroyl-CoA, caffeoyl-CoA, feruloyl-CoA, and sinapoyl-CoA) are produced from cinnamic acid by a series of enzymes, including cinnamate-4-hydroxylase, coumarate-3-hydroxylase, caffeic acid O-methyltransferase, ferulate-5-hydroxylase, and hydroxycinnamate: CoA ligase (Douglas, 1996). Aromatic amines include tyramine, octopamine, dopamine, noradrenaline, and tryptamine. Tyramine and tryptamine are produced by decarboxylation of Tyr and Trp, respectively.
The broad substrate specificity of THT is accompanied by a broad range of affinity for these substrates. The kinetics of THT from potato, tobacco, poppy, maize, pepper (Capsicum annuum), and tomato were studied in detail (Hohlfeld et al., 1995; Negrel and Javelle, 1997; Yu and Facchini, 1999; Ishihara et al., 2000; Back et al., 2001; Von Roepenack-Lahaye et al., 2003). These THTs have a relatively high affinity for feruloyl-CoA, sinapoyl-CoA, 4-coumaroyl-CoA, and caffeoyl-CoA, but tobacco and poppy THT do not utilize caffeoyl-CoA as a substrate (Negrel and Martin, 1984; Yu and Facchini 1999), and maize THT does not utilize cinnamoyl-CoA as a substrate. Similarly, THTs preferentially utilize specific amines as acyl acceptors in the presence of feruloyl-CoA. Tyramine is bound by THT with the highest affinity, followed by octopamine and dopamine. Potato THT binds noradrenaline, but tobacco THT does not. Interestingly, maize THT has higher affinity for tryptamine than for tyramine.
p-Coumaroylserotonin and feruloylserotonin are another class of HCAA found in plants. They have been reported to be antioxidants (Zhang et al., 1996) and to inhibit injury associated with ischemia-reperfusion (Hotta et al., 2002). These serotonin derivatives have only been detected in certain families of plant seeds or in the twigs of the diseased bamboo (Pavlik et al., 2002; Tanaka et al., 2003), and the pathway for their biosynthesis has not been identified.
This study characterizes transgenic rice (Oryza sativa) plants that overexpress pepper N-(hydroxycinnamoyl)transferase possessing THT activity to see whether the heterologous expression of N-(hydroxycinnamoyl)transferase enhances the synthesis of HCAA in rice. The N-(hydroxycinnamoyl)transferase overexpressing transgenic rice plants produce HCAA, such as feruloyltyramine, upon the foliar treatment of exogenous amines. Fortuitously, it was discovered that these transgenic rice plants also overproduce novel N-hydroxycinnamoylserotonins, such as p-coumaroylserotonin and feruloylserotonin. The overproduction of these serotonin derivatives in transgenic rice plants requires the serotonin N-hydroxycinnamoyltransferase (SHT) transgene and involves a relatively complex pathway that reflects the intrinsic characteristics of pepper SHT. The properties and accumulation mechanism of serotonin derivative in rice are discussed.
RESULTS
Transgenic Rice Plants Expressing Pepper N-(Hydroxycinnamoyl)transferase Harboring THT Activity
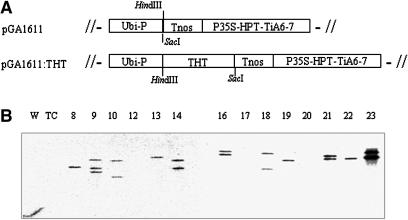
Transgenic rice plants expressing a pepper THT gene were generated by transforming wild-type rice (O. sativa cv Nakdong) with the binary vector pGA1611:THT (Fig. 1A). The full-length cDNA encoding pepper THT was subcloned into pGA1611 under the control of constitutive maize ubiquitin promoter; the vector carries the hygromycin phosphotransferase gene as a selectable marker. This construct was transferred into rice using a standard Agrobacterium tumefaciens-mediated rice transformation protocol (Lee et al., 2000). Fourteen independent transgenic lines (T0) were isolated and transferred to a greenhouse for production of T1 seeds. T1 transgenic rice plants surviving on Murashige and Skoog medium containing hygromycin (50 mg/L) were transplanted into a paddy field for production of T2 seeds. Twelve transgenic lines demonstrated stable integration of one to three copies of the pepper THT gene by Southern blot (Fig. 1B). Two additional lines, 12 and 20, had no detectable THT transgene on Southern blot. Line 17 also had a faint single band of THT. Transgene insertion locus was estimated by counting the number of hybridizing HindIII fragments and by evaluating segregation of resistance to hygromycin. All lines except line 9 segregated hygromycin resistance in a 3:1 ratio (resistant:susceptible); line 9 has two independently segregating copies of the hygromycin resistance gene (data not shown), and all other lines appear to have one functional genetic locus. Nevertheless, lines 10, 14, 16, 18, 21, and 23 have two copies of the THT gene by Southern blot.
Figure 1.
Schematic diagram of T-DNA region in binary vector (A) and Southern analysis of transgenic lines (B). Ubi-P, maize ubiquitin promoter; Tnos, nopaline synthase terminator; P35S, cauliflower mosaic virus 35S promoter; HPT, hygromycin phosphotransferase; TiA6-7, terminator of TiA6-7. HindIII-digested rice genomic DNA was probed with THT cDNA. DNA of the wild type (W) and of the transgenic control (TC) expressing a vector only (pGA1611) were included as negative and positive controls, respectively.
Expression of the THT Transgene in Transgenic Rice
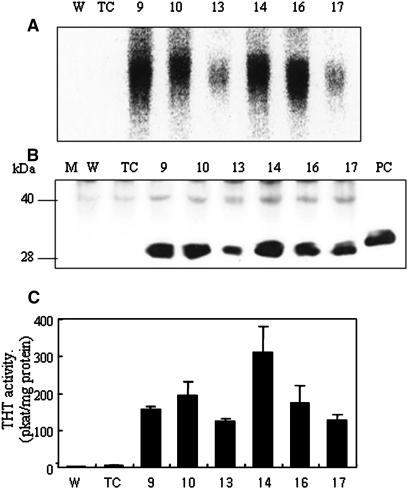
Six independent transgenic lines (T1) were selected for further analysis. Expression of the pepper THT gene was analyzed by northern blot using total RNA from wild-type and transgenic rice leaves and a pepper THT cDNA probe. Pepper THT mRNA was detected in all THT transgenic plants but not in wild-type or transgenic control plants (Fig. 2A). The expression level varied significantly: lines 13 and 17 expressed a low level and lines 9, 10, 14, and 16 expressed a relatively high level of pepper THT mRNA. Western-blot analysis also showed strong cross-reactivity with THT antibody in THT transgenic but not in wild-type or transgenic control plants, suggesting that THT mRNA was translated efficiently in the transgenic rice plants (Fig. 2B).
Figure 2.
Transgene expression in wild-type and THT-overexpressing transgenic rice plants. A, Northern-blot analysis. Ten micrograms of total RNA was separated on formamide gel, blotted, and probed with pepper THT cDNA as described in “Materials and Methods.” B, Western-blot analysis. Protein was extracted from the seedlings of independent transgenic lines (T1) and the wild-type control. Equal amounts of denatured total soluble protein (50 μg) were separated on SDS-PAGE, electrotransferred onto polyvinylidene difluoride membrane, and incubated with primary antibody raised against a purified recombinant pepper THT protein. M, protein molecular size marker; PC, purified THT (20 ng). C, THT enzyme activity. THT enzyme activity was measured in extracts of leaves of wild-type and transgenic rice (T1). THT activity is expressed as pkat (mg protein)−1.
The THT polypeptide produced in transgenic plants was also tested for enzymatic activity. THT activity, assayed in vitro by measuring production of feruloyltyramine, correlated well with the absolute amount of THT protein (Fig. 2C). Lines 10 and 14 express a higher level of THT protein and show higher levels of enzyme activity in vitro, while lines 13 and 17 express a low level of THT protein and activity. The enzyme activity of lines 10 and 14 (approximately 260 pkat mg−1 protein) is as high as the peak activity reported in maize after wound treatment (Ishihara et al., 2000).
Product Analyses in Transgenic Rice Plants
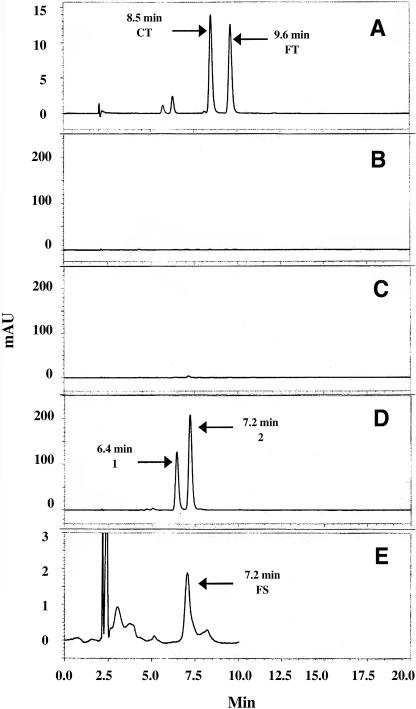
The in vivo activity of pepper THT in transgenic rice plants was also assessed. Leaves of the wild type and the homozygous T2 transgenic line (line 14) were extracted with methanol, and the extract was separated on a Sep-Pak cartridge followed by HPLC to quantify HCAA (Fig. 3). No HCAA were detected in wild-type or transgenic leaves (Fig. 3, B and C), and no HCAA were detected in wild-type or transgenic leaves of 8- to 10-week-old plants grown in field (data not shown). However, when plants (line 14) were grown in the presence of 50 μm tyramine (Fig. 3D), two compounds (compound 1, 6.4 min; compound 2, 7.2 min) were detected that elute faster than p-coumaroyltyramine (8.5 min) and feruloyltyramine (9.6 min; Fig. 3A).
Figure 3.
HPLC profiles of THT products from wild-type and transgenic rice (line 14, T2 plants). A, Authentic compounds. CT, p-coumaroyltyramine; FT, feruloyltyramine. HPLC chromatograms of extracts from leaves of wild-type (B) and transgenic rice (C and D). Sterilized seeds were sown and grown in Murashige and Skoog medium. Leaves of 15-d-old rice plants were extracted with methanol, and the extract analyzed by HPLC as described in “Materials and Methods.” D, Two unknown compounds detected in HPLC profile from transgenic plants, which were grown in Murashige and Skoog medium supplemented by 50 μm tyramine, not in HPLC profile of transgenic rice plants grown in the absence of tyramine (C). Extracts were prepared as described above. Purified recombinant pepper THT (0.5 μg) was coincubated in vitro with feruloyl-CoA and serotonin, and the reaction product was analyzed by HPLC (E).
Identification of Compounds 1 and 2
Compounds 1 and 2 were identified by the following procedure. Ion-spray liquid chromatography/mass spectrometry (MS) identified the Mr of compound 1 as 322 (the protonated molecule [M+H]+ has an mass-to-charge ratio [m/z] equal to 323), and a fragment of compound 1 was identified with m/z equal to 147; this fragment is characteristic of esters and amides of p-coumaric acid, suggesting that compound 1 is a p-coumaroyl derivative. Similar analysis of compound 2 indicated an Mr of 352 and the presence of a feruloyl moiety. The remaining moiety, common to compounds 1 and 2, was deduced to have an Mr of 176. The UV spectra of compounds 1 and 2 were similar to those of p-coumaroyltyramine and feruloyltyramine. If compounds 1 and 2 have an even molecular mass and are amides, the unknown moiety should have an even number of nitrogen atoms on the basis of the nitrogen rule (Silverstein and Webster, 1997). It was concluded that compounds 1 and 2 may be p-coumaroylserotonin and feruloylserotonin, respectively, suggesting that pepper THT might synthesize these compounds in vivo.
The latter possibility was tested by examining whether pepper THT could synthesize feruloylserotonin in vitro from feruloyl-CoA and serotonin. The in vitro enzyme product was analyzed by HPLC and compared to the unknown compounds produced in vivo (Fig. 3E). The in vitro reaction product had a retention time of 7.2 min, comigrating with unknown compound 2, suggesting that compound 2 is feruloylserotonin.
Compounds 1 and 2 were positively identified to be p-coumaroylserotonin and feruloylserotonin, respectively, by comparing their chromatographic properties and ion-spray MS and UV spectra with the authentic compounds synthesized chemically. p-Coumaroylserotonin and feruloylserotonin were synthesized from p-coumaroyl- and feruloyl-N-hydroxysuccinimide esters with serotonin, similar to the procedure used to synthesize hydroxycinnamoyl-CoA (Stökigt and Zenk, 1975).
Kinetic Studies of Purified Recombinant Pepper N-(Hydroxycinnamoyl)transferase
In a previous study, recombinant His6-tagged pepper THT was purified to homogeneity by Ni+2 affinity chromatography (Back et al., 2001). The same homogenous recombinant enzyme was used in the following experiments on the kinetics and substrate specificity of SHT. Various cinnamoyl-CoA esters and aromatic amines were used as acyl donors and acceptors, respectively (Table I). In addition to the p-coumaroyl-CoA (Km = 23.2 μm; Vmax = 35 nkat mg−1 protein) and feruloyl-CoA (Km = 3.5 μm; Vmax = 7 nkat mg−1 protein), the enzyme utilized other cinnamoyl-CoA esters, such as caffeoyl-CoA (Km = 12.7 μm; Vmax = 31 nkat mg−1 protein) and cinnamoyl-CoA (Km = 31.9 μm; Vmax = 23 nkat mg−1 protein) with serotonin as acyl acceptor. The Km for various substrates differed significantly within the range 3.5 to 32 μm. Variable Km values have also been detected with other THT enzymes. Notably, the Km for tyramine and serotonin differed significantly when feruloyl-CoA was the acyl donor. Pepper N-(hydroxycinnamoyl)transferase has a higher affinity for serotonin (Km = 73 μm) than for any other amine substrate and the second lowest affinity for tyramine (Km = 1,165 μm) when feruloyl-CoA is acyl donor. Thus, Km for serotonin is 16-fold lower than for tyramine with this acyl acceptor. The Vmax/Km is similar for p-coumaroyl-CoA (4.3) and feruloyl-CoA (5.8), which is consistent with the observation that p-coumaroylserotonin and feruloylserotonin were produced in similar amounts in vivo (Table III). It is likely that transgenic rice plants produce serotonin derivatives and do not produce N-hydroxycinnamoyltyramines because of the relative Vmax/Km of SHT for serotonin and tyramine.
Table I.
Substrate specificity of pepper SHT
| Substrates | Km | Relative Vmax | Vmax/Km |
|---|---|---|---|
| μm | % | ||
| Acyl donorsa | |||
| Caffeoyl-CoA | 12.7 | 89 | 7.0 |
| Feruloyl-CoA | 3.5 | 20 | 5.8 |
| p-Coumaroyl-CoA | 23.2 | 100 | 4.3 |
| Cinnamoyl-CoA | 31.9 | 66 | 2.1 |
| Acyl acceptorsb | |||
| Serotonin | 73 | 22 | 0.302 |
| Phenethylamine | 1389 | 100 | 0.072 |
| Dopamine | 721 | 27 | 0.038 |
| Tryptamine | 848 | 17 | 0.020 |
| Tyramine | 1165 | 19 | 0.016 |
Serotonin (1 mm) was used as the acyl acceptor.
Feruloyl-CoA (250 μm) was used as the acyl donor.
Table III.
Leaf contents of serotonin derivatives in transgenic rice plants
| Plants | Coumaroylserotonin | Feruloylserotonin | Total Serotonin Derivatives |
|---|---|---|---|
| μg g−1 fresh weight | |||
| WT | 0.8 ± 0.4 | 1.7 ± 0.8 | 2.5 ± 0.6 |
| TC | 0.9 ± 0.3 | 1.1 ± 0.4 | 2.0 ± 0.3 |
| 9 | 24 ± 05 | 24 ± 08 | 48 ± 07 |
| 10 | 86 ± 13 | 114 ± 20 | 200 ± 11 |
| 13 | 224 ± 25 | 200 ± 30 | 424 ± 27 |
| 14 | 46 ± 04 | 78 ± 10 | 124 ± 07 |
| 16 | 50 ± 08 | 70 ± 04 | 120 ± 06 |
| 17 | 36 ± 03 | 96 ± 06 | 132 ± 05 |
Rice seeds were grown in half-strength medium for 15 d in the presence of tyramine (50 μm), and the leaves of the rice were harvested and subjected to HPLC analyses. WT, Wild type; TC, transgenic control; 9 to 17; transgenic rice. Data represent the means ± sd of duplicates of the same sample. −, No detection.
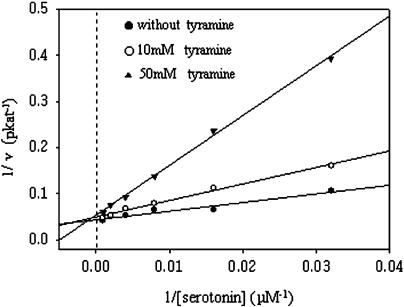
The interdependency of THT and SHT activities was tested by Lineweaver-Burk analysis (Fig. 4). SHT activity was measured in the presence of various concentrations of tyramine. SHT activity was inhibited by 10 to 50 mm tyramine but not by low concentrations of tyramine (i.e. 3 mm). In the presence of tyramine, SHT activity had an increased Km but constant Vmax. This result implies that tyramine competes for serotonin as acyl acceptor, although it must be present in molar excess for effective competition.
Figure 4.
Inhibition of SHT activity by tyramine. SHT activity was measured in the absence or presence of different concentrations of tyramine; data was analyzed using a Lineweaver-Burk plot. Acyl donor was feruloyl-CoA (250 μm), and serotonin was acyl acceptor.
Substrate Effects on Synthesis of Hydroxycinnamic Acid Amides in Vivo
Pepper SHT synthesizes HCAA efficiently in vitro, thus it was surprising that HCAA were not produced in transgenic rice plants grown under standard conditions. This phenomenon was possibly caused by limiting substrate in vivo because HCAA were produced only when the transgenic plants were sprayed with tyramine or serotonin (Table II). However, the reason for production of serotonin derivatives and not tyramine derivates was not clear (Fig. 3). Thus, parameters that might determine the nature of the reaction product were examined.
Table II.
Synthesis of phenolic amides in leaf tissues by substrate application to the transgenic rice plants
| Products
|
|||
|---|---|---|---|
| Substrates | Feruloyltyramine | p-Coumaroylserotonin | Feruloylserotonin |
| nmol g−1 fresh weight | |||
| Leaf spray methoda | |||
| Wild-type | |||
| Tyramine | – | – | – |
| Serotonin-HCl | – | – | – |
| Transgenic rice | |||
| p-Coumaric acid | – | – | – |
| Tyramine | 6.6 ± 1.2 | – | – |
| Serotonin-HCl | – | – | 7.9 ± 2.0 |
| MS media containing various substratesb | |||
| MS medium | – | – | – |
| CuSO4 | – | – | – |
| Chitin | – | – | – |
| Cinnamic acid | – | 340 ± 45 | 462 ± 47 |
| p-Coumaric acid | – | 81 ± 10 | 153 ± 15 |
| Caffeic acid | – | 125 ± 20 | 157 ± 16 |
| Ferulic acid | – | 178 ± 30 | 275 ± 28 |
| Serotonin-HCl | – | – | – |
| Dopamine-HCl | – | – | – |
| Tryptamine | – | 127 ± 24 | 185 ± 21 |
| Tyramine | – | 186 ± 16 | 290 ± 40 |
| Coumaric acid + tyramine | − | 56 ± 07 | 165 ± 12 |
One millimolar substrates were sprayed onto the leaves of wild and transgenic rice (line 14), harvested after 24 h, and then quantified the products by HPLC.
Transgenic rice seeds were grown in half-strength Murashige and Skoog medium for 15 d in the presence of various substrates (50 μm), CuSO4 (0.5 mm), and chitin (10 μg/mL of N-acetylchitiheptaose; Seikagaku, Tokyo). The leaves of the rice were harvested and subjected to HPLC analyses. Each experiment was carried out at least twice and expressed as mean ± sd. –, No detection.
Transgenic rice plants (15 d old) were sprayed with various SHT substrates (1 mm), and reaction products were examined. HCAA were produced only when amine substrates were applied by spraying, suggesting that amine substrates are deficient in rice shoot tissues (Table II). Feruloyltyramine and feruloylserotonin were not produced in plants sprayed with p-coumaric acid but were produced in plants sprayed with amines. By contrast, no HCAA were detected upon foliar treatment with amine substrates in wild-type plant. Different results were obtained when substrates were included in the growth medium. For example, transgenic rice plants produced large amounts of feruloylserotonin and p-coumaroylserotonin when grown in the presence of phenolic acids, including cinnamic acid, p-coumaric acid, caffeic acid, and ferulic acid. By contrast, only tyramine or tryptamine induced serotonin derivative production among the various amine substrates tested. The addition of either serotonin or dopamine did not result in the synthesis of serotonin derivatives. In particular, there were no induction effects of nonspecific elicitors such as CuSO4 or chitin for the production of HCAA.
Serotonin Derivatives in Transgenic Rice Plants
To confirm that pepper SHT-carrying T2 homozygous transgenic plants have the capacity to synthesize serotonin derivatives when growth media is supplemented with 50 μm tyramine (free base), extracts of six independent transgenic lines were analyzed by HPLC. As shown in Table III, all SHT transgenic produced p-coumaroylserotonin and feruloylserotonin. For different transgenic strains, the amount of the serotonin derivatives varied from 48 to 424 μg per gram fresh weight. Unlike foliar treatment, the preferential production of feruloylserotonin was not observed when rice plants were grown for a long time in the presence of tyramine. The relative level of p-coumaroylserotonin and feruloylserotonin was similar in each line, which matched well with its similar Vmax/Km value between p-coumaroyl-CoA and feruloyl-CoA for serotonin (Table I). Interestingly, control plants were able to produce low levels of serotonin derivatives ranging from 2.0 to 2.5 μg per gram of fresh weight shoot tissues. The relative level of SHT protein did not correlate well with the production of serotonin derivatives. For example, transgenic line 14 had the highest level of SHT protein, but it produced the third lowest quantity of serotonin derivatives; by contrast, line 13 had the lowest SHT mRNA and protein but produced the highest quantity of serotonin derivatives.
Effects of Tyramine on Enzyme Activities, Serotonin Levels, and Phenolic Amide Contents
To determine whether tyramine supplementation in the medium affects enzyme activities responsible for serotonin derivatives synthesis, we analyzed SHT and Trp decarboxylase (TDC) activities as well as serotonin and phenolic amide contents in both the shoots and the roots of wild-type and transgenic rice.
SHT activities in both the shoots and the roots of wild-type and transgenic rice plants grown in the 50 μm tyramine did not increase compared to those grown in the absence of tyramine, demonstrating that the enormous accumulation of serotonin derivatives in transgenic shoots has nothing to do with the induction of SHT enzyme activity (Table IV). Interestingly, we found for the first time, to our knowledge, that SHT activity in the wild-type rice was present predominantly in the roots, showing a 10-fold higher level (3.4 pkat mg−1 protein) than in the shoots (0.3 pkat mg−1 protein). The higher expression of SHT in the roots was in agreement with previous findings that the peak THT activity of wheat (Triticum aestivum) and barley (Hordeum vulgare) and the abundant level of a potato THT mRNA were present in the roots (Louis and Negrel, 1991; Schmidt et al., 1999). A slightly higher level of SHT activity in the shoots rather than in the roots of transgenic rice seems to be ascribed to the functional characteristics of a maize ubiquitin promoter (Cornejo et al., 1993).
Table IV.
Effects of tyramine on SHT, TDC enzyme activities, serotonin, and phenolic amide contents
| Phenolic Amides
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| SHT Activity | TDC Activity | Serotonin | CT | FT | CS | FS | |||
| nmol g−1 fw | μg g−1 fw | ||||||||
| Wild-type rice | |||||||||
| MS | Shoot | 0.3 | 0.36 | 3.39 | – | – | – | – | |
| Root | 3.4 | 1.01 | – | – | – | 0.31 | 0.13 | ||
| MS + Tyra | Shoot | 0.2 | 0.79 | 2.13 | – | – | 1.25 | 2.29 | |
| Root | 2.5 | 1.87 | – | 0.12 | 1.08 | 0.55 | 0.09 | ||
| Transgenic rice (line 14) | |||||||||
| MS | Shoot | 78 | 0.95 | – | – | – | – | – | |
| Root | 52 | 1.83 | – | – | – | 0.36 | 0.16 | ||
| MS + Tyra | Shoot | 73 | 0.68 | – | 0.17 | 0.24 | 43.5 | 74.3 | |
| Root | 40 | 1.37 | – | 4.03 | 8.77 | 1.56 | 0.16 | ||
Rice seeds were grown in half-strength Murashige and Skoog medium for 7 d in the presence or absence of tyramine (50 μm), and the rice was harvested and subjected to HPLC analyses. SHT and TDC activities are expressed as pkat mg−1 protein. fw, Fresh weight; Tyra, tyramine; CT; coumaroyltyramine; FT, feruloyltyramine; CS, coumaroylserotonin; FS, feruloylserotonin. Data represent the average of duplicates of the same sample. –, No detection.
An accumulation of serotonin derivatives such as p-coumaroylserotonin and feruloylserotonin were obviously observed in the roots of wild-type and transgenic rice grown in the absence of tyramine. These levels were similar between wild-type and transgenic rice plants showing an average of 0.44 μg and 0.52 μg of the total serotonin derivatives per gram of fresh weight root material, respectively, regardless of the levels of SHT activities. By contrast, the roots grown in the presence of tyramine resulted in the accumulation of tyramine derivatives such as p-coumaroyltyramine and feruloyltyramine as well as the serotonin derivatives. This result of tyramine derivatives production was in agreement with the foliar application of tyramine in transgenic shoots but disagreed with the result of an exclusive production of feruloyltyramine in transgenic shoots sprayed with tyramine (Table II).
Neither the TDC activities nor serotonin contents increased upon tyramine treatments, indicating that tyramine does not play a role as an elicitor responsible for the abundant synthesis of serotonin derivatives in shoot tissues (Table IV). TDC activity was about 2-fold higher in the roots than in the shoots and ranged from 0.36 to 1.83 pkat mg−1 protein, which was consistent with the values reported by Lopez-Meyer and Nessler (1997) and Ueno et al. (2003). As for a negative control of TDC assay, we employed tobacco, which was reported to lack TDC gene (Fiore et al., 2002). TDC activities from leaves and suspension-cell culture of tobacco were not detected at all. Neither incubations omitting the protein extract nor the incubation conducted with a boiled extract catalyzed the TDC reaction.
Only a low level of serotonin was detected in the shoot tissues of the wild-type rice, suggesting that free serotonin seems to be metabolized rapidly by either endogenous or transgene SHT enzymes, especially in the transgenic rice and the root tissues of wild-type rice.
As for the control wild-type rice, the total serotonin derivatives (0.44 μg) accumulated due to its high SHT activity (3.4 pkat mg−1 protein) in the root tissues but not in the shoot tissues. By contrast, an enhanced accumulation of serotonin derivatives was obtained in the shoot tissues (3.54 μg) grown in the presence of tyramine, even though SHT activities were low. When rice seedlings were grown in the presence of tyramine, the total levels of p-coumaroylserotonin and feruloylserotonin in the shoot tissues of the wild-type and transgenic rice were significantly enhanced approximately 5-fold and 68-fold compared to those of the root tissues, respectively.
Thus, the high accumulation of the serotonin derivatives in the shoot tissues both in wild-type and transgenic rice plants may be accounted for by the transport of serotonin derivatives from the root to shoot tissues.
DISCUSSION
This study characterizes transgenic rice plants that express pepper SHT, an enzyme that synthesizes HCAA such as p-coumaroylserotonin and feruloylserotonin. This SHT property was overlooked in the preliminary characterization of the recombinant protein in which pepper N-(hydroxycinnamoyl)transferase has a high affinity toward various cinnamoyl-CoA esters, including cinnamoyl-CoA, sinapoyl-CoA, feruloyl-CoA, caffeoyl-CoA, p-coumaroyl-CoA, and amine substrates such as tyramine and octopamine (Back et al., 2001). The rediscovery of SHT was achieved by characterizing the transgenic rice plants expressing pepper N-(hydroxycinnamoyl)transferase that had the ability to synthesize p-coumaroylserotonin and feruloylserotonin in abundance. Overproduction of these serotonin derivatives in transgenic rice was catalyzed by the heterologous pepper SHT, which accepts many aromatic amines as substrates, including serotonin. From our studies, it is now well established that recombinant pepper N-(hydroxycinnamoyl)transferase has higher affinity for serotonin than tyramine, as shown by the exclusive synthesis of serotonin derivatives instead of tyramine derivatives in vivo. This is also evident from pepper SHT kinetic parameters for serotonin and tyramine: pepper SHT has a 16-fold lower Km for serotonin than for tyramine with feruloyl-CoA as acyl donor, and SHT activity is only inhibited by molar excess (i.e. 10 mm) of tyramine. It is worth noting that maize THT has a very high affinity for tryptamine, which is similar in structure to serotonin (i.e. 5-hydroxytryptamine; Ishihara et al., 2000).
From the transgenic rice plants expressing pepper SHT, it was formally possible that HCAA would have been constitutively produced; by contrast, no HCAA were produced in the shoot tissues. This is most likely due to either limiting amine substrates in the rice (Table II) or the limited synthesis of Tyr or Trp, precursors of amine compounds, by an extremely low-level expression of corresponding enzymes (Table IV; Ueno et al., 2003).
Amine substrates such as tyramine, octopamine, tryptamine, serotonin, and dopamine are produced from amino acids (e.g. Tyr, Trp, and dihydroxyphenylalanine) by the activity of specific decarboxylases. There are two putative rice Tyr decarboxylases (accession nos. BAB56067 and AAM74316) and one rice TDC (Ueno et al., 2003), indicating that rice cells may have enzymes with the capacity to synthesize tyramine and tryptamine, respectively.
In addition, rice grains also have a high concentration of serotonin and melatonin (N-acetyl-5-methoxytryptamine), a metabolite of serotonin (Badria, 2002), which suggests that rice cells have genes responsible for the synthesis of serotonin.
Transgenic rice plants expressing pepper SHT did not produce HCAA in response to foliar treatment with 1 mm jasmonic acid or wounding (data not shown). Thus, jasmonic acid and wounding may not stimulate synthesis of serotonin (or other amines) in rice; alternatively, amines may be produced in rice cells in response to jasmonic acid but not be a substrate for pepper SHT expressed in rice possibly due in part to either the compartmentalization of aromatic amine formation or extremely low levels of synthesis.
Serotonin derivatives, such as p-coumaroylserotonin and feruloylserotonin, have been detected in several plant species, including Carthamus tinctorius, Echinochloa utilis, and Amorphophallus konjac (Zhang et al., 1996; Watanabe, 1999; Niwa et al., 2000). Serotonin derivatives may function as antibacterial compounds, antioxidants, suppressors of proinflammatory cytokine production, antitumor compounds, or inhibitors of ischemia-reperfusion (Kawashima et al., 1998; Nagatsu et al., 2000; Hotta et al., 2002; Kumarasamy et al., 2003), but little is known about how their activities are regulated.
In particular, no highly homologous rice SHT is present in the GenBank or in the MAFF Rice DNA Bank (Japan), which is consistent with our western-blot analysis showing that the pepper SHT polyclonal antibodies did not detect immunoreactive polypeptide in the protein extract of wild-type rice. However, there is one N-acetyltransferase-like gene (AK073240; MAFF Rice DNA Bank) showing a 26% amino acid identity with pepper SHT. Therefore, we assayed whether rice exhibits SHT activity from different tissues of rice plants. Surprisingly, it was found that SHT activity was confined to the root tissues accompanied with the constitutive synthesis of p-coumaroylserotonin and feruloylserotonin, albeit a low level, suggestive of the definite existence of functional SHT in rice. The functional role of a putative rice N-acetyltransferase-like gene as SHT remains to be studied.
In this study, high concentrations of serotonin derivatives were found in the shoot tissues of both wild-type and transgenic rice plants upon treatments with amines or phenolic acids in the medium. The mechanism by which tyramine and/or phenolic acids induce production of serotonin derivatives is ascribed to neither the induced synthesis of serotonin nor the induction of TDC or SHT (Table IV). The additions of tyramine significantly stimulated serotonin derivative production, especially in the shoot tissues rather than in the root tissues, irrespective of the SHT specificity. Because of an inverse correlation between the accumulation of serotonin derivatives and the SHT enzyme activity, we speculate that the serotonin derivatives biosynthesized in the root tissues may be transported to the shoot tissues. In plants, there was a well-known example of transporting secondary metabolite from the root to the shoot. Nicotine is synthesized in the root and then transported to aerial parts of the plant via the xylem (Wink and Roberts, 1998). However, it is not clear how amine/phenolic treatment induces serotonin derivative formation. Further study of the effects of aromatic amines or phenolic acids on the shikimate/phenylpropanoid pathways and on the regulation of Trp biosynthesis would be necessary for a better understanding of serotonin derivatives biosynthesis in plants (Yao et al., 1995; Guillet et al., 2000). Also, considering that indole-3-acetic acid is synthesized from Trp, we cannot rule out an interference with auxin biosynthesis (Tanaka et al., 2003).
The different patterns of serotonin derivative production between the foliar treatment and the media supplementation on the effects of tyramine in rice plants can be attributed to the difference of substrate absorption. Although tyramine was absorbed and bioavailable to pepper SHT when applied to rice by foliar treatment, a low level of tyramine was absorbed into the shoot tissues and thus utilized as a substrate for SHT transgene, resulting in the preferential synthesis of feruloyltyramine in the shoot tissues. This result was also consistent with a finding that higher ratios of feruloyltyramine to p-coumaroyltyramine were obtained in the root tissues when absorbed through the plant root (Table IV).
Based on the kinetics of SHT, tyramine is employed as a substrate, but tyramine-derived amides are not produced except in the tissues treated with the tyramine exogenously. This phenomenon may be attributed to a variety of reasons, such as the great difference in Km between tyramine and serotonin, the inability to produce tyramine under our experimental conditions, and the lack of functional gene coding for tyramine synthesis in rice.
Given the SHT cDNA cloned from pepper, it is of interest to determine whether pepper is able to synthesize serotonin derivatives. For this purpose, we analyzed the synthesis of serotonin derivatives in pepper challenged with wounding. It was found that p-coumaroylserotonin (500 ng g−1 fruit) and feruloylserotonin (291 ng g−1 fruit) were only detected in the wounded fruit but not in the unwounded fruit, whereas the wounded or the control leaves had undetectable levels of serotonin derivatives (data not shown). Unfortunately, no known data on the synthesis of serotonin derivatives in pepper have been found elsewhere, other than a report on the tyramine derivatives accumulated in response to pathogen and wounding (Pearce et al., 1998; Newman et al., 2002). The physiological and biochemical significance between SHT expression and the synthesis of serotonin derivatives in pepper remain to be clarified.
In conclusion, this study shows that transgenic rice plants expressing pepper SHT can overproduce p-coumaroylserotonin and feruloylserotonin. This is the first report that recombinant pepper transferase possessing THT activity has SHT activity in vivo, and this result was confirmed by using purified recombinant pepper SHT in vitro. Additionally, for the first time to our knowledge, we have shown that serotonin derivatives are synthesized in the root tissues of rice in parallel with the high SHT activity. Further work will focus on elucidating the possible functions of serotonin derivatives against pathogen attack (Kumarasamy et al., 2003; Tanaka et al., 2003), as well as the kinetic analysis of a putative rice N-acetyltransferase.
MATERIALS AND METHODS
Construction of Binary Vector and Transgenic Rice Plants
Agrobacterium tumefaciens-mediated transformation was utilized to generate transgenic rice (Oryza sativa) plants. Scutellum-derived rice calli (O. sativa cv Nakdong) were cocultured with A. tumefaciens strain LBA4404 harboring pGA1611:THT binary vector. The pepper (Capsicum annuum) THT gene was amplified with the following primers: 5′-ATCAAGCTTATGGCTTCTGCTCCTCAA-3′ (HindIII site underlined) and 5′GGTGGAGCTCCTAACAGCTTCCTGCACC-3′ (SacI site underlined). The PCR product was digested with HindIII and SacI, gel purified, and ligated into the same restriction sites within the pBluescript SK− (Stratagene, La Jolla, CA). After verifying the DNA sequence, the HindIII and SacI fragments of THT were ligated into HindIII/SacI cleaved pGA1611 between the maize (Zea mays) ubiquitin promoter and the nopaline synthase 3′ terminator. The resulting pGA1611:THT was transformed into A. tumefaciens LBA4404. Rice transformation was performed as described previously (Lee et al., 2000).
Isolation and Analysis of Nucleic Acids
Total RNA (10 μg) was isolated from leaves of transgenic or wild-type rice plants using TRI reagent (Sigma, St. Louis) and fractionated on a 1% agarose gel containing formaldehyde using 20 mm 3-(N-morpholino)propanesulfuric acid as a running buffer. The gel was blotted to a nylon membrane and hybridized with the pepper THT cDNA. RNA blot hybridizations were performed with the pepper THT cDNA clone radiolabeled using a Prime-It kit (Stratagene) at 60°C in 0.25 m sodium phosphate buffer (pH 7.5), 7% SDS, 1% bovine serum albumin, and 1 mm EDTA. After hybridization, the RNA blot was washed at 55°C, twice with 2× SSC/0.1% SDS and twice with 0.2× SSC/0.1% SDS (Sambrook et al., 1989; Lee et al., 2000). RNA was stained with ethidium bromide prior to blotting. Genomic DNA was isolated using the DNAzol ES (Molecular Research Center, Cincinnati). Five micrograms of genomic DNA was digested with HindIII, size-fractionated by electrophoresis in an 0.8% agarose gel, and blotted to nylon membrane (Nylon 66 plus; Pharmacia Biotech, San Francisco). Hybridizations were performed as described above.
Immunoblotting
A polyclonal mouse antiserum was raised against purified pepper THT protein (Back et al., 2001). The pepper THT protein was detected in extracts from rice leaves by western blot. Total soluble proteins (50 μg) were homogenized in a mortar and pestle with 1 mL of homogenization buffer: 80 mm Tris/HCl (pH 7.0), 20% (w/v) glycerol, 10 mm sodium metabissulfate, 10 mm sodium ascorbate, 1% (w/v) polyvinylpyrrolidone, 5 mm β-mercapthoethanol, and 2 mm EDTA. After centrifuging for 10 min at 13,500g, the supernatant extracts were employed as total soluble proteins. Proteins were separated by 11% SDS-PAGE and electroblotted to polyvinylidene difluoride membrane. Immunodetection was performed according to standard procedures (Boehringer Mannheim, Basel).
Analyses of Products in Transgenic Rice Plants
The N-(hydroxycinnamoyl)-amines were analyzed as described previously (Ishihara et al., 2000). Briefly, rice leaves (250 mg) were ground to powder in liquid nitrogen and extracted with 3 mL of methanol. The samples were centrifuged at 12,000g for 10 min. The supernatants were passed through a Sep-Pak C18 cartridge (Waters, Milford, MA) and further fractionated with a Sep-Pak Silica cartridge (Waters). The fraction eluted in chloroform:methanol (30:1) was evaporated to dryness and dissolved in 0.5 mL of methanol. This solution was analyzed by a reversed-phase HPLC (Shimadzu 10A equipped with a photodiode array detector, Shimadzu SPD-M 10A VP; Columbia, MD). Compounds were separated on a Wakosil II 3C18HG column (4.6 × 150 mm; Wako Pure Chemical , Osaka) with isocratic elution with 35% (v/v) methanol in water containing 0.5% trifluoroacetic acid at a flow of 0.8 mL/min. Elution of compounds was detected at 320 nm. Two unknown compounds (1 and 2) were found in this chloroform:methanol (30:1) fraction. The UV spectra of the compounds were recorded with the HPLC system equipped with photodiode array detector. The positive ion-spray MS spectra were measured with a Perkin-Elmer Sciex API-165 instrument (ion-spray voltage, 5 kV; orifice voltage, 30 V; nebulizer gas, air, curtain gas, nitrogen; Foster City, CA) combined with a Shimadzu 10A HPLC system.
Compound 1 is as follows: UV λmax (relative intensity), 293 (100), 310 (99); ion-spray MS, m/z (relative intensity), 147 (100, [M-C10H11N2O]+), 323 (25, [M + H]+). Compound 2 is as follows: UV λmax (relative intensity), 294 (89), 316 (100); ion-spray MS, m/z (relative intensity), 177 (100, [M-C10H11N2O]+), 353 (34, [M + H]+).
Assay for THT Activity
Rice leaves were homogenized in a mortar and pestle at 4°C with a 100 mm sodium phosphate buffer (pH 7.5) and 14.4 mm mercaptoethanol. The homogenates were centrifuged for 10 min at 12,000g, and the supernatants were employed as a crude enzyme solution. Unless otherwise indicated, the 10 μL of crude enzyme solution were assayed in a total of 10 μL of 1 mm feruloyl-CoA, 10 μL of 10 mm tyramine (or serotonin for SHT assay), and 70 μL of 100 mm Tris-HCl buffer (pH 8.5). After a 10-min incubation at 30°C, the reaction was stopped with 20 μL of acetic acid. The mixture was filled up to 500 μL with methanol, and a 10-μL aliquot was subjected to HPLC analysis. Protein concentration was determined by the Bradford method using the Bio-Rad protein assay dye (Hercules, CA).
Chemicals and Synthesis of Serotonin Derivatives
Hydroxycinnamoyl-CoA thioesters were prepared from hydroxycinnamoyl N-hydroxysuccinimide esters and CoA according to the method of Stökigt and Zenk (1975). HCAA with tyramine were synthesized by using dicyclohexylcarbodiimide as described by Villegas and Brodelius (1990). p-Coumaroylserotonin was synthesized from p-coumaroyl N-hydroxysuccinimide ester and serotonin hydrochloride. Serotonin hydrochloride (42.5 mg, 0.2 mm) was dissolved in 4 mL of water, and the pH of the solution was adjusted to pH 8.0 with NaHCO3 (33 mg, 0.4 mm). p-Coumaroyl N-hydroxysuccinimide ester (57.4 mg, 0.22 mm) in 4 mL of acetone was added to the solution, and the mixture was left in the dark at room temperature for 24 h. After neutralization with acetic acid, the acetone was evaporated by a nitrogen stream. The aqueous phase was extracted with ethyl acetate, and the extract was dried over magnesium sulfate, followed by evaporation to dryness. Purification of the residue by silica gel column chromatography using hexane:ethyl acetate (1:5) as eluent afforded p-coumaroylserotonin (46.1 mg, yield 72%). Feruloylserotonin was synthesized from feruloyl N-hydroxysuccinimide ester (85 mg, 0.4 mm) and serotonin hydrochloride (128 mg, 0.44 mm) using the same protocol (109 mg, yield 77%).
p-Coumaroylserotonin is as follows: UV λmax (MeOH) nm (ɛ), 225 (35000), 293 (27500), 310 (27000); ion-spray MS, m/z (relative intensity), 147 (100), 323 (25). Feruloylserotonin is as follows: UV λmax (MeOH) nm (ɛ), 220 (35000), 295 (21000), 317 (24000); ion-spray MS, m/z (relative intensity), 177 (100), 353 (34). 1H NMR, 13C NMR, and electron ionization-MS data of p-coumaroylserotonins and feruloylserotonins showed good correlations with literature values (Sakamura et al., 1980; Sarker et al., 1997; Watanabe, 1999).
Serotonin Analysis and Trp Decarboxylase Assay
Seeds of wild-type and transgenic rice were sown in an 0.5% Murashige and Skoog medium in the presence or absence of 50 μm tyramine and incubated at 25°C under 12 h light for 7 d. The seedlings were separated into shoot and root, frozen by liquid nitrogen, and homogenized to a fine powder by mortar and pestle. Methanol was added to the powder. The mixture was further homogenized for 5 min and was passed through a filter (Millex-LG; Millipore, Bedford, MA). Water (100 μL) was added to a 400-μL aliquot of the filtrate. The mixture was passed through a Sep-Pak Light C18 cartridge that was equilibrated with 80% MeOH. The cartridge was washed with 500 μL of 80% MeOH. The column through fraction and 80% MeOH fraction were combined and concentrated in a vacuum centrifuge. The resulting residue was dissolved in 40 μL of 50% MeOH. This solution was analyzed by a reversed-phase HPLC (Shimadzu). Compounds were separated on a Wakosil II 5C18HG column (4.6 × 150 mm) with isocratic elution of 5% (v/v) methanol in water containing 0.3% trifluoroacetic acid at a flow of 0.8 mL/min. The eluate was detected at 280 nm. TDC was determined by monitoring the conversion of l-[methylene-14C]Trp to [14C]tryptamine according to De Luca et al. (1988). Serotonin content and TDC activity measurements were performed in duplicate.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AF329463.
This work was supported by the Korea Science and Engineering Foundation (KOSEF; Basic Research Program grant no. R05–2001–000–00732–0 and Agricultural Plant Stress Research Center Program grant no. R11–2001–09203001–0).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.038372.
References
- Back K, Jang SM, Lee BC, Schmidt A, Strack D, Kim KM (2001) Cloning and characterization of a hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl) transferase induced in response to UV-C and wounding from Capsicum annuum. Plant Cell Physiol 42: 475–481 [DOI] [PubMed] [Google Scholar]
- Badria FA (2002) Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J Med Food 5: 153–157 [DOI] [PubMed] [Google Scholar]
- Clarke DD (1982) The accumulation of cinnamic acid amides in the cell walls of potato tissue as an early response to fungal attacks. In RKS Wood, ed, Active Defense Mechanisms in Plants. Plenum, New York, pp 321–332
- Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23: 567–581 [DOI] [PubMed] [Google Scholar]
- De Luca V, Alvarze-Fernandez F, Campbell D, Kurz WGW (1988) Developmental regulation of enzymes of indole alkaloid biosynthesis in Cathranthus roseus. Plant Physiol 86: 447–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CJ (1996) Phenylpropanoid metabolism and lignin biosynthesis: from weeds to trees. Trends Plant Sci 1: 171–178 [Google Scholar]
- Farmer MJ, Czernic P, Michael A, Negrel J (1999) Identification of cDNA clones encoding hydroxycinnamoyl-CoA:tyramine N-hydroxycinnamoyltransferase from tobacco. Eur J Biochem 263: 686–694 [DOI] [PubMed] [Google Scholar]
- Fiore SD, Li Q, Leech MJ, Schuster F, Emans N, Fischer R, Schillberg S (2002) Targeting tryptophan decarboxylase to selected subcellular compartments of tobacco plants affects enzyme stability and in vivo function and leads to a lesion-mimic phenotype. Plant Physiol 129: 1160–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandmaison J, Olah GM, Van Calsteren MR, Furlan V (1993) Characterization and localization of plant phenolics likely involved in the pathogen resistance expressed by endomycorrhizal roots. Mycorrhiza 3: 155–164 [Google Scholar]
- Guillet G, Poupart J, Basurco J, De Luca V (2000) Expression of tryptophan decarboxylase and tyrosine decarboxylase genes in tobacco results in altered biochemical and physiological phenotypes. Plant Physiol 122: 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol 40: 347–369 [Google Scholar]
- Hohlfeld H, Schurmann W, Scheel D, Strack D (1995) Partial purification and characterization of hydroxycinnamoyl-coenzyme A:tyramine hydroxycinnamoyltransferase from cell suspension cultures of Solanum tuberosum. Plant Physiol 107: 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Nagatsu A, Liu W, Muto T, Narumiya C, Lu X, Yajima M, Ishikawa N, Miyazeki K, Kawai N, et al. (2002) Protective effects of antioxidative serotonin derivatives isolated from safflower against postischemic myocardial dysfunction. Mol Cell Biochem 238: 151–162 [DOI] [PubMed] [Google Scholar]
- Ishihara A, Kawata N, Matsukawa T, Iwamura H (2000) Induction of N-hydroxycinnamoyltyramine synthesis and tyramine N-hydroxycinnamoyltransferase (THT) activity by wounding in maize leaves. Biosci Biotechnol Biochem 64: 1025–1031 [DOI] [PubMed] [Google Scholar]
- Kawashima S, Hayashi M, Takii T, Kimura H, Zhang HL, Nagatsu A, Sakakibara J, Murata K, Oomoto Y, Onozaki K (1998) Serotonin derivative, N-(p-coumaroyl) serotonin, inhibits the production of TNF-alpha, IL-1 alpha, IL-1 beta, and IL-6 endotoxin-stimulated human blood monocytes. J Interferon Cytokine Res 18: 423–428 [DOI] [PubMed] [Google Scholar]
- Kumarasamy Y, Middleton M, Reid RG, Nahar L, Sarker SD (2003) Biological activity of serotonin conjugates from the seeds of Centaurea nigra. Fitoterapia 74: 609–612 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee SB, Chung JS, Han SU, Han O, Guh JO, Jeon JS, An G, Back K (2000) Transgenic rice plants expressing a Bacillus subtilis protoporphyrinogen oxidase gene are resistant to diphenyl ether herbicide oxyfluorfen. Plant Cell Physiol 41: 743–749 [DOI] [PubMed] [Google Scholar]
- Lopez-Meyer M, Nessler CL (1997) Tryptophan decarboxylase is encoded by two autonomously regulated genes in Camptotheca acuminata which are differentially expressed during development and stress. Plant J 11: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Louis V, Negrel J (1991) Tyramine hydroxycinnamoyl transferase in the roots of wheat and barley seedlings. Phytochemistry 30: 2519–2522 [Google Scholar]
- Miyagawa H, Ishihara A, Lim CH, Ueno T, Furuichi N (1998) Induction of N-ρ-coumaroyloctopamine in potato tuber disks by β-1,3-glucooligosaccharide. J Pestic Sci 23: 49–53 [Google Scholar]
- Nagatsu A, Zhang HL, Mizukami H, Okuyama H, Sakakibara J, Tokuda H, Nishino H (2000) Tyrosinase inhibitory and anti-tumor promoting activities of compounds isolated from safflower (Carthamus tinctorius L.) and cotton (Gossypium hirsutum L.) oil cakes. Nat Prod Lett 14: 153–158 [Google Scholar]
- Negrel J, Javelle F (1997) Purification, characterization and partial amino acid sequencing of hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase from tobacco cell-suspension cultures. Eur J Biochem 247: 1127–1135 [DOI] [PubMed] [Google Scholar]
- Negrel J, Javelle F, Paynot M (1993) Wound-induced tyramine hydroxycinnamoyltransferase in potato (Solanum tuberosum) tuber discs. J Plant Physiol 142: 518–524 [Google Scholar]
- Negrel J, Jeandet P (1987) Metabolism of tyramine and feruloyltyramine in TMV-inoculated leaves of Nicotiana tabacum. Phytochemistry 26: 2185–2190 [Google Scholar]
- Negrel J, Lherminier J (1987) Peroxidase-mediated integration of tyramine into xylem cell walls of tobacco leaves. Planta 172: 494–501 [DOI] [PubMed] [Google Scholar]
- Negrel J, Martin C (1984) The biosynthesis of feruloyltyramine in Nicotiana tabacum. Phytochemistry 23: 2797–2801 [Google Scholar]
- Newman MA, Roepenack-Lahaye E, Parr A, Daniels MJ, Dow JM (2002) Prior exposure to lipopolysaccharide potentiates expression of plant defenses in response to bacteria. Plant J 29: 487–495 [DOI] [PubMed] [Google Scholar]
- Niwa T, Etoh H, Shimizu A, Shimizu Y (2000) Cis-N-(ρ-coumaroyl)serotonin from konnyaku, Amorphophallus konjac K. koch. Biosci Biotechnol Biochem 64: 2269–2271 [DOI] [PubMed] [Google Scholar]
- Pavlik M, Laudova V, Gruner K, Vokac K, Harmatha J (2002) High-performance liquid chromatographic analysis and separation of N-feruloylserotonin isomers. J Chromatogr 770: 291–295 [DOI] [PubMed] [Google Scholar]
- Pearce G, Marchand PA, Griswold J, Lewis NG, Ryan CA (1998) Accumulation of feruloyltyramine and ρ-coumaroyltyramine in tomato leaves in response to wounding. Phytochemistry 47: 659–664 [Google Scholar]
- Sakamura S, Terayama Y, Kawakatsu S, Ichihara A, Saito H (1980) Conjugated serotonins and phenolic constituents in safflower seed (Carthamus tinctorius L.). Agric Biol Chem 44: 2951–2954 [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sarker SD, Savchenko T, Whiting P, Sik V, Dinan LN (1997) Moschamine, cis-moschamine, moschamindolol: Four novel indole alkaloids from Centaurea moschata. Nat Prod Lett 9: 189–199 [Google Scholar]
- Schmidt A, Grimm R, Schmidt J, Scheel D, Strack D, Rosahl S (1999) Cloning and expression of a potato cDNA encoding hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferase. J Biol Chem 274: 4273–4280 [DOI] [PubMed] [Google Scholar]
- Silverstein RM, Webster FX (1997) Spectrometric Identification of Organic Compounds, Ed 6. John Wiley & Sons, Hoboken, NJ
- Stökigt J, Zenk MH (1975) Chemical synthesis and properties of hydroxycinnamoyl coenzyme A derivatives. Z Naturforsch 30c: 352–358 [DOI] [PubMed] [Google Scholar]
- Tanaka E, Tanaka C, Mori N, Kuwahara Y, Tsuda M (2003) Phenylpropanoid amides of serotonin accumulate in witchs' broom diseased bamboo. Phytochemistry 64: 965–969 [DOI] [PubMed] [Google Scholar]
- Ueno M, Shibata H, Kihara J, Hnda Y, Arase S (2003) Increased tryptophan decarboxylase and monoamine oxidase activities induce Sekiguchi lesion formation in rice infected with Magnaporthe grisea. Plant J 36: 215–228 [DOI] [PubMed] [Google Scholar]
- Villegas M, Brodelius PE (1990) Elicitor-induced hydroxycinnamoyl-CoA:tyramine hydroxycinnamoyltransferase in plant cell suspension cultures. Physiol Plant 78: 414–420 [Google Scholar]
- Von Roepenack-Lahaye E, Newman MA, Schornack S, Hammond-Kosack KE, Lahaye T, Jones JDG, Daniels MJ, Dow JM (2003) p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogen. J Biol Chem 278: 43373–43383 [DOI] [PubMed] [Google Scholar]
- Watanabe M (1999) Antioxidative phenolic compounds from Japanese barnyard millet (Echinochloa utilis) grains. J Agric Food Chem 47: 4500–4505 [DOI] [PubMed] [Google Scholar]
- Wink M, Roberts MF (1998) Compartmentation of alkaloid synthesis, transport, and storage. In MF Roberts, M Wink, eds, Alkaloids. Plenum Press, New York, pp 239–262
- Yao K, De Luca V, Brisson N (1995) Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell 7: 1787–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Facchini PJ (1999) Purification, characterization, and immunolocalization of hydroxycinnamoyl-CoA: tyramine N-(hydroxycinnamoyl)transferase from opium poppy. Planta 209: 33–44 [DOI] [PubMed] [Google Scholar]
- Zhang HL, Nagatsu A, Sakakibara J (1996) Antioxidative compounds isolated from Safflower (Carthamus tinctorius L.) oil cake. Chem Pharm Bull 44: 874–876 [DOI] [PubMed] [Google Scholar]