Abstract
In this review, the clinical manifestations of urinary schistosomiasis are displayed from a pathogenetic perspective. According to the prevailing host’s immune response profile, urinary schistosomiasis may be broadly categorized into cell-mediated and immune-complex-mediated disorders. The former, usually due to Schistosoma haematobium infection, are attributed to the formation of granulomata along the entire urinary tract. As they heal with excessive fibrosis, they may lead to strictures, calcifications and urodynamic abnormalities. The main impact is lower urinary, the site of heaviest ovi-position. Secondary bacterial or viral infection is common, any may be incriminated in secondary stone formation of the development of bladder malignancy. Immune-complex mediated lesions are usually associated with hepatosplenic schistosomiasis due to Schistosoma mansoni infection. Circulating complexes composed of schistosomal gut antigens and different classes of immunoglobulins deposit in the kidneys leading to several patterns of glomerular pathology. The latter have been categorized under six classes based on the histological and immunofluorescence profile. These classes have been linked to respective clinical manifestations and depend on the stage of evolution of the host’s immune response, extent of associated hepatic fibrosis and co-infection with salmonella or hepatitis C. Secondary amyloidosis develops in 15% of such patients, representing a critical impairment of macrophage function. Conclusion: The wide clinicopathological spectrum of urinary schistosomiasis mirrors the evolution of the host’s immune response according to chronicity of infection, bacterial or viral co-infection and, in the case of glomerulonephritis, to the extent of hepatic co-morbidity.
Keywords: Glomerulonephritis, Hepatosplenic schistosomiasis, Amyloidosis, Bladder cancer, Salmonellosis, Hepatitis C
Introduction
Schistosomes are well-preserved parasites that have been documented to cause urinary disease in humans since ancient times, being mostly documented in Egyptian papyri, notably the Eber’s and Edwin Smith’s [1]. A wealth of knowledge about urinary schistosomiasis has accumulated over the succeeding centuries. This encompassed every aspect of the disease including its epidemiology, clinical syndromes, pathogenicity and therapy. In this article, I shall focus on the clinicopathological syndromes as they relate to prevailing pathogenetic mechanisms.
Like with most other manifestations of the disease, pathogenicity is attributed to perturbation of the host’s immune system by parasitic antigens. Accordingly, urinary disease may be categorized under cellular (Type IV hypersensitivity reaction) or humoral (mainly Type III) immune response to infection, and the latter’s oncogenic potential.
Manifestations of cell-mediated immune response
The schistosomal granuloma is the typical “unit” of the cellular immune response to schistosomal infection. As described elsewhere in this issue of the Journal, it is composed of different blood and tissue cells being recruited by specific chemo-attractants and serving specific functions. Although this reaction is basically cellular in nature, the role of antibodies and complement has been documented in experimental models since many decades [2].
The clinicopathological impact of schistosomal granulomata differs according to the phase of their evolution. Inflammation dominates the early phase when granulomata are cellular and actively secreting chemokines, cytokines and other pro-inflammatory mediators, while parenchymal–mesenchymal transformation dominates the later phases when granulomata are modulated, fibrotic or calcified.
Inflammatory lesions
S.hematobium infection typically involves the bladder, lower ureters, seminal vesicles, and, less frequently, the vas deferens, prostate, and the female genital system. The initial lesions are mucosal granulomas which coalesce to form tubercles, nodules or masses which usually ulcerate (Fig. 1). The surrounding mucosa is hyperemic. The submucosa and muscle layers are also involved in the inflammatory process, which may lead to transient back pressure if the urterovesical junctions are affected.
Fig. 1.

Cystoscopic appearances of common bilharzial lesions in the urinary bladder. (A) Bilharzial pseudotubercles and adjacent ulcer; (B) Bilharzial sessile mass covered by psudotubercles; (C) Sandy patches; (D) cystitis cystica; (E) malignant ulcer (squamous cell carcinoma) with adjacent phosphate encrustations and sandy patches; and (F) fungating malignant mass (squamous cell carcinoma). Hand painted images, courtesy of Professor Naguib Makar, Cairo University. Reproduced from Barsoum [3], with permission.
The characteristic clinical presentation is terminal haematuria, usually associated with increased frequency of micturition and dysuria. Diagnosis is made by finding the characteristic ova in the urine. Cystoscopic examination (Fig. 1), which is usually unnecessary in an endemic area, may show one or more of the mentioned lesions.
Fibrotic lesions
Bladder
As the bladder lesions dry up, they leave a pale mucosa with patches of granular floor, descriptively known as “sandy patches” which are characteristic of healed schistosomiasis (Fig. 1C). These often calcify, leading to a typical linear opacity in a plain radiological examination (Fig. 2). The patchy nature of the lesion may spare relatively healthy mucosa that becomes encysted by the surrounding fibrosis, leading to a typical pathological picture known as “cystitis cystica” (Fig. 1D). These healed lesions may be totally asymptomatic, though secondary bacterial infection usually supervenes due to urological instrumentation, leading to chronic cystitis. In certain endemic areas, salmonella organisms are notorious causes of resistant secondary bacterial cystitis [4], owing to the known symbiotic association between schistosomes and certain salmonella strains [5].
Fig. 2.
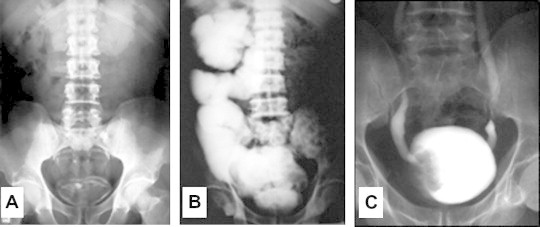
Radiographic appearances in advanced urinary schistosomiasis: (a) Linear calcifications of the urinary bladder. (b) Intravenous urography showing massive right hydronephrosis and hydroureter with a non-functioning left kidney. (c) Ascending cystography showing an irregular filling defect due to a fungating malignant tumor, and bilateral grade I vesicoureteric reflux. Reproduced from Barsoum [3].
Involvement of the submucosa may lead to contraction of the bladder capacity. Fibrosis of the muscle layer may contribute in the bladder contraction, and may also lead to urodynamic disorders including an “irritable”, a “hypertonic” or an “atonic” bladder. Respective clinical presentations are frequency of micturition or retention of urine, although symptoms may overlap when there is chronic retention with overflow.
Urethra
The bladder outlet is one of the favorite sites for oviposition, being at the apex of the vesical trigone. It is therefore notorious of developing intense fibrosis which induces a bladder neck obstruction. In an occasional patient, involvement of the internal sphincter may lead to incontinence. The latter, however, is usually a complication of urological procedures intended to dilate the bladder neck. Bladder neck obstruction often confounds the urodynamic disorders resulting from the detrusor pathology. Chronic retention is the most frequent clinical expression of this scenario.
Urethral lesions may extend beyond the bladder neck, leading to strictures or fistulae, which again are usually iatrogenic, resulting from instrumentation rather than the disease.
Ureterovesical junctions
The ureterovesical junctions hallmark the base of the bladder trigone, hence their vulnerability to dense schistosomal lesions. During the initial phase of the disease, they often become congested and edematous, which may lead to configurational changes causing functional obstruction and/or reflux. These consequences may lead to transient back pressure changes in the upper urinary tract, which are typically reversed by anti-schistosomal treatment.
Persistent reflux is usually iatrogenic, due to urological procedures as cystoscopic dilatation, surgical incision or uretero-vesical implantation. It is noteworthy that such procedures are rarely necessary, in view of the natural compensation which overcomes the obstruction in the majority of case.
Owing to the slow development of back pressure, these conditions may be asymptomatic. A few patients may complain of loin aches or occasional colics, that may be provoked by straining during micturition. In many others, back pressure is detected only during the work up for acute or recurrent urinary infection of urolithiasis.
Ureters
Bilharzial lesions are typically limited to the lower halves of the ureters, corresponding to the lower border of the third lumbar vertebra. This has been attributed to anastomotic channels at this site, in-between the inferior mesenteric and the periureteric and perivesical veins. These communications are believed to be the main route through which S. hematobium worms migrate to the urinary system.
The lower ureteric lesions in schistosomiasis mirror those in the bladder, including the early tubercles and ulcers, and subsequently the sandy patches and cysts, known here as “ureteritis cystica”. Fibrosis of the lower ureteric musculosa may lead to partial obstruction. It is remarkable, though, that the upper ureter compensates by dilatation hypertrophy that generates enough bolus pressure to overcome the distal obstruction, thereby protecting the kidneys from back pressure.
Since the ureteric lesions are often associated with bladder or renal pathology, they do not lead any specific symptoms, although some patients with severely inflamed or ulcerating ureters may complain of suprapubic or deep pelvic discomfort or pain.
Genital structures
Inflammatory lesions, and subsequent fibrosis, may develop in the seminal vesicles and prostate in males, and the uterine cervix, vagina and vulva in females. Most of these lesions are asymptomatic, though the subsequent fibrosis may lead to sterility in men. Calcification of the seminal vesicles is one of the characteristic radiological signs in schistosomiasis.
Obstructive nephropathy
Back pressure effects may extend to the kidneys in those with failure of ureteric urodynamic compensation, or those with vesico-ureteric reflux. Like with obstructive nephropathy due to other causes, ascending infection further may complicate the scenario.
It is noteworthy that, owing to the associated fibrosis of renal parenchyma, relief of obstruction may not correct the back pressure appearances as shown radiologically. In these cases, pressure measurements are necessary to avoid unjustified or even harmful intervention. The gold standard procedure here is the Whitaker test, which correlates with the less invasive isotopic diuresis renogram in about 75% of cases.
Chronic pyelonephritis
As a result of obstruction, reflux, infection and possibly parasite-specific immune-mediated tubular injury [6] the kidneys often end up in chronic interstitial nephritis with fibrosis and impaired function. Loss of concentrating ability, sodium wasting and tubular acidosis constitute a typical early triad. Although urinary calcium excretion is increased due to associated bone resorption, and citrate concentration is reduced due to tubular acidosis, stone formation is unusual owing to the associated polyuria. However, the incidence of infective stones is increased in schistosomiasis.
The glomeruli may show “alternative” changes associated with periglomerular fibrosis or concomitant immunological damage (see later). Eventually, glomerular function declines, ending with renal failure. Like with other end stage renal disease due to chronic interstitial disease, anemia is fairly severe, acidosis is remarkable as it combines acid retention with base loss, and bone disease is prominent due to early calcium wasting and subsequent hyper-parathyroidism.
Immune-Complex-mediated disease
Immune complexes are blamed for two categories of schitosoma-associated morbidity, namely Katayama fever (described elsewhere in this issue of the Journal) and glomerulonephritis. The latter has been most consistently associated with advanced S. mansoni hepatosplenic schistosomioasis, and occasionally with early S. hematobium lower urinary schistosomiasis.
S. mansoni-associated glomerulonephritis
This has been reported from Africa (Egypt, The Sudan, Algeria, Nigeria, Madagascar) and Latin America (Brazil, Peru, Costa Rica). According to post-mortem [7] and clinical studies from Latin America [6,8] and Egypt [9], 12–15% of patients with hepatosplenic schistosomiasis develop histologically documented glomerular lesions. This relative frequency has not changed over the years [10,11], though its epidemiological impact has regressed owing the decline in the overall prevalence of schistosomiasis [12]. The role of schistosomiasis in the pathogenesis of ‘steroid-resistant’ nephrotic syndrome in Subsaharan Africa remains controversial.
Clinically, the disease is encountered as occult, overt, or endstage glomerulopathy. Among the factors that define the severity of glomerular lesions are species and strains of the parasite, associated infections, racial and genetic host factors [13], and the extent of associated hepatic involvement [14]. The latter seems essential for the progression of glomerular lesions, largely due to impairment of hepatic macrophage function by parasite-related antigens and Th2 cytokines [15]. Since macrophages are essential for the clearance of schistosomal antigens transported from the gut via the portal veins, infringement of this function provides direct access of these antigens to the systemic circulation, and subsequently the glomeruli [13].
Serum IgA level is increased in advanced hepatosplenic schistosomiasis, which explains the relatively high frequency of circulating immune complexes containing this immunoglobulin in association with schistosomal antigens. High serum IgA was initially thought to be non-specific, resulting from impaired hepatocyte sialoglycoprotein receptors encountered in many forms of chronic hepatitis [16]. However, more recent evidence suggests that increased circulating IgA in hepatosplenic schistosomiasis is largely attributed to switching of lymphocyte immunoglobulin synthesis pathways in favor of IgA under the influence of IL-10, a predominant Th2 cytokine in chronic schistosomiasis [17].
Six distinct clinico-pathological classes are identified (Table 1, Fig. 3). Class I represents the non-specific glomerular response to immune complex deposits in most parasitic nephropathies. Class II is a subacute response to co-infection with salmonella infection [18], reflecting an augmented pro-inflammatory cytokine profile where bacterial activation of Toll-like receptors seems to play an important role [19]. Classes III and IV occur in 15–20% of patients with established hepatosplenic schistosomiasis, [20] usually due to S. mansoni infection, though also described in humans [21,22] and experimental animals [23] with S. hematobium-associated hepatic fibrosis. Although IgA deposits are predominantly seen in the glomeruli in both classes, the histological patterns are distinct, being mesangiocapillary in Class III and Focal sclerosing in Class IV. The main factor in defining the glomerular response seems to be genetic, with predominance of the latter in blacks [24]. That such lesions are distinct from conventional cirrhotic glomerulopathy is shown by the different glomerular sites of immune deposits, progressive course and lack of correlation with hepatocellular function or injury markers [14].
Table 1.
Classification of schistosomal glomerulopathies.
| Class | Histology | Immuno-fluorescence | Etiologic agent | Prevalence | Clinical findings | Treatment of renal disease |
|---|---|---|---|---|---|---|
| I | Mesangioproliferative | IgM, C3 Schistosomal gut antigens | S. hematobium S. mansoni S. Japonicum | 27–60% of asymptomatic patients, 10–40% of patients with renal disease | Microhematuria Proteinuria | No |
| II | Diffuse proliferative | C3, Salmonella antigens | S. hematobium S. mansoni + Salmonella sp | Salmonella infections Reduced serum C3 | Acute nephritic syndrome, Toxemia | Treatment of salmonella infection |
| III | Membranoproliferative | IgG, IgA, C3 Schistosomal antigens | S. mansoni (S. hematobium?) | 7–20% of asymptomatic patients and in 80% of patients with overt renal disease | Hepatosplenomegaly nephrotic syndrome hypertension, renal failure | No |
| IV | Focal segmental glomerulosclerosis | IgM, IgG (occasionally IgA) | S. mansoni | 11–38% | Hepatosplenomegaly nephrotic syndrome hypertension, renal failure | No |
| V | Amyloid | AA protein | S. mansoni S. hematobium | 16–39% | Hepatosplenomegaly nephrotic syndrome hypertension, renal failure | No |
| VI | Cryoglobulinemic | IgM, C3 | S. mansoni + HCV | Unknown | Hepatosplenomegaly nephrotic syndrome, purpura, vasculitis, arthritis, hypertension, renal failure | ? Interferon + ribavirin corticosteroids, Immunosuppression Plasmapheresis |
Fig. 3.

Histological patterns of schistosomal glomerular lesions. Classes I–VI in sequence from A to F (Explanation n Table 1). Adapted from Barsoum [20,26]with permission.
The presence of AA amyloid deposits in the affected glomeruli distinguishes Class V, which may be encountered in S. hematobium as well as S. mansoni infection of long duration [25]. Class VI is a more recently described pattern where cryoglobulin deposits are superimposed on Class V lesions in cases of combined schistosomal and hepatitis C viral infection [26].
S. hematobium-associated glomerulonephritis
A few reports have documented the occurrence of a transient nephritic syndrome during the early phases of S. hematobium infection of the lower urinary tract [21]. This is often missed within the “noise” of bladder disease, dominated by hematuria and dysuria. Such patients may develop mild to moderate hypertension, facial puffiness or mild edema, and their urine contains significant amounts of albumin, dysmorphic red cells and blood casts.
Malignancy
Bladder neoplasia can be experimentally induced in baboons by S. haematobium infections [27]. Its reported incidence in patients may be as high as 4.5% of those with urinary bilharziasis in Nigeria [28]. The typical histological lesion reported in many studies over the years is a squamous cell carcinoma in roughly 60% (Fig. 4). Transitional cell carcinoma was reported in about 20%, adenocarcinoma in 10% and mixed in 10%. Schistosomal ova were detected in more than 85% of bladder cancers in an Egyptian series of 1026 cases subjected to surgical cystectomy [30]. The tumor, particularly when of the squamous-cell type, remains localized for a long time before spreading to the surrounding pelvic tissues or distant site, thanks to the occlusion of lymphatics by the preceding fibrotic process.
Fig. 4.

Bladder cancer. Surgical specimen showing an extensive ulcerating growth occupying the bladder vault. Reproduced from Barsoum [29]with permission.
There seems to be a significant change in the breakdown of bladder cancer in Egypt. In a recent study on almost 10,000 patients, schistosomal association dropped to 55.3%. The predominant lesion became a transitional cell carcinoma (65.8%), while that of squamous cell carcinoma dropped to 28.4% [31]. This may be attributed to better preventive measures and effective early treatment of schistosomiasis at large.
Associated bacterial and viral infections, rather than parasitic products, are suggested to be the main pathogenetic factors. Associated infection with Human Papilloma Virus has received considerable recent attention in this respect [32], being encountered in about one fourth of cases. Specific p53 gene mutations have been shown in one third of cases [33], being attributed to the effect of neutrophil-generated reactive oxygen molecules, cleavage of conjugated urinary carcinogens or the production of nitrosamines by bacterial enzymes [34].
While bladder malignancy may be discovered accidentally during the assessment of persistent cystitis, it may be predicted if the symptoms of chronic cystitis are augmented or changed in character or distribution, or if necrotic tissues are passed with urine (“necroturia”). The tumor may be felt by pelvic examination, which can also identify local extravesical lymphatic spread. Distant metastasis into the lungs, liver or bones can be detected by specific imaging as described elsewhere in this issue of the Journal.
Conclusion
Science had enough time and motivation to study schistosomiasis, as once of most ancient diseases known to affect humans on a very large scale. Most of the progress occurred during the past decade, as the clinical manifestations became well categorized, pathogenicity understood and treatment standardized. This overview summarizes the principal clinical syndromes of urinary schistosomiasis, viewed from a pathogenetic perspective.
The notion that many infectious diseases result from the host’s immune response, rather than from the direct effect of the bugs themselves, is very well illustrated in schistosomiasis. Were it not for the host’s response, adult worms would have lived in the blood circulation for decades and laid hundreds of eggs every day without directly causing any damage. It is the host’s cell-mediated response to the few trapped ova, which fail to find their way out of the body, that triggers the cascade of cell-mediated immunity, granuloma formation, fibrosis and calcification. This scenario is responsible for S. hematobium-induced lower urinary tract morbidity and subsequent upstream complications.
Similarly, the worm antigens would have been totally harmless if adequately handled by the hepatic macrophages. Failing this, as a result of advanced hepatosplenic schistosomiasis, worm antigens get exposed to the host’s immune system, provoking a humoral response. The latter ultimately leads to glomerular deposition of immune complexes, hence the glomerulonephritis associated with S. mansoni-induced hepatic fibrosis.
Interestingly, certain schistosomal antigens modulate the host’s immune system targeting a state of tolerance to the parasite. While this may be a fair compromise that maintains peace in between the two partners, it may also lead to impaired ability of the macrophages to re-uptake one of its own chemo-attractants, the Amyloid-A protein. This may thus accumulate and infiltrate parenchymal organs, the kidney being a major target.
A similar mechanism explains the vulnerability of patients with chronic schistosomiasis to co-infection with other bacteria or viruses. Some of these result in well-defined syndromes as the schistosoma–salmonella and schistosoma–HCV classes of glomerulonephritis. Along the same line, co-infection with the papilloma virus is incriminated in the development of bilharzial bladder cancer.
Although “genus schistosoma” has been too cruel to humans, it has provided a unique opportunity to scientists, physicians and surgeons in endemic areas to expand their relevant knowledge, experience and research. Not only did this enlighten the world about a most amazing pathogen, but also to extrapolate precious information about the immune response to infection at large.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ghalioungui Paul. Barnes and Noble, Inc.; New York: 1965. Magic and medical science in ancient Egypt. [Google Scholar]
- 2.de Brito P.A., Kazura J.W., Mahmoud A.A. Host granulomatous response in Schistosomiasis mansoni Antibody and cell-mediated damage of parasite eggs in vitro. J Clin Invest. 1984;74(5):1715–1723. doi: 10.1172/JCI111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsoum RS. Schistosomiasis. In: Davison A, Cameron JS, Ritz E, et al., editors. Oxford textbook of clinical nephrology. 3rd ed. New York: Oxford University Press; 2005. p. 1173–84.
- 4.Hathout S.el-D., Abd el-Ghaffar.Y., Awny A.Y. Salmonellosis complicating schistosomiasis in Egypt. A new clinical appreciation. Am J Trop Med Hyg. 1967;16(4):462–472. doi: 10.4269/ajtmh.1967.16.462. [DOI] [PubMed] [Google Scholar]
- 5.Muniz-Junqueira M.I., Tosta C.E., Prata A. Schistosoma-associated chronic septicemic salmonellosis: evolution of knowledge and immunopathogenic mechanisms. Rev Soc Bras Med Trop. 2009;42(4):436–445. doi: 10.1590/s0037-86822009000400015. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Rahman H.M., Nessim L., Francis M., El-Rouby O.A., Barsoum R. Immune-mediated tubulopathy in patients with schistosomal hepatic fibrosis: Egypt. J Bilharziasis. 1994;16(1–2):17–35. [Google Scholar]
- 7.Rocha H., Cruz T., Brito E., Susin M. Renal involvement in patients with hepatosplenic Schistosomiasis mansoni. Am J Trop Med Hyg. 1976;251:108–115. doi: 10.4269/ajtmh.1976.25.108. [DOI] [PubMed] [Google Scholar]
- 8.Andrade Z.A., Rocha H. Schistosomal glomerulopathy. Kidney Int. 1979;16(1):23–29. doi: 10.1038/ki.1979.99. [DOI] [PubMed] [Google Scholar]
- 9.Barsoum R.S., Bassily S., Baligh O.K., Eissa M., El-Sheemy N., Affify N. Renal disease in hepatosplenic schistosomiasis: a clinicopathological study. Trans Roy Soc Trop Med Hyg. 1977;71(5):387–391. doi: 10.1016/0035-9203(77)90035-9. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues V.L., Otoni A., Voieta I., Antunes C.M., Lambertucci J.R. Glomerulonephritis in Schistosomiasis mansoni: a time to reappraise. Rev Soc Bras Med Trop. 2010;43(6):638–642. doi: 10.1590/s0037-86822010000600007. [DOI] [PubMed] [Google Scholar]
- 11.Correia E.I., Martinelli R.P., Rocha H. Is glomerulopathy due to Schistosomiasis mansoni disappearing? Rev Soc Bras Med Trop. 1997;30(4):341–343. doi: 10.1590/s0037-86821997000400012. [DOI] [PubMed] [Google Scholar]
- 12.King C.H. Toward the elimination of schistosomiasis. N Engl J Med. 2009;360(2):106–109. doi: 10.1056/NEJMp0808041. [DOI] [PubMed] [Google Scholar]
- 13.Barsoum R.S. Schistosomal glomerulopathy: selection factors. Nephro Dial Transplan. 1987;2(6):488–497. [PubMed] [Google Scholar]
- 14.Barsoum R.S., Sersawy G., Haddad S., Hashem M.B., Kamel M., Wassef N. Hepatic macrophage function in schistosomal glomerulopathy. Nephrol Dial Transpl. 1988;3(5):612–616. doi: 10.1093/oxfordjournals.ndt.a091715. [DOI] [PubMed] [Google Scholar]
- 15.Flores-Villanueva P.O., Zheng X.X., Strom T.B., Stadecker M.J. Recombinant IL-10 and IL-10/Fc treatment down-regulate egg antigen-specific delayed hypersensitivity reactions and egg granuloma formation in schistosomiasis. J Immunol. 1996;156(9):3315–3320. 1. [PubMed] [Google Scholar]
- 16.Stockert R.J., Kressner M.S., Collinst J.C., Sternlieb I., Morell A.G. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci USA. 1982;79(20):6229–6231. doi: 10.1073/pnas.79.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsoum R.S., Nabil M., Saady G., Genin C., Saleh E., Francis M. Immunoglobulin-A and the pathogenesis of schistosomal glomerulopathy. Kidney Int. 1996;50(3):920–928. doi: 10.1038/ki.1996.392. [DOI] [PubMed] [Google Scholar]
- 18.Bassily S., Farid Z., Barsoum R.S., Soliman L.A., Higashi G.I., Miner W.F. Renal biopsy in Schistosoma–Salmonella associated nephrotic syndrome. J Trop Med Hyg. 1976;79(11):256–258. [PubMed] [Google Scholar]
- 19.Coburn B., Grassl G.A., Finlay B.B. Salmonella, the host and disease: a brief review. Immunol Cell Biol. 2007;85(2):112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 20.Barsoum R.S. Schistosomal glomerulopathies. Kidney Int. 1993;44(1):1–12. doi: 10.1038/ki.1993.205. [DOI] [PubMed] [Google Scholar]
- 21.Ezzat E, Tohamy M, El-Sherif A, Omer AH. Immunopathological study of glomerulonephritis associated with Schistosoma haematobium infection. In: Proceedings of the international conference on schistosomiasis, Cairo; 1978. p. 625–628.
- 22.Soliman M, Abdel-Salam E, Higashi GI, Abdel-Meguid AE, El-Ghadban H. Schistosomiasis haematobium glomerulopathy: a clinical or a pathological entity? In: Abstracts of the 10th international congress of nephrology, London; 1987. p. 362.
- 23.Cheever A.W., Duvall R.H., Hallack T.A., Jr. Hepatic fibrosis in Schistosoma haematobium-infected mice. Trans Roy Soc Trop Med Hyg. 1983;77(5):673–679. doi: 10.1016/0035-9203(83)90202-x. [DOI] [PubMed] [Google Scholar]
- 24.Lopes A.A., Port F.K., James S.A., Silveira M.A., Martinelli R., Brito E. Race and glomerulonephritis in patients with and without hepatosplenic Schistosomiasis mansoni. Clin Nephrol. 2002;58(5):333–336. doi: 10.5414/cnp58333. [DOI] [PubMed] [Google Scholar]
- 25.Barsoum R.S., Bassily S., Soliman M.M., Ramzy M.F., Milad M., Hassaballa A.M. Renal amyloidosis and schistosomiasis. Trans Roy Soc Trop Med Hyg. 1979;73(4):367–374. doi: 10.1016/0035-9203(79)90156-1. [DOI] [PubMed] [Google Scholar]
- 26.Barsoum R. The changing face of schistosomal glomerulopathy. Kidney Int. 2004;66(6):2472–2484. doi: 10.1111/j.1523-1755.2004.66042.x. [DOI] [PubMed] [Google Scholar]
- 27.Hicks R.M., James C., Webbe G. Effect of Schistosoma haematobium and N-butyl-N-14-hydroxybutylnitrosamine on the development of urothelial neoplasia in the baboon. Brit J Cancer. 1980;42(5):730–755. doi: 10.1038/bjc.1980.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chugh K.S., Harries A.D., Dahniya M.H., Nwosu A.C., Gashau A., Thomas J. Urinary schistosomiasis in Maiduguri, north east Nigeria. Ann Trop Med Parasitol. 1986;80(6):593–599. doi: 10.1080/00034983.1986.11812073. [DOI] [PubMed] [Google Scholar]
- 29.Barsoum RS. The Kidney in Schistosomiasis. In: Floege J, Johnson R, Feehally J, editors. Comprehensive clinical nephrology. 4th ed. St. Louis: Saunders, Elsvier; 2010. p. 654–61.
- 30.Ghoneim M.A., El-Mekresh M.M., El-Baz M.A., el-Attar I.A., Ashamallah A. Radical cystectomy for carcinoma of the bladder, critical evaluation of the results in 1026 cases. J Urol. 1997;158(2):393–399. [PubMed] [Google Scholar]
- 31.Gouda I., Mokhtar N., Bilal D., El-Bolkainy T., El-Bolkainy N.M. Bilharziasis and bladder cancer: a time trend analysis of 9843 patients. J Egypt Natl Canc Inst. 2007;19(2):158–162. [PubMed] [Google Scholar]
- 32.el-Mawla N.G., el-Bolkainy M.N., Khaled H.M. Bladder cancer in Africa: update. Semin Oncol. 2001;28(2):174–178. doi: 10.1053/sonc.2001.21961. [DOI] [PubMed] [Google Scholar]
- 33.Warren W., Biggs P.J., El-Baz M., Ghoneim M.A., Stratton M.R., Venitt S. Mutations in p53 gene in schistosomal bladder cancer. Carcinogenesis. 1995;16(5):1181–1189. doi: 10.1093/carcin/16.5.1181. [DOI] [PubMed] [Google Scholar]
- 34.Mostafa M.H., Sheweita S.A., O’Connor P.J. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12(1):97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]



