Abstract
With each passing year since the Chernobyl accident of 1986, more questions arise about the potential for organisms to adapt to radiation exposure. Often this is thought to be attributed to somatic and germline mutation rates in various organisms. We analyzed the adaptability of native Arabidopsis plants collected from areas with different levels of contamination around the Chernobyl nuclear power plant from 1986 to 1992. Notably, progeny of Chernobyl plants resisted higher concentrations of the mutagens Rose Bengal and methyl methane sulfonate. We analyzed the possible molecular mechanisms of their resistance to mutagens and found a more than 10-fold lower frequency of extrachromosomal homologous recombination, significant differences in the expression of radical scavenging (CAT1 and FSD3) and DNA-repair (RAD1 and RAD51-like) genes upon exposure to mutagens (Rose Bengal and x-rays), and a higher level of global genome methylation. This data suggests that adaptation to ionizing radiation is a complex process involving epigenetic regulation of gene expression and genome stabilization that improves plants' resistance to environmental mutagens.
Constant exposure to mutagens, such as UV and ionizing radiation, chemicals, heat, drought, and cold, forces plants to either adapt or die. Nuclear testing, leakage of radioactive wastes, and nuclear accidents such as the Chernobyl disaster have compelled scientists to confront the effects of chronic ionizing radiation exposure on all organisms (Dubrova et al., 1996; Ellegren et al., 1997; Kovalchuk et al., 1998). Plants deserve special attention relative to their susceptibility to ionizing radiation. In radioactively contaminated environments, plants are particularly vulnerable to chronic radiation since they are stationary and unable to leave the contaminated zone; thus, they cannot avoid harmful environmental influences and must adapt to life in harsh environments. Plants do not have a predetermined germline; the germ cells are produced during plant development from somatic cells, and, thus, mutations occurring during somatic development can be inherited (Walbot, 1985). Research into the mechanisms of plant adaptation to environmental stresses and increased radiation levels still lags far behind many other areas of plant molecular biology. Adaptation is a complex process by which populations of organisms respond to long-term environmental stresses by permanent genetic change. Data about adaptation of various organisms, including plants to chronic radiation, are controversial (Dmitrieva, 1996). Plants seem to be an excellent system to study the molecular mechanisms of adaptation to chronic radiation exposure, as they are higher eukaryotes, have fast growth rates, provide a large number of offspring, and are ethically acceptable to work with, in contrast to animals.
Ionizing radiation is known to have general effects on plant growth and development, ranging from stimulatory effects at very low doses, increasingly harmful effects for vegetative growth at intermediate levels, and pronounced decreases in reproductive fitness and yields at high radiation levels. The severity of the effects varies across different plants and is dependent upon the species, age, plant morphology, physiology, and genome organization (Holst and Nagel, 1997). Several studies have been conducted to analyze plant performance under acute and chronic radiation exposure. They revealed a high frequency of chromosome aberrations in Crepis tectorum, winter rye (Secale cereale), and wheat (Triticum aestivum) populations grown in the Chernobyl exclusion zone (Shevchenko and Grinikh, 1990; Shkvarnikov, 1990; Ziablitskaia et al., 1996), increased incidences of DNA single-strand breaks (Syomov et al., 1992), increased frequency of embryonic lethal mutants (Abramov et al., 1992), as well as pronounced dose-dependent genome destabilization (Kovalchuk et al., 1998, 2000b).
Previous adaptation studies were conducted using primarily a single generation of laboratory plants. Studies of multiple generations exposed to radiation were rarely undertaken due to the difficulties of creating a suitable model to study the effects of chronic exposure. Almost 18 years have passed since the enormous release of radioactivity from the Chernobyl accident (International Atomic Energy Agency, 1996; Izrael et al., 1997). Interestingly, plants have continued to grow even in the most radioactively contaminated areas. The boost of active vegetation in the Chernobyl area has attracted a great deal of interest in the question of how organisms adapt to ionizing radiation. Adaptation is a complex process by which populations of organisms respond to long-term environmental stresses by permanent genetic or epigenetic change. Ecological surveys have revealed the existence of specific communities of endemic plant species that adapted to survival in the radioactively polluted soil (Abramov et al., 1992). This has prompted us to investigate the existence of highly debated adaptation phenomena, as well as their possible molecular mechanisms. In the course of the unique natural open-field radiation adaptation experiment, we analyzed several generations of Arabidopsis plants native to the Chernobyl reactor area. These plants were collected from 1986 to 1992 in the Chernobyl area, where they received different doses of radiation (Abramov et al., 1992). Genetic analysis of embryonic lethal mutations in these plants has shown significant dose dependence (Abramov et al., 1992).
We evaluated the resistance of chronically exposed plants to DNA-damaging agents—MMS, free radical-producing agent Rose Bengal (RB), as well as to ionizing radiation. In parallel, we studied the genome stability of the exposed plants using transient recombination assay and assayed the expression of genes involved in DNA repair and genome maintenance. Finally, we investigated epigenetic changes, such as methylation pattern induced by prolonged chronic exposure in several generations. Such detailed analysis of several generations of plants exposed to radiation provides some insight into possible molecular mechanisms of plant adaptation to chronic radiation exposure.
RESULTS
Collection of Plants from Chernobyl Exclusion Zone
Seeds from several groups of naturally growing Arabidopsis plants were collected from the areas with different contamination levels around the Chernobyl nuclear power plant each year from 1986 to 1992. Table I shows the radiation exposure to β- and γ-radiation (mR h-1) in the air at the time the seeds were harvested and also the estimated absorbed dose rate (Gy). The plants that were used in the current work were collected from experimental plots of Tolsty Les, Yanov 12-6, Chernobyl, and control plot. As shown in Table I, plants grown at Yanov 12-6 were exposed to the highest level of radiation. No seeds were collected from this plot from 1986 to 1987, as exposure levels were very high. Plants grown at the control plot were exposed to relatively low, near background radiation levels. It was shown previously that plants grown at Yanov 12-6 had 2- to 3-fold higher incidences of embryonic lethal mutants compared to plants grown at Tolsty Les and 10 to 15 times higher compared to Chernobyl (Abramov et al., 1992). Plants collected from all experimental plots had similar phenotypic appearance. Moreover, profiling of several microsatellite loci revealed a similar pattern from all lines and most closely approximated ecotype Landsberg erecta (data not shown).
Table I.
Radiological characteristics of plant grown in Chernobyl area
| Plot | 1986
|
1987
|
1988
|
1989
|
1990
|
1991
|
1992
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | AD | E | AD | E | AD | E | AD | E | AD | E | AD | E | AD | |
| TL | 240 | 5.0 | 2.5 | 2.0 | 2.5 | 1.9 | 1.4 | 1.1 | 1.2 | 0.7 | 0.9 | 0.2 | ||
| Ch | 0.5 | 0.5 | 0.7 | 0.4 | 0.5 | 0.2 | 0.2 | 0.05 | 0.2 | 0.03 | ||||
| Ya | 90 | 248 | 70 | 211 | 24 | 74 | 9 | 23 | ||||||
Plants were collected from Tolsty Les (TL), Chernobyl (Ch), and Yanov 12-6 (Ya) experimental plots. E stands for γ and β radiation exposure (mR h−1), and AD stands for absorbed dose (Gy). Note that plants of TL harvested in 1986 were exposed for 1 month only. No seeds were collected from Yanov 12-6 plot from 1986 to 1987, as exposure levels were very high. No plants were found in TL in 1991 and in Ch and Ya in 1992.
Chernobyl Arabidopsis Plants Are Changed in Their Sensitivity to Mutagens
To analyze whether any possible adaptation processes have taken place in these plants, we evaluated their resistance to methyl methane sulfonate (MMS), often referred to as radiomimetic agent (Singer, 1986), and the free radical-producing agent RB (Kim et al., 2001). In previous studies, we identified the concentration range that allowed the visualization of sensitivity of Arabidopsis plants. Plants were initially germinated on solid sterile medium and at the age of 10 d were transferred to the liquid medium containing various RB and MMS concentrations.
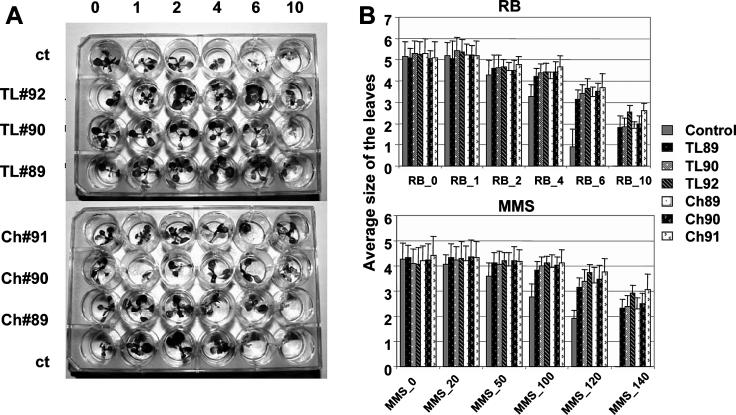
Interestingly, we found that the progeny of plants grown in areas with high contamination were more resistant to mutagens, surviving concentrations that killed the progeny of control plants grown in the area with very low contamination (10 μm RB; Fig. 1A). Moreover, the progeny of plants that were collected from the same experimental plot (either Tolsty Les or Chernobyl) in years 1991 and 1992 were more resistant to mutagens than the progeny of plants collected in 1989 and 1990 (Fig. 1). Statistically significant differences were observed between plants collected in Tolsty Les from 1989 to 1992 grown on MMS (120–140 μm) and RB (6.0–10.0 μm) and between plants collected in Chernobyl from 1989 to 1991 grown on 140 μm MMS and 10 μm RB (P < 0.001 in all cases, ANOVA; Supplemental Tables I and II). With respect to the MMS treatment data, it is important to remember that alkylation is a primary source of DNA modification; therefore, the damaging effects that MMS produces are not the same as those produced by radiation.
Figure 1.
Progeny of plants collected at Tolsty Les plot in 1989, 1990, and 1992 (TL#89, TL#90, and TL#92) and at Chernobyl plot in 1989, 1990, and 1991 (Ch#89, Ch#90, Ch#91) or at control area were initially germinated on solid sterile medium and at the age of 10 d were transferred to the liquid medium containing 0, 1, 2, 4, 6, and 10 μm RB or 0, 20, 50, 100, 120, and 140 μm MMS. Viability of the plants after the treatment was analyzed by measuring the size (perpendicular to the middle vein) of the third and fourth true leaves. Plants grown on RB are shown (A). The average (from 5–6 plants) size of the leaves in mm is shown (B). Bars represent sd.
Table II.
Real-time PCR analysis of the gene expression
| Gene | Basic Medium
|
Rose Bengal
|
x-rays, 1 Gy
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| CT | CH90 | TL90 | CT | CH90 | TL90 | CT | CH90 | TL90 | |
| CAT | 1.0 | 0.4 | 0.3 | 3.1 | 3.3 | 4.8 | 0.3 | 1.1 | 2.3 |
| FSD3 | 1.0 | 0.4 | 0.4 | 2.4 | 2.2 | 2.1 | 0.3 | 0.5 | 1.5 |
| RAD1 | 1.0 | 1.1 | 0.9 | 1.6 | 1.1 | 1.1 | 1.3 | 1.1 | 3.2 |
| RAD54 | 1.0 | 1.6 | 0.5 | 0.4 | 0.2 | 0.6 | 1.4 | 0.6 | 5.4 |
The progeny of plants that were collected in 1990 from control (CT), Chernobyl (Ch), and Tolsty Les (TL), experimental plots was germinated in sterile conditions. In the course of this experiment, one set of 14-d-old control and Chernobyl plants were placed in 100 μM RB for 2 h. The other set of plants was irradiated with x-rays (1 Gy) and frozen 2 h after exposure. The RNA and cDNA samples were prepared from two individual experiments, and the averages of real-time PCR readings were taken. The data is presented as fold of induction of gene activity in CT, CH90, and TL90 plants after exposure to mutagens (RB, x-rays) to gene activity in plants grown on basic medium. The data for CH90 and TL90 plants grown on basic medium are standardized to the data for CT plants.
Genome Stability of the Exposed Plants: Study of Homologous Recombination Frequency
A possible mechanism of plant adaptation to radiation is genome stabilization (Kovalchuk et al., 2003b). As a reflection of genome stability, we measured the homologous recombination (HR) frequency. HR is an efficient repair process that frequently results in gene and segmental duplication, gene loss, or inactivation. This contributes significantly to genome rearrangements and, thus, to genome instability (Gorbunova and Levy, 1997; Critchlow and Jackson, 1998; Kirik et al., 2000; Smith et al., 2001).
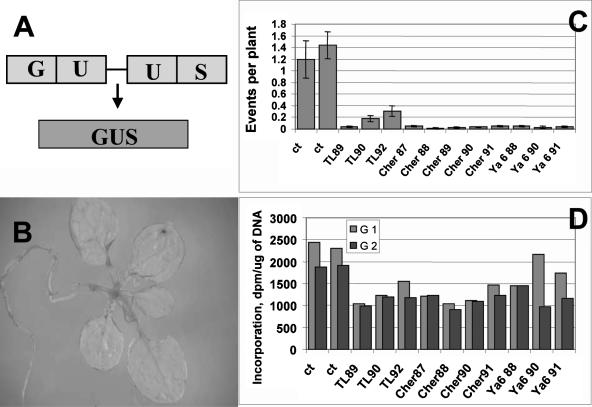
To analyze the HR frequency, we used a transgenic recombination reporter uidA gene coding for β-glucuronidase (Fig. 2A). Delivery of the two overlapping nonfunctional copies of the transgene to plants via Agrobacterium-mediated transformation allowed the visualization of extrachromosomal HR events (Fig. 2B). Transient recombination assay (Puchta et al., 1995; Kovalchuk et al., 2000a) revealed a 12- to 16-fold lower frequency of recombination in Chernobyl plants compared to control plants (P < 0.0001, ANOVA; Fig. 2C). Interestingly, plants that were collected from Tolsty Les from 1990 to 1992 had higher recombination levels than plants collected from earlier years. Recombination frequency in plants collected in Tolsty Les from 1990 and 1992 was higher than in plants collected in 1989 (P < 0.01 in both cases, ANOVA). Recombination frequency was also higher in plants collected from Chernobyl plot (P < 0.01 in all cases, ANOVA) from 1990 to 1992. Notably, recombination frequencies in Yanov plants collected in 1988 were significantly higher than in plants collected from 1990 to 1991 (P < 0.05, ANOVA). Overall, the frequencies of extrachromosomal HR in exposed plants were very low. Such a low recombination frequency observed in all Chernobyl plants could be an indicator of adaptation since lower HR frequency may protect against excessive genome rearrangements.
Figure 2.
A, Structure of recombination substrate. Two nonfunctional truncated overlapping copies of uidA transgene serve as an HR substrate. Repair of the damage that occurs to either region of the homology (U) via HR results in activation of the transgene. B, Arabidopsis plants with recombination events (blue spots). C, Transient recombination assay was performed on the progeny of plants collected from control (years 1989 and 1992) and contaminated (TL, Tolsty Les plot; Cher, Chernobyl plot; Ya, Yanov plot; years 1987–1992) areas. The y axis shows the average number of recombination spots per plant as counted on 300 plants. The results are shown as mean values ± sd. D, Global genome methylation status of progeny of control and irradiated plants. The y axis shows the incorporation of [3H]dCTP (dpm/μg DNA). The x axis shows the experimental plots. Two independent experiments were performed (G1 and G2).
Expression of Stress-Response Genes
The increased resistance to mutagens we observed could also be due to changes in expression of the important housekeeping and DNA-repair genes acquired by plants over several generations of radiation exposure. This may lead to stronger plants, more capable of tackling environmental challenges.
We analyzed the expression of oxidative stress-responsive (CAT and FSD3) and DNA-repair (Rad1 and Rad54-like) genes upon treatment of control, CH90, and TL90 plants with RB (100 μm for 2 h), and x-rays (1 Gy). FSD3, a superoxide dismutase, and CAT, a catalase, are the major reactive oxygen species scavenging enzymes (Mittler, 2002). The DNA-repair genes chosen for analysis are involved in strand break repair (Rad54-like, a member of SNF2/RAD54 family [ETL subfamily]) and nucleotide excision repair (Rad1; Gallego et al., 2000). Strand breaks are the main type of DNA damage caused by radiation; however, radiation also results in various types of nucleotide damage primarily due to radical production (Gros et al., 2002).
We found CAT and FSD3 genes in CH90 and TL90 plants to be expressed at lower levels in noninduced conditions but to be similarly up-regulated by RB as compared to control plants (Table II). Expression of CAT and FSD3 genes after x-ray exposure was unchanged in CH90 but was induced in TL90 plants and repressed in control plants. A very different result was obtained after x-ray irradiation: a relatively small induction of Rad1 and Rad54-like was observed in control plants, whereas no change was observed in CH90 plants. By contrast, progeny of plants collected from Tolsty Les in 1990 showed the strong induction of all genes tested. This suggests that plants of Tolsty Les that were exposed to stronger radiation for several generations built a much stronger response compared to plants exposed to lower radiation in the Chernobyl plot. Interestingly, although Rad54 was up-regulated, the extrachromosomal HR frequency was not affected (Fig. 2). Since Rad54 exerts its activity through chromatin remodeling, its enhanced expression could not stimulate recombination of exogenously suppliedβ-glucuronidase recombination substrate.
Epigenetic Changes in the Exposed Plants
As suggested from our previous studies, plants may regulate their adaptation to environmental stimuli through an epigenetic control mechanism (Kovalchuk et al., 2003b). A major mechanism may involve the change of methylation patterns. We have evaluated global genome DNA methylation in two generations of control and Chernobyl radiation-exposed Arabidopsis plants using the method based on the use of methylation-sensitive HpaII restriction endonuclease. This endonuclease leaves a 5′ guanine overhang after DNA cleavage, with subsequent single nucleotide extension with labeled [3H]dCTP. The first generation was grown under laboratory conditions from seeds harvested in Chernobyl, and the second generation was grown from seeds of the first generation. We have found that genomic DNA of both generations of Arabidopsis plants was considerably hypermethylated in comparison to control plants (Fig. 2D). Plants of the first generation collected in Tolsty Les plot in 1989 had a significantly higher global genome DNA methylation level than those collected during 1991 and 1992 (P < 0.05 and P < 0.01, respectively, ANOVA). For the second generation, this trend was less pronounced, and the difference in the methylation levels was significant only between plants collected during 1989 and 1990 (P < 0.05, ANOVA). Very interesting changes in global DNA methylation were observed in plants from the Chernobyl plot. The first generation of plants harvested in 1987 had significantly lower methylation levels compared to plants harvested in 1988 and 1989 (P < 0.01 in both cases, ANOVA). In 1991, methylation levels dropped (P < 0.0001, ANOVA). Notably, comparable trends were observed in the second generation as well. Plants from the first generation grown in the Yanov plot in 1988 had higher methylation levels than the ones from 1990 to 1991 (P < 0.0001 in both bases, ANOVA). In the second generation, the situation was quite the opposite—plants grown during 1990 and 1991 had less methylated genomic DNA (P < 0.001, ANOVA). This may be due to the fact that the contamination levels in the Yanov plot were the highest in the early years. Overall, in the majority of cases, DNA from plants collected in earlier years (1988–1990) was more hypermethylated than that of plants collected from later years (1991–1992). This could suggest that hypermethylation of the progeny's DNA is an immediate plant response to radiation, whereas the slow return of methylation patterns to normal suggests adaptation of plants grown longer at contaminated areas. Alternatively, it is possible that the decreased intensity of radiation resulted in restoration of normal methylation patterns.
DISCUSSION
Ionizing radiation is known to affect plants in many ways and to varying extents, depending upon the species, plant physiology, and, of course, genome organization (Holst and Nagel, 1997). Woody species seem to be generally more sensitive to acute irradiation than herbaceous species (Holst and Nagel, 1997). Thus, acute irradiation (60 Gy) of pine (Pinus sylvestris) stands resulted in death of pine trees near the Chernobyl Nuclear Power Plant (Arkhipov et al., 1994). By contrast, the lethal dose for Arabidopsis was estimated to be more than 150 Gy in our studies (our unpublished data). The molecular basis for the differing sensitivity of various plant species to radiation remains unknown.
We present the results of a natural open-field radiation adaptation experiment in the Chernobyl area—a unique natural laboratory to study plant adaptation to radiation. We studied the molecular basis of adaptation using Arabidopsis plants grown in the vicinity of the Chernobyl reactor for several generations. We took an integrated approach and studied physiological, genetic, and epigenetic changes in several generations of these plants.
The exposed Arabidopsis plants presumably have developed different, efficient mechanisms to tolerate chronic radiation exposure. Mutations, although important and powerful tools of adaptation, are definitely not the sole basis for development of plant adaptive responses, as most of them are deleterious. Many excellent papers appearing during the last 5 to 7 years have demonstrated the harmful effects of Chernobyl radiation on the genetic apparatus of plant, animal, and human populations exposed for different periods of time (Syomov et al., 1992; Dubrova et al., 1996; Satoh and Kodaira, 1996; Ellegren et al., 1997; Kovalchuk et al., 1998, 2000b, 2003a, 2003b). Here, we present, to our knowledge, the first evidence describing how plants adapt to living in a radiation-contaminated environment and propose possible molecular mechanisms of such adaptation.
The major finding of the study was the extremely low recombination levels in Chernobyl plants. This could serve as an indicator of adaptation, since low frequency of recombination may prevent excessive genome rearrangements. Another possibility, however, is that plants grown at contaminated areas shift their strand break repair mechanism toward non-HR, a more efficient but more error-prone mechanism. Higher recombination in plants collected from later years from Tolsty Les and Chernobyl plots could suggest that these plants started adapting to the environmental conditions. It is also possible that plants with higher recombination levels do not germinate as efficiently as plants with lower recombination levels.
Although the picture of adaptation is far from complete, we think that epigenetic regulation resulting in genome stability plays a major role in adaptation. Hypermethylation in this case could be considered as a stress response and a general defense mechanism of plants that prevents genome rearrangements. Indeed, we observed significantly lower levels of transgene rearrangements in progeny of plants collected from contaminated areas. The fact that DNA methylation and homologous recombination exhibited a tendency to slowly return to control levels (Fig. 2) in plants collected in later years suggests that plants acquired yet another unidentified mechanism of protection. However, it is possible that this is a reflection of a decrease in the level of contamination present (Table I). Similar parallels were found in pine trees native to the Chernobyl zone—a tendency to release the hypermethylation was observed with the decrease of absorbed dose by the plants (Kovalchuk et al., 2003b). Genome stabilization as a part of adaptation process was also observed for viruses, organisms with highly unstable genomes (Holland et al., 1992). The cold adaptation of live influenza vaccine was believed to be a result of a greater genetic stability for the highly variable RNA genome (Herloher et al., 1993). Further studies are clearly needed to analyze the adaptation ability of natural plant populations grown in Chernobyl and to understand the extent of epigenetic control in the genome of these plants.
MATERIALS AND METHODS
Plant Material
Arabidopsis seeds were collected in various experimental plots of the exclusion zone around the Chernobyl reactor from 1987 to 1992. The radiological parameters of the experimental plots are given in Table I. The β- andγ-rays exposure (mR h-1) was determined in the air at the level of 15 cm from the ground using an SRB9-1-ship beta-gamma radiometer. Absorbed dose (Gy) was estimated from the readings as well as from weighed leaf powder samples. For more detailed information, see Abramov et al. (1992).
DNA Extraction
Total DNA was prepared from whole plants using Nucleon PhytoPure total DNA isolation kit (Amersham Life Science, Arlington Heights, IL) in accordance with the manufacturer's protocol.
RNA Extraction and Real-Time PCR
Total RNA was extracted using TRIzol reagent (Gibco-BRL, Cleveland) in accordance with the manufacturer's protocol. After quantification, 1 μg of RNA was taken for cDNA preparation (You-Prime-First-Strand, ready-to-go PCR beads; Amersham, Buckinghamshire, UK). The following primers were used for amplification: FSD3 (At5g23310) forward 5′-TTGTGTTGTGACGACAAGC-3′, reverse 5′-ATCAATCTGCTCAAGAACACC-3′; CAT (X64271) forward 5′-TATGGAACAACAACTCCTCC-3′, reverse 5′-TCTCTGAGTATCGGCATAGG-3′; AtRAD1 (AF089003) forward 5′-AATGGATGCTTGTCTCAAGGAG-3′, reverse 5′-TTCCATTTTGGTGCCTCTTCC-3′; AtRAD54-like (At2g02090) forward 5′-GGTGAGGATTTGTTGTTAGAGG-3′, reverse5′-GCTGAAATCAGAATCCTCTGC-3′. Real time PCR was performed in a total volume of 25 μL using 1 μL of the first-strand cDNA synthesis mixture as a template, 300 nm forward primer, 300 nm reverse primer, and 12.5 μL of 2× SYBRGreen PCR Master Mix (Applied Biosystems, Foster City, CA). The duplicate reactions were carried out with the 1:3 and 1:15 dilutions of the first-strand cDNA synthesis mixture. A SmartCycler (Cepheid, Sunnyvale, CA) was used to perform the PCR cycles, and fluorescence was quantified against standards. The cDNAs were amplified under the following conditions: (1) 95°C for 5 min for one cycle; (2) 94°C for 30 s, 58°C for 30 s, 72°C for 1 min for 30 cycles; and (3) 72°C for 10 min for one cycle. The melting temperatures were estimated for every gene product. The standards for the expression of each gene were amplified from the cDNA of following dilutions: 1:1, 1:4, 1:20, and 1:100. Equal loading of each amplified sample was determined by the control AtActin-1 PCR product (forward primer 5′TGGACAAGTCATAACCATCGGAGC3′; reverse primer 5′TGTGAACAATCGATGGACCTGAC3′).
Transient Recombination Assay
The assay was performed essentially as described (Rossi et al., 1993). About 300 Arabidopsis plants (3 weeks old) were incubated for 3 d with 10 mL of germination medium containing Agrobacterium tumefaciens (a final optical density of 0.6 at 600 nm) carrying uidA recombination substrate. Cloning of the substrate was described previously (Swoboda et al., 1994). The mixture of plants and bacteria was exposed to reduced pressure (0.15 atm) in a sterile vacuum chamber for 5 min. The seedlings were placed on germination medium plates and further cocultivated with Agrobacterium for 3 d in a growth chamber at 25°C with 16-h-light period. The plantlets were washed in sterile 10 mm MgSO4 and blotted dry in a sterile vacuum chamber for 5 min. Transient expression of recombined uidA gene was determined by histochemical staining as described (Kovalchuk et al., 2000a). Transient recombination assay revealed spontaneous extrachromosomal recombination events observed as sectors of blue upon the histochemical staining.
Cytosine Extension Assay to Detect Sequence-Specific Changes in DNA Methylation
DNA was prepared from control and Chernobyl plants and digested overnight with 10-fold excess of HpaII endonuclease according to manufacturer's protocol (New England Biolabs, Beverly, MA). Additional DNA aliquot was incubated without restriction enzyme addition and served as background control. The single nucleotide extension reaction was performed on a 25 μg of DNA, 1× PCR bufferII, 1.0 mm MgCl2, 0.25 units of Amplitaq DNA polymerase (Perkin-Elmer, Foster City, CA), and [3H]dCTP (57.4 Ci/mmol; NEN, Boston). The reaction mixtures were incubated at 56°C for 1 h and then placed on ice. Duplicate aliquots from each reaction were placed on Whatman DE-81 ion-exchange filters (Clifton, NJ) and washed three times with sodium phosphate buffer (pH 7.0) at the room temperature. The filters were dried and processed by scintillation counting. Background label incorporation was subtracted from enzyme-digested samples, and results were expressed as relative [3H]dCTP incorporation/1 μg of DNA or as percent change from control (Pogribny et al., 1999). The radioactive incorporation (Fig. 2D, y axis) is shown in dpm/μg of DNA. Incorporation is directly dependent on the completeness of the DNA digestion with methylation-sensitive enzymes: the higher the methylation, the lower the digestion rate and the lower the amount of incorporated radioactively labeled cytosin ([3H]dCTP). Two independent experiments were performed (G1 and G2).
Statistical Analysis
Most of the statistical procedures used here were described by Sokal and Rohlf (1995). For each group of samples, the mean values were calculated. Data were presented as means ± se of the mean. Statistical analysis of DNA methylation and homologous recombination was performed using ANOVA (with TREATMENT as main factor) and post-hoc analysis using Excel 2000 software. The statistical significance of the experiments with growing plants on MMS and RB was confirmed by performing single factor ANOVA for the following groups: TL89/90/92 and Ch89/90/91 grown on 120 and 140 μm MMS as well as 6.0 and 10.0 μm RB (Supplemental Tables I and II).
Supplementary Material
Acknowledgments
We thank Alicja Ziemienowicz and Crystal Snyder for critical comments on the manuscript and Paula Burke for help with methylation analysis.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (Establishment Grant to O.K.) and the Alberta Heritage Foundation for Science and Engineering Research (Alberta Ingenuity Grants).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040477.
References
- Abramov VI, Fedorenko OM, Shevchenko VA (1992) Genetic consequences of radioactive contamination for populations of Arabidopsis. Sci Total Environ 112: 19–28 [DOI] [PubMed] [Google Scholar]
- Arkhipov NP, Kuchma ND, Askbrant S, Pasternak PS, Musica VV (1994) Acute and long-term effects of irradiation on pine (Pinus sylvestris) stands post-Chernobyl. Sci Tot Environ 157: 383–386 [PubMed] [Google Scholar]
- Critchlow S, Jackson S (1998) DNA end-joining: from yeast to man. Trends Biochem Sci 23: 394–402 [DOI] [PubMed] [Google Scholar]
- Dmitrieva SA (1996) The adaptation of natural plant populations to chronic irradiation due to the accident at the Chernobyl Atomic Electric Power Station. Tsitol Genet 30: 3–8 [PubMed] [Google Scholar]
- Dubrova YE, Nesterov VN, Krouchinsky NG, Ostapenko VA, Neumann R, Neil DL, Jeffreys AJ (1996) Human minisatellite mutation rate after the Chernobyl accident. Nature 380: 683–686 [DOI] [PubMed] [Google Scholar]
- Ellegren H, Lindgren G, Primmer CR, Møller AP (1997) Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature 389: 593–596 [DOI] [PubMed] [Google Scholar]
- Gallego F, Fleck O, Li A, Wyrzykowska J, Tinland B (2000) AtRAD1, a plant homologue of human and yeast nucleotide excision repair endonucleases, is involved in dark repair of UV damages and recombination. Plant J 21: 507–518 [DOI] [PubMed] [Google Scholar]
- Gorbunova V, Levy AA (1997) Non-homologous DNA end-joining in plant cells is associated with deletions and filler DNA insertions. Nucleic Acids Res 25: 4650–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros L, Saparbaev MK, Laval J (2002) Enzymology of the repair of free radicals-induced DNA damage. Oncogene 21: 8905–8925 [DOI] [PubMed] [Google Scholar]
- Herlocher ML, Maassab HF, Webster RG (1993) Molecular and biological changes in the cold-adapted “master strain” A/AA/6/60 (H2N2) influenza virus. Proc Natl Acad Sci USA 90: 6032–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JJ, de la Torre JC, Steinhauer DA (1992) RNA virus populations as quasispecies. Curr Top Microbiol Immunol 176: 1–20 [DOI] [PubMed] [Google Scholar]
- Holst RW, Nagel DJ (1997) Radiation effects on plants. In W Wang, JW Gorsuch, JS Hughes, eds, Plants for Environmental Studies. Lewis Publishers, Boca Raton, FL, pp 37–81
- International Atomic Energy Agency (1996) Proceedings of the International Conference: One Decade after Chernobyl: Summing up the Consequences of the Accident. Austria Center, April 8–12, 1996, Vienna
- Izrael YA, De Cort M, Jones AR, Nazarov IM, Fridman SD, Kvasnikova EV, Stukin ED, Kelly GN, Matveenko II, Poumeiko YM, et al. (1997) The atlas of Caesium-137 contamination of Europe after the Chernobyl accident. In A Karaoglu, G Desmet, GN Kelly, HG Menzel, eds, The Radiological Consequences of the Chernobyl Accident. ECSC-EC-EAEC, Brussels, pp 1–10
- Kim SY, Kwon OJ, Park JW (2001) Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie 83: 437–444 [DOI] [PubMed] [Google Scholar]
- Kirik A, Salomon S, Puchta H (2000) Species-specific double-strand break repair and genome evolution in plants. EMBO J 19: 5562–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk I, Kovalchuk O, Arkhipov A, Hohn B (1998) Transgenic plants are sensitive bioindicators of nuclear pollution caused by the Chernobyl accident. Nat Biotechnol 16: 1054–1059 [DOI] [PubMed] [Google Scholar]
- Kovalchuk I, Kovalchuk O, Hohn B (2000. a) Genome-wide variation of the somatic mutation frequency in transgenic plants. EMBO J 19: 4431–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O, Burke P, Arkhipov A, Kuchma N, James SJ, Kovalchuk I, Pogribny I (2003. b) Genome hypermethylation in Pinus silvestris of Chernobyl – a mechanism for radiation adaptation? Mutat Res 529: 13–20 [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Dubrova Y, Arkhipov A, Hohn B, Kovalchuk I (2000. b) Wheat mutation rate after Chernobyl. Nature 407: 583–584 [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Kovalchuk I, Arkhipov A, Hohn B, Dubrova Y (2003. a) Extremely complex pattern of microsatellite mutation in the germline of wheat exposed to the post-Chernobyl radioactive contamination. Mutat Res 525: 93–101 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Puchta H, Swoboda P, Hohn B (1995) Induction of homologous DNA recombination in whole plants. Plant J 7: 203–210 [Google Scholar]
- Pogribny I, Yi P, James SJ (1999) A sensitive new method for rapid detection of abnormal methylation patterns in global DNA and within CpG islands. Biochem Biophys Res Commun 262: 624–628 [DOI] [PubMed] [Google Scholar]
- Rossi L, Escudero J, Hohn B, Tinland B (1993) Efficient and sensitive assay for T-DNA-dependent transient gene expression. Plant Mol Biol Rep 11: 220–229 [Google Scholar]
- Satoh C, Kodaira M (1996) Effects of radiation on children. Nature 383: 226. [DOI] [PubMed] [Google Scholar]
- Shevchenko VV, Grinikh LI (1990) Cytogenetic effects in native populations of Crepis tectorum exposed to chronic irradiation in the vicinity of the Chernobyl Nuclear Power Station. Induction of chromosome aberrations during the first 2 years following the accident. Radiobiologiia 30: 728–734 [PubMed] [Google Scholar]
- Shkvarnikov PK (1990) A cytological study of plants growing under exposure to different radiation levels. Tsitol Genet 24: 33–37 [PubMed] [Google Scholar]
- Singer B (1986) O-Alkyl pyrimidines in mutagenesis and carcinogenesis: occurrence and significance. Cancer Res 46: 4879–4885 [PubMed] [Google Scholar]
- Smith J, Baldeyron C, De Oliveira I, Sala-Trepat M, Papadopoulo D (2001) The influence of DNA double-strand break structure on end-joining in human cells. Nucleic Acids Res 29: 4783–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R, Rohlf FJ (1995) Biometry. Freeman, New York
- Swoboda P, Gal S, Hohn B, Puchta H (1994) Intrachromosomal homologous recombination in whole plants. EMBO J 13: 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syomov AB, Ptitsyna SN, Sergeeva SA (1992) Analysis of DNA strand break induction and repair in plants from the vicinity of Chernobyl. Sci Total Environ 112: 1–8 [DOI] [PubMed] [Google Scholar]
- Walbot V (1985) On the life strategies of plants and animals. Trends Genet 1: 165–170 [Google Scholar]
- Ziablitskaia EI, Geras'kin SA, Udalova AA, Spirin EV (1996) An analysis of the genetic sequelae of the contamination of winter rye crops by the radioactive fallout from the Chernobyl Atomic Electric power station. Radiats Biol Radioecol 36: 498–505 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.