Abstract
During cold acclimation, winter rye (Secale cereale) plants accumulate pathogenesis-related proteins that are also antifreeze proteins (AFPs) because they adsorb onto ice and inhibit its growth. Although they promote winter survival in planta, these dual-function AFPs proteins lose activity when stored at subzero temperatures in vitro, so we examined their stability in solutions containing CaCl2, MgCl2, or NaCl. Antifreeze activity was unaffected by salts before freezing, but decreased after freezing and thawing in CaCl2 and was recovered by adding a chelator. Ca2+ enhanced chitinase activity 3- to 5-fold in unfrozen samples, although hydrolytic activity also decreased after freezing and thawing in CaCl2. Native PAGE, circular dichroism, and Trp fluorescence experiments showed that the AFPs partially unfold after freezing and thawing, but they fold more compactly or aggregate in CaCl2. Ruthenium red, which binds to Ca2+-binding sites, readily stained AFPs in the absence of Ca2+, but less stain was visible after freezing and thawing AFPs in CaCl2. We conclude that the structure of AFPs changes during freezing and thawing, creating new Ca2+-binding sites. Once Ca2+ binds to those sites, antifreeze activity, chitinase activity and ruthenium red binding are all inhibited. Because free Ca2+ concentrations are typically low in the apoplast, antifreeze activity is probably stable to freezing and thawing in planta. Ca2+ may regulate chitinase activity if concentrations are increased locally by release from pectin or interaction with Ca2+-binding proteins. Furthermore, antifreeze activity can be easily maintained in vitro by including a chelator during frozen storage.
To become freezing-tolerant, overwintering cereals must first undergo a complex adjustment of plant morphology and metabolism known as cold acclimation. As winter rye (Secale cereale) plants acclimate to cold temperatures, they secrete pathogenesis-related (PR) proteins, including endochitinases, β-1,3-glucanases, and thaumatin-like proteins into intercellular spaces and the xylem (Hon et al., 1995; Antikainen et al., 1996; Pihakaski-Maunsbach et al., 1996, 2001). These apoplastic PR proteins are thought to provide a preemptive defense against psychrophilic fungi, such as snow molds (Snider et al., 2000), that are able to infect overwintering cereals at low temperatures and reduce their winter survival (Ergon et al., 1998). Moreover, the PR proteins that accumulate in response to cold temperature in winter rye are also antifreeze proteins (AFPs) because they are able to adsorb onto the surfaces of ice crystals and inhibit their growth (Hon et al., 1994, 1995). AFPs may enhance the survival of overwintering plants by lowering their freezing temperature, by modifying the growth of intercellular ice as plants freeze, and by inhibiting the recrystallization of ice in frozen plants (Griffith and Antikainen, 1996; Chun et al., 1998). During the recrystallization of ice, water molecules migrate from smaller crystals to larger ones, which reduces the free energy of the system by minimizing the surface area of ice (Knight et al., 1984) but may damage tissues physically by forming large masses of ice (Griffith and Antikainen, 1996).
The dual-function PR proteins/AFPs must be stable to freezing and thawing if they are to provide resistance to pathogens and modify the growth of ice in planta over the course of a typical winter. However, in the course of our experiments, we observed a significant decrease in antifreeze activity when AFPs extracted from winter rye leaves were frozen to −20°C and thawed repeatedly. AFPs isolated from the taproots of carrots also lost activity when they were stored at −80°C or at −196°C for 10 weeks (Hong Wang et al., 2002). Therefore, the goal of this study was to examine factors that could affect the activity and stability of PR proteins/AFPs from winter rye.
We usually extract AFPs from the apoplast of cold-acclimated (CA) winter rye leaves by intercellular washing (Hon et al., 1994). Apoplastic extracts from CA winter rye leaves contain nine secreted native proteins (NPs), six of which exhibit antifreeze activity (NP2 to NP7) and are oligomeric complexes composed of β-1,3-endoglucanases, endochitinases, thaumatin-like proteins, and other polypeptides (Yu and Griffith, 1999). The apoplastic proteins are typically stored in the extraction solution, which is composed of 20 mm CaCl2 and 20 mm ascorbic acid, pH 3 (Hon et al., 1994), and so low pH and/or the presence of ions may influence the stability of PR proteins and AFPs. In preliminary studies, we found that the proteins were stable over a wide pH range (pH 3–7; data not presented). Therefore, the present study was undertaken to examine the effects of ions on the stability of winter rye AFPs to freezing and thawing. We elected to work with the whole apoplastic extract in order to obtain the proteins in their native, oligomeric state and have the opportunity to examine interactions between them. We also took advantage of the fact that many of the apoplastic proteins have dual functions and monitored both antifreeze and chitinase activities in response to cations and freeze-thaw cycles. Moreover, we examined the effects of cations and freeze-thaw cycles on the structure of the apoplastic proteins using native PAGE, circular dichroism (CD), and Trp fluorescence. We now show that Ca2+ affects both the activities and structure of PR proteins/AFPs after freezing and thawing.
RESULTS
Antifreeze Activity Decreases When Ca2+ Is Present During Freezing and Thawing
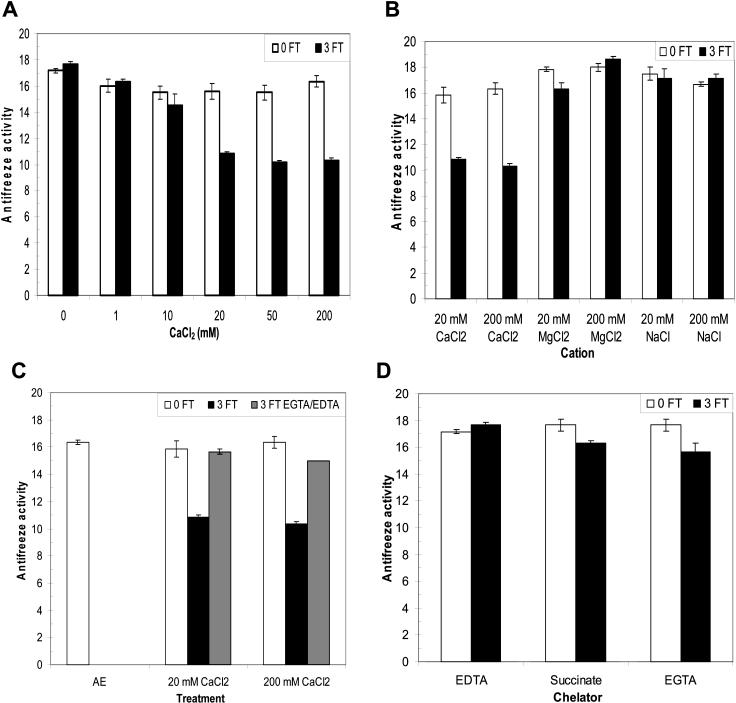
To determine the effect of cations on antifreeze activity, apoplastic proteins extracted from CA winter rye leaves were dialyzed in CaCl2 solutions ranging from 10 to 200 mm at pH 5.5, then assayed for their ability to alter the shape of an ice crystal grown in solution. For a negative control treatment, the proteins were dialyzed in 5 mm EDTA to achieve 0 mm Ca2+. When the solutions were maintained at 4°C, there was no effect (P > 0.05) of CaCl2 on antifreeze activity over the entire concentration range of 0 to 200 mm CaCl2 (Fig. 1A). Freezing and thawing AFPs in solutions of 0 or 10 mm CaCl2 also had no effect on antifreeze activity (Fig. 1A). However, there was a statistically significant interaction (P < 0.01) between cation concentration and freeze-thaw cycles. Antifreeze activity decreased by 36% when the apoplastic proteins were subjected to three freeze-thaw cycles in the presence of 20 mm or more CaCl2 (Fig. 1A). The total protein concentrations in our experiments were approximately 5 μm, so the concentrations of cation concentrations used were all in excess of the protein concentrations.
Figure 1.
Effect of cations and freeze-thaw (FT) cycles on antifreeze activity. A, Apoplastic proteins in solutions containing 1 to 200 mm CaCl2 at pH 5.5 (0FT), were assayed for antifreeze activity, and then frozen and thawed three times (3FT) and reassayed. The absence of Ca2+ was achieved by dialyzing proteins in 5 mm EDTA. B, Apoplastic proteins were dialyzed in 20 or 200 mm CaCl2, MgCl2, or NaCl at pH 5.5, then frozen and thawed three times. C, To determine if the effect of Ca2+ was reversible, apoplastic proteins were frozen and thawed in the presence of 20 or 200 mm CaCl2, then dialyzed in either 5 mm EGTA or 50 mm EDTA at pH 5.5. AE is the activity of the original apoplastic extract in 20 mm CaCl2 and 20 mm ascorbate, pH 3. D, Apoplastic proteins were dialyzed in 5 mm EDTA, sodium succinate, or EGTA at pH 5.5, then frozen and thawed three times. Antifreeze activity was quantified by determining changes in ice morphology with dilution as described in “Materials and Methods”. Data are presented as means ± se, n = 3.
To determine whether the cation effect was specific for Ca2+, apoplastic proteins were also dialyzed in solutions containing 20 or 200 mm MgCl2 or NaCl in place of CaCl2 (Fig. 1B). There were no significant effects (P > 0.05) of freezing and thawing winter rye AFPs in the presence of 20 or 200 mm NaCl or 200 mm MgCl2 upon antifreeze activity, but there was a 13% decrease (P < 0.05) in antifreeze activity when apoplastic proteins were frozen and thawed in 20 mm MgCl2 (Fig. 1B).
By using CaCl2, MgCl2, and NaCl in the freeze-thaw experiments, we were able to discount the Cl− anion and the cation Na+ as factors affecting antifreeze activity. Our results show that freezing and thawing in the presence of Ca2+ caused a much larger decrease in antifreeze activity compared with the effect of Mg2+. The loss of antifreeze activity was not caused by protein denaturation as the salt concentration increased during freezing (salting out) because freezing AFPs in solutions containing 200 mm MgCl2 or NaCl did not inhibit antifreeze activity (Fig. 1B). Moreover, the effect of Ca2+ on antifreeze activity was reversible in the presence of chelators (Fig. 1C). Antifreeze activity was restored to the level of the unfrozen control samples by dialyzing the 20 mm-CaCl2 samples in 5 mm EGTA after freezing and thawing (Fig. 1C).
Because antifreeze activity was affected by Ca2+ or Mg2+ only in samples that had been frozen and thawed, we predicted that the addition of chelators before freezing would prevent the loss of antifreeze activity observed after freezing. In fact, antifreeze activity was not affected (P > 0.05) by repeated freezing and thawing in the presence of 5 mm EDTA, 5 mm sodium succinate, or 5 mm EGTA, all at pH 5.5 (Fig. 1D).
Chitinase Activity Is Enhanced by Calcium
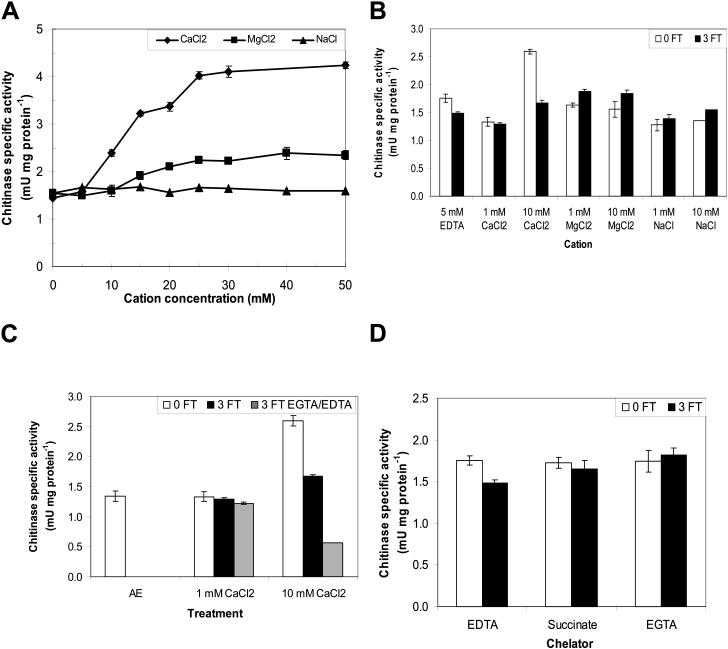
The effects of cations and freeze-thaw cycles on apoplastic proteins were also monitored by assaying chitinase activity. There are two chitinases present in apoplastic extracts obtained from CA winter rye leaves (Hon et al., 1995; Yeh et al., 2000). One is a 35-kD, class I chitinase with a lectin-like chitin-binding domain in addition to the catalytic domain that binds and hydrolyzes chitin, whereas the other is a 28-kD, class II chitinase that lacks the chitin-binding domain (Hon et al., 1995; Yeh et al., 2000). Chitinase activity was measured as the hydrolysis of the substrate 4-methylumbelliferyl β-d-N, N′, N″-triacetylchitotrioside to yield the fluorescent product free methylumbelliferone (Hollis et al., 1997). The specific activity in apoplastic extracts from CA winter rye leaves which contained both chitinases increased 2.8-fold (statistically significant at P < 0.01) in the presence of 25 to 50 mm CaCl2 when compared with the 0 mm CaCl2 (5 mm EDTA) treatment (Fig. 2A). NaCl had no effect on chitinase specific activity (P > 0.05), but the hydrolysis of the chitinase substrate was slightly enhanced (1.5-fold, P < 0.05) in the presence of 25 to 50 mm MgCl2 (Fig. 2A).
Figure 2.
Effects of cations and freeze-thaw (FT) cycles on chitinase activity. A, Apoplastic proteins were dialyzed in solutions containing 20 to 200 mm CaCl2, MgCl2, or NaCl at pH 5.5. Proteins were dialyzed in 5 mm EDTA to achieve 0 mm cation. B, Apoplastic proteins were dialyzed in solutions containing 20 to 200 mm CaCl2, MgCl2, or NaCl at pH 5.5 (0FT), then frozen and thawed three times (3FT) and reassayed. C, To determine if the effect of Ca2+ was reversible, apoplastic proteins frozen and thawed in the presence of 20 or 200 mm CaCl2 were then dialyzed in 5 mm EGTA or 50 mm EDTA at pH 5.5. AE is the activity of the original apoplastic extract in 20 mm CaCl2 and 20 mm ascorbate, pH 3. D, Apoplastic proteins were dialyzed in 5 mm EDTA, sodium succinate, or EGTA at pH 5.5, then frozen and thawed three times. In all experiments, each sample was diluted in a solution containing the substrate 4-MU(GlcNAc)3 and chitinase activity was measured by the release of free 4-MU as described in “Materials and Methods”. The dependence of chitinase activity on cation concentration was calculated using the final cation concentration in the assay medium. Data are presented as means ± se, n = 3.
Each of the three cations had a different effect on chitinase specific activity after three freeze-thaw cycles (Fig. 2B). Adding 10 mm CaCl2 resulted in a loss of 36% of the chitinase specific activity following three freeze-thaw cycles (P < 0.01), and the effect was not reversed by adding 5 mm EDTA (Fig. 2C). Surprisingly, the specific activity of the chitinases was enhanced by 12% (P < 0.01) following three freeze-thaw cycles in the presence of 1 or 10 mm MgCl2 (Fig. 2B). There was no effect of freeze-thaw cycles on chitinase specific activity in the presence of 1 or 10 mm NaCl (P > 0.05).
When apoplastic proteins were dialyzed in 5 mm Na-succinate or EGTA at pH 5.5, the chitinase specific activity was low (Fig. 2D) and was unaffected by three freeze-thaw cycles (P > 0.05). However, chitinase specific activity decreased by 16% (P < 0.05) when apoplastic proteins were dialyzed into 5 mm EDTA at pH 5.5 and exposed to three freeze-thaw cycles (P < 0.05).
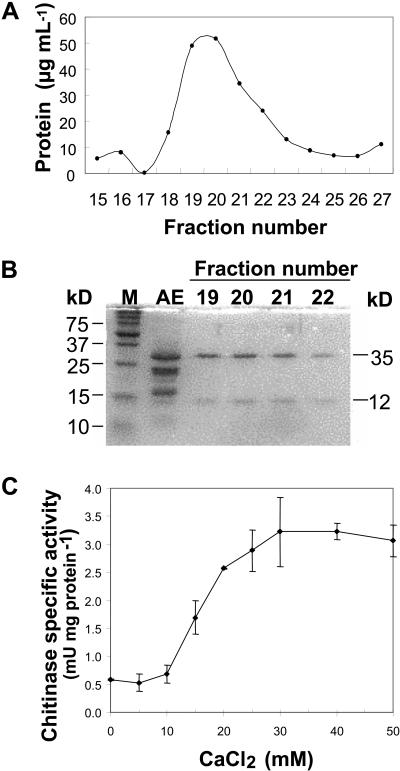
To ascertain that the effects of cations on chitinase specific activity were not confounded by the presence of two chitinases in the apoplastic extracts, we purified the 35-kD, class I chitinase in its native state by affinity chromatography on a colloidal chitin column. As shown by SDS-PAGE, fractions eluted from the column in 20 mm acetic acid, pH 3, contained a single native protein (NP3) with major polypeptides at 35- and 12-kD (Fig. 3, A and B). This oligomer has previously been shown by immunoblotting to contain a 35-kD chitinase, a 35-kD glucanase, and a 12-kD thaumatin-like protein (Yu and Griffith, 1999). The chitinase specific activity of this oligomeric complex increased 5.4-fold when the CaCl2 concentration was raised from 0 to 30 mm (Fig. 3C), thus demonstrating that the activity of a single chitinase from CA winter rye is enhanced by Ca2+.
Figure 3.
Effect of Ca2+ on activity of a purified native chitinase (NP3). A, NP3 was eluted from a colloidal chitin-affinity column with 20 mm acetic acid, pH 3. B, The original apoplastic extract (AE) and the eluted fractions were examined by SDS-PAGE. The apparent molecular masses of Bio-Rad Precision Protein Standards are shown on the left and of the prominent polypeptides are shown on the right. C, Aliquots of NP3 were dialyzed in solutions containing up to 200 mm CaCl2, and then chitinase activity was assayed as described in “Materials and Methods”. The dependence of chitinase activity on cation concentration was calculated using the final cation concentration in the assay medium. Data are presented as means ± sd, n = 3.
Calcium Induces Aggregation of Native Apoplastic Proteins
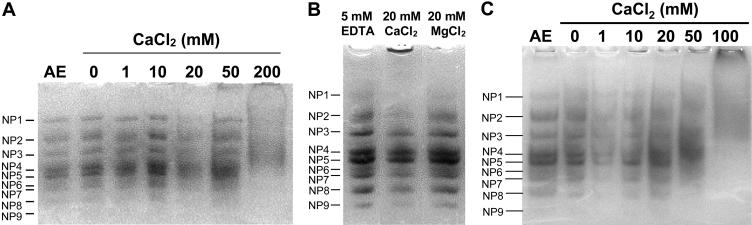
To determine the effect of cations on the structure of apoplastic proteins, apoplastic extracts were dialyzed into solutions of CaCl2, MgCl2, and NaCl, and separated by native PAGE. As shown in Figure 4A, apoplastic extracts dialyzed against low concentrations (0–10 mm) of CaCl2 exhibited nine native proteins (described previously by Yu and Griffith, 1999). However, in CaCl2 concentrations of 20 mm or higher, some proteins did not enter the gel, other proteins migrated more slowly within the gel, and the banding patterns on the gel were less distinct, all of which indicated either changes in protein charge or aggregation of the proteins. Aggregation of the native proteins was more apparent when AFPs were dialyzed into 20 mm CaCl2 and compared with the 20 mm MgCl2 and the 5 mm EDTA treatments (Fig. 4B).
Figure 4.
Effect of cations and freeze-thaw cycles on the organization of apoplastic proteins as determined by native PAGE. Proteins were separated in 9% polyacrylamide gels and stained with Coomassie Blue. A, Apoplastic proteins were dialyzed in 0 to 200 mm CaCl2, then 5 μg protein were loaded per lane. Nine native proteins (NPs) were visible in the original apoplastic extract, AE. B, Separation of apoplastic proteins (10 μg per lane) dialyzed in 5 mm EDTA, 20 mm CaCl2, and 20 mm MgCl2. C, Apoplastic proteins were dialyzed in 0 to 200 mm CaCl2, frozen and thawed three times, then 10 μg protein were loaded per lane.
When apoplastic proteins were frozen and thawed three times in the presence of 1 to 200 mm CaCl2 and then separated by native PAGE, the same native proteins were observed, but it was not possible to resolve the bands as clearly as in unfrozen samples (Fig. 4C). Because proteins in native PAGE are separated by charge as well as by size, the lack of resolution after freezing and thawing may be caused by changes in protein charge after binding Ca2+ or by changing the composition or aggregation of the oligomers.
Calcium Binds to Apoplastic Proteins
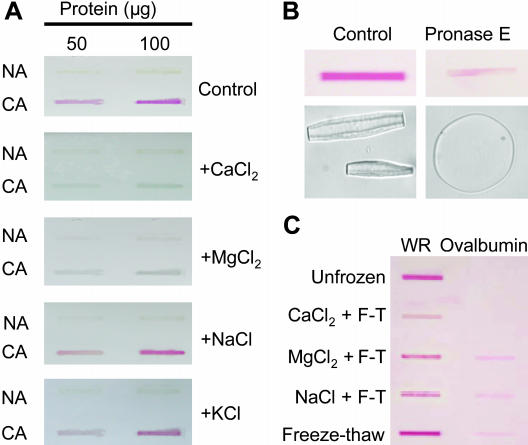
In order to confirm that Ca2+ interacts directly with apoplastic proteins, we examined the staining of apoplastic proteins with ruthenium red, which is known to bind at the same sites that bind Ca2+ (Achenbach and Ewart, 2002). Ovalbumin, a known Ca2+-binding protein, was used as a positive control to demonstrate that ruthenium red stains Ca2+-binding proteins a red color under our experimental conditions (Fig. 5C). Apoplastic proteins extracted from CA winter rye leaves and dialyzed in Tris-EDTA buffer to remove previously bound Ca2+ stained a bright red color when they bound ruthenium red, but apoplastic proteins isolated from nonacclimated (NA) winter rye leaves did not (Fig. 5A, control).
Figure 5.
Use of ruthenium red to observe binding of Ca2+ to apoplastic proteins extracted from NA and CA winter rye leaves. A, Apoplastic proteins (50 or 100 μg per sample) were blotted onto nitrocellulose and stained with ruthenium red. The ability of cations to displace ruthenium red was determined after washing blots with 100 mm solutions of CaCl2, MgCl2, NaCl, or KCl. Unstained proteins appear yellow-gray in color and stained proteins appear red. B, Apoplastic proteins (100 μg) from CA winter rye leaves were incubated with Pronase E for 2 h, and then blotted and stained with ruthenium red. Antifreeze activity was assayed by observing the shape of growing ice crystals. The c-axis of the ice crystals is parallel to the plane of the page for the control sample and normal to the plane of the page for the Pronase E sample. C, Effect of freeze-thaw cycles on ruthenium red binding to apoplastic proteins from CA winter rye leaves (WR). Apoplastic proteins (100 μg) were dialyzed in Tris-EDTA, subjected to three freeze-thaw cycles (F-T) in the presence of 40 mm CaCl2, MgCl2, or NaCl, and then blotted and stained with ruthenium red. Unfrozen and freeze-thawed controls in Tris-EDTA buffer were also included. Ovalbumin (100 μg) was the positive control for a Ca2+-binding protein.
Ruthenium red was completely displaced from apoplastic proteins isolated from CA winter rye leaves by incubating the ruthenium red-stained membranes in a solution containing 100 mm CaCl2 or 100 mm MgCl2 (Fig. 5A). This effect was due to binding of divalent cations because ruthenium red was not displaced when the CA apoplastic proteins were incubated in 100 mm NaCl or KCl (Fig. 5A). When the proteins were degraded by incubation with Pronase E, antifreeze activity disappeared and the binding of ruthenium red decreased (Fig. 5B), thus demonstrating that cation binding and antifreeze activity were both associated with proteinaceous components of the apoplastic extract.
Ruthenium red readily bound to apoplastic proteins that were dialyzed in Tris-EDTA buffer and were either held unfrozen or subjected to freeze-thaw cycles (Fig. 5C). Ruthenium red could still stain the proteins when MgCl2 or NaCl were present during the freeze-thaw cycles. However, freezing and thawing the proteins in the presence of CaCl2 reduced their subsequent ability to bind ruthenium red, thus indicating that Ca2+ binding sites were inaccessible to the dye (Fig. 5C).
Cations and Freeze-Thaw Cycles Affect Protein Tertiary Structure
CD was used in the near UV (240–320 nm) to examine the effects of cations and freeze-thaw cycles on the tertiary structure of AFPs (Strickland, 1974; Campbell and Dwek, 1984). Although the apoplastic extracts from winter rye leaves contain a complex mixture of proteins, each protein has a very low content of hydrophobic amino acids (Hon et al., 1994). For example, the genes encoding the two chitinase-AFPs have been cloned, and their translated sequences contain less than 13% Phe, Tyr, Trp, and His (Yeh et al., 2000). These low aromatic amino acid contents may explain why we were able to obtain spectra of complex protein mixtures with such low noise.
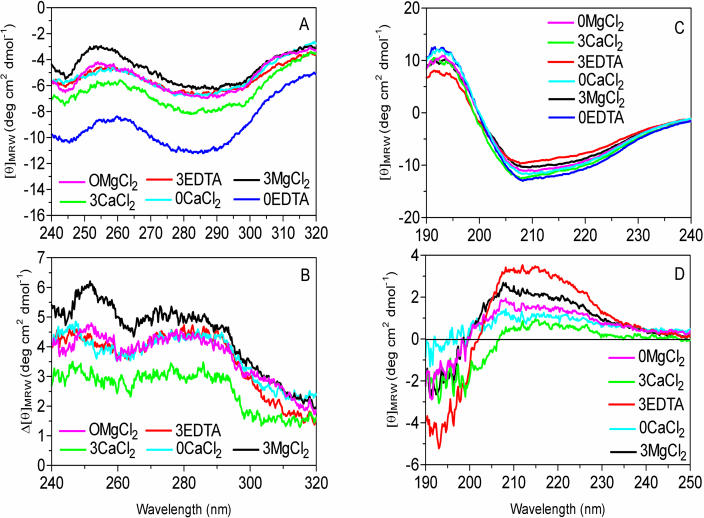
Apoplastic proteins dialyzed into 5 mm EDTA to minimize cation concentrations and stored at 4°C exhibited the most negative near-UV spectrum (0EDTA, Fig. 6A). Freezing and thawing the apoplastic proteins three times in the absence of cations resulted in a dramatic increase in the mean residue ellipticity (3EDTA, Fig. 6A). When the apoplastic proteins were dialyzed into 20 mm CaCl2 or MgCl2, they also exhibited an increase in mean residue ellipticity that was similar to the effect of freeze-thaw cycles (0CaCl2, 0MgCl2; Fig. 6A). We calculated difference spectra in the near UV by subtracting the 0EDTA treatment from all the other treatments to examine the effects of three freeze-thaw cycles on the interaction of cations with apoplastic proteins (Fig. 6B). Interestingly, freezing and thawing the proteins in 20 mm MgCl2 (3MgCl2, Fig. 6B) resulted in a more pronounced positive shift of the near-UV CD spectrum than freezing and thawing apoplastic proteins dialyzed into CaCl2 (3CaCl2, Fig. 6B).
Figure 6.
Effect of cations and freeze-thaw cycles on the structure of apoplastic proteins determined by CD. Apoplastic proteins (1 mg mL−1) were dialyzed in 5 mm EDTA (pH 5.5), 20 mm CaCl2, or 20 mm MgCl2. Half the samples were stored at 4°C as unfrozen controls, and the remaining samples were subjected to three freeze-thaw cycles, then they were maintained at 4°C until analysis. Treatments are labeled by the number of freeze-thaw cycles followed by the salt, e.g. 0EDTA indicates unfrozen, control samples dialyzed in EDTA, and 3EDTA indicates that samples dialyzed in EDTA were subjected to 3 freeze-thaw cycles. A, Near-UV CD spectra. B, Near-UV CD difference spectra calculated by subtracting the 0EDTA control from the other spectra shown in graph A. In order to examine secondary structure in far-UV, samples were diluted to 50 μg apoplastic protein mL−1. C, Far-UV CD spectra. D, Far-UV CD difference spectra calculated by subtracting the 0EDTA control from the other spectra shown in graph C.
To better understand the nature of the structural changes in apoplastic proteins caused by freeze-thaw cycles and cations, we also used Trp fluorescence to examine the degree of solvent exposure of the chromophore and polarization of the Trp ring (Reshetnyak et al., 2001; Vivian and Callis, 2001). Proteins dialyzed into 5 mm EDTA exhibited two fluorescence maxima, one at 360 nm reflecting Trp residues fully exposed to solvent, and one at 328 reflecting buried Trp residues (Table I). These results confirmed previous studies of chitinases purified from rye seeds, which showed that their Trp residues vary in susceptibility to oxidation and that a Trp involved in binding the chitin substrate is fully exposed on the protein surface (Yamagami and Funatsu, 1995, 1997). When the proteins in EDTA were frozen and thawed, they exhibited only one fluorescence maximum at 355 nm, indicating that all the Trp residues were exposed to solvent. Upon addition of either CaCl2 or MgCl2, the apoplastic proteins exhibited a blue shift of the Trp absorbance maxima to 335 to 336 nm, indicating less solvent exposure or polarization by electric fields from either solvent or protein residues (Table I). We interpreted this change as showing the formation of a more compact or aggregated AFP structure in the presence of divalent cations.
Table I.
Effect of cations and freeze-thaw cycles on the structure of apoplastic proteins from winter rye leaves as determined by Trp fluorescence
| Salt | No. of Freeze-Thaw Cycles | λmax |
|---|---|---|
| nm | ||
| EDTA | 0 | 327.7 ± 0.3, 360.3 ± 0.3 |
| EDTA | 3 | 355.2 ± 0.2 |
| CaCl2 | 0 | 336.5 ± 0.2 |
| CaCl2 | 3 | 335.5 ± 0.0 |
| MgCl2 | 0 | 336.3 ± 0.2 |
| MgCl2 | 3 | 334.8 ± 0.4 |
Proteins were dialyzed in 5 mm EDTA, 20 mm CaCl2, or 20 mm MgCl2. Half the samples were stored at 4°C until analysis as unfrozen controls, and the remaining samples were subjected to three freeze-thaw cycles in which they were frozen at −20°C for 2 h and thawed at 20°C for 5 min. Trp residues were excited at 280 nm, and the wavelengths of maximum fluorescence emission (λmax) were recorded. Data are presented as means ±se, n = 3.
Effects of Cations and Freeze-Thaw Cycles on Secondary Structure of Apoplastic Proteins
The effects of cations and freeze-thaw cycles on the secondary structure of AFPs were examined by CD in the far-UV range (Fig. 6C; Campbell and Dwek, 1984). The difference spectra ranging from 190 to 250 nm were calculated using the unfrozen, cation-free apoplastic proteins (0EDTA) as the control (Fig. 6D). The largest changes in protein secondary structure were observed after freezing and thawing in the absence of cations. Proteins that were frozen and thawed three times in 5 mm EDTA (3EDTA) exhibited more negative mean residue ellipticity at 193 nm and more positive mean residue ellipticity at 208 and 222 nm when compared with the 0EDTA control, thus indicating a loss of α-helical structure (Fig. 6D). The 3EDTA proteins also showed more negative mean residue ellipticity at 198 nm and more positive mean residue ellipticity at 217 nm relative to the 0EDTA control, which we interpreted as a loss of β structure. Moreover, the more negative mean residue ellipticity observed at 198 nm indicated an increase in random coil. Therefore, freezing and thawing in the absence of cations resulted in a loss of secondary structural elements in apoplastic proteins isolated from CA winter rye leaves.
The addition of CaCl2 to the apoplastic proteins resulted in the smallest observable change in protein secondary structure compared with the 0EDTA control (0CaCl2, Fig. 6D). The small increase in mean residue ellipticity at 208 and 222 nm coupled with the small decrease in mean residue ellipticity at 193 nm indicated a loss of α-helical structure. However, this was not accompanied by a more negative signal at 198 nm, indicating a possible conversion of α-helix to a β structure rather than random coil. After three freeze-thaw cycles in the presence of CaCl2, the proteins showed a small increase in random coil. Moreover, they also showed a decrease in mean residue ellipticity in the 200- to 205-nm range that was not observed in any other treatment and may involve modification of a β-turn (Perczel and Fasman, 1992; 3CaCl2, Fig. 6D). Adding MgCl2 to the apoplastic proteins had a different effect than adding CaCl2 (Fig. 6D). Proteins in MgCl2 lost some α-helical and β structure with a corresponding increase in random coil both with and without exposure to freeze-thaw cycles (Fig. 6D).
DISCUSSION
Antifreeze activity has been observed in many overwintering organisms, including bacteria, fungi, invertebrates, and vertebrates, as well as plants (DeVries, 1986; Duman and Olsen, 1993). AFPs have no common evolutionary origin as even their ice-binding domains lack a consensus sequence or a common structure (Ewart et al., 1999). To date, we know little about the thermal stability and ionic interactions of the AFPs from plants, which limits our understanding of both their physiological functions and their potential applications for inhibiting the recrystallization of ice in frozen foods and cryopreservation (Griffith and Ewart, 1995; Feeney and Yeh, 1998; Fletcher et al., 1999). The advantage of conducting studies on stability of AFPs from winter rye is that these proteins have dual functions, so we are able to compare several activities to gain a better understanding of the structure and function of these proteins.
Freezing and Cations Induce Structural Changes in Winter Rye Apoplastic Proteins
Winter rye plants accumulate PR proteins at warm temperatures when the plants are treated with pathogenic fungi, salicylic acid, or abscisic acid (Hiilovaara-Teijo et al., 1999; Wiseman, 2001; Yu and Griffith, 2001; Yu et al., 2001), but these proteins lack antifreeze activity. We have been unable to identify any specific changes in amino acid sequence or posttranslational modifications of the cold-induced PR proteins that could confer antifreeze activity (Wiseman, 2001). Therefore, it was interesting to observe that apoplastic proteins from NA plants were unable to bind ruthenium red, whereas it was readily bound by the proteins extracted from CA plants (Fig. 5A). The ability of PR proteins produced during cold acclimation to interact with Ca2+ may reflect changes in their surfaces that allow them to adsorb to ice.
Both freeze-thaw cycles and cations affected the structure or organization of the apoplastic proteins from CA winter rye leaves. Freeze-thaw cycles alone caused a large positive shift of the near-UV CD spectrum (Fig. 6), the disappearance of the Trp fluorescence peak at 328 nm (Table I), and the loss of secondary structural elements coupled with an increase in random coil (Fig. 6), perhaps by partially unfolding the proteins or rearranging the complexes. However, these structural changes did not affect antifreeze activity (Fig. 1), nor did they inhibit binding of ruthenium red (Fig. 5). Adding Ca2+ did not affect antifreeze activity, but did change the structure of apoplastic proteins extracted from CA winter rye leaves, as shown by decreased migration in native PAGE (Fig. 4), a large positive shift in near-UV CD (Fig. 6), the blue shift of the Trp fluorescence peak to 336.5 nm (Table I), and a small change in secondary structure (Fig. 6). Only the combination of freezing and thawing apoplastic proteins in the presence of Ca2+ decreased antifreeze activity (Fig. 1), chitinase activity (Fig. 2), and ruthenium red staining (Fig. 5). One interpretation of these results is that apoplastic proteins undergo a structural change during freezing that creates new Ca2+-binding sites. Once Ca2+ binds to those sites, the structure or organization of the oligomers becomes more compact, and antifreeze and chitinase activities are both inhibited.
AFPs from other organisms are known to undergo structural changes at cold temperatures that affect their ice-binding domains. For example, upon cooling, helical and β-structures increase in an AFP isolated from the beetle Dendroides canadensis (Li et al., 1998b), the α-helical structure increases in type I AFPs from winter flounder (Graether et al., 2001), and the ice-binding surface of the AFP isolated from spruce budworm increases its regularity (Graether et al., 2003). When cooled to subzero temperatures, antifreeze glycoproteins (AFGPs) from Antarctic fish form a variety of conformers that may expose more ice-binding groups which bind to different surfaces of ice (Tsvetkova et al., 2002). Freezing and thawing also affects the oligomerization of some AFPs. For example, AFPs from the insect spruce budworm form oligomers at 30°C, but these largely dissociate at 5°C, presumably due to weakening of hydrophobic forces (Graether et al., 2003). However, it is not known whether monomerization of the AFPs affects antifreeze activity. On one hand, monomerization increases the number of AFPs that can subsequently bind to more sites on the ice surface. On the other hand, protein complexes with greater mass are thought to cause more freezing point depression due to an increased Kelvin effect (Wu et al., 1991a, 1991b). If the winter rye apoplastic proteins dissociated to form monomers at cold temperatures, we would have expected these smaller proteins to have migrated rapidly through the native gels. Instead, the protein patterns remained largely the same after freezing and thawing (Fig. 4), but the blurred patterns could indicate differences in composition or charge of the oligomers after freezing and thawing.
Effect of Calcium on Winter Rye AFPs
The winter rye AFPs and type II AFPs from North Atlantic fish all exhibit homology to proteins that bind carbohydrates (Ewart et al., 1999). Another characteristic of type II AFPs is that they require Ca2+ for antifreeze activity. After binding a single Ca2+, a type II AFP adopts a new conformation that creates the ice-binding domain (Ewart et al., 1996, 1998, 1999, 2000; Achenbach and Ewart, 2002). However, Ca2+ had no effect on antifreeze activity in unfrozen winter rye extracts (Fig. 1). Instead, Ca2+ inhibited winter rye antifreeze activity after freezing, thus demonstrating that the ice-binding domains are not created in the same way in winter rye and type II AFPs.
Cations may also inhibit AFPs from other organisms. Li and coworkers (1998a) observed that the activity of AFPs isolated from the hemolymph of the overwintering beetle D. canadensis was enhanced 3- to 6-fold in the presence of 0.25 to 1.0 m concentrations of small molecules such as citrate, succinate, malate, Asp, glycerol, sorbitol, and Ala. Only glycerol was found at high enough concentrations in the hemolymph of overwintering beetles to function as an enhancer of AFPs in vivo (Duman and Serianni, 2002). It is not known how these molecules affect antifreeze activity; however, our results raise the possibility that the organic acids function as chelators that minimize interactions between AFPs and cations.
Effect of Calcium on the Activity of Winter Rye Chitinases
The specific activity of chitinases isolated from CA winter rye leaves was enhanced 3- to 5-fold in the presence of more than 10 mm CaCl2 or MgCl2 (Figs. 2A and 3C). Only a few studies have reported the effect of cations on chitinase activity. In one case, a chitinase isolated from the marine bacterium Alteromonas sp. was activated 1.6-fold by 1 mm Mg2+ and 1.3-fold by 1 mm Na+, K+, or Ca2+ (Tsujibo et al., 1992). In a second example, the activity of a chitinase isolated from pineapple stems was not affected by 5 mm NaCl, MgCl2, or CaCl2 (Hung et al., 2002).
Although Ca2+ enhances chitinase activity before freezing, it inhibits chitinase activity after freezing and thawing (Figs. 2 and 3). Chitinase activity could not be restored by chelators after freezing and thawing in the presence of Ca2+ (Fig. 2). These results indicate that the Ca2+ binding site(s) involved in activating the chitinases is different from the Ca2+ binding site(s) that inhibit both antifreeze and chitinase activities. Our observation that Mg2+ enhances chitinase activity (Fig. 2), but has little effect on antifreeze activity before or after freeze-thaw cycles (Fig. 1), shows that Mg2+ can bind to the regulatory site normally occupied by Ca2+, but not to the Ca2+-binding sites exposed by freezing and thawing. We were unable to localize the Ca2+-binding sites because searches using the tools available at the InterProScan and ExPASy sites (http://www.ebi.ac.uk/interpro/scan.html, June 2003; and http://ca.expasy.org/tools/scanprosite/, June 2003; respectively) did not find any known Ca2+-binding domains in the amino acid sequences of cold-induced winter rye class I and class II chitinases with antifreeze activity (Yeh et al., 2000).
The free Ca2+ concentration in the apoplast usually ranges from 0.1 to 1 mm (Bush, 1995; Mühling et al., 1998; Felle, 2001) at a pH of 4.6 to 5.9 (Yu et al., 2000; Felle, 2001). Although these conditions are ideal for maintaining stable antifreeze activity, the concentration of Ca2+ is too low to activate chitinase activity. However, the majority of the Ca2+ found in plants is bound to pectin, so it is possible that winter rye plants may acidify the cell wall to release proton-exchangeable Ca2+ into the apoplast and enhance chitinase activity in planta (Bush, 1995). Alternatively, if Ca2+-transport proteins are present in the apoplast, they could interact cooperatively with winter rye chitinases and increase the affinity of their Ca2+-binding sites (Zielinski, 1998).
SUMMARY
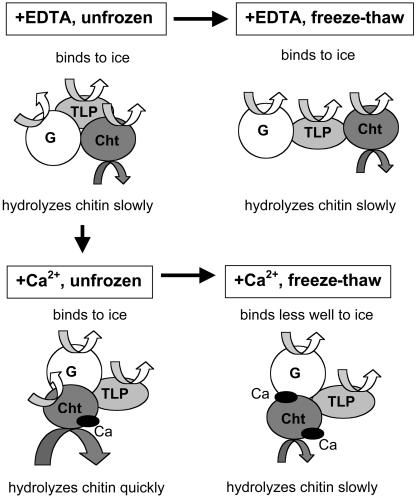
The effects of freeze-thaw cycles and Ca2+ on AFPs are summarized in Figure 7. AFPs/PR proteins accumulate as a population of oligomeric proteins in the apoplast of winter rye leaves during cold acclimation and exhibit ice-binding activity and antifungal activities such as chitin hydrolysis. When frozen and thawed in the absence of cations, the AFPs/PR proteins maintain both antifreeze and chitinase activities (Figs. 1 and 2) even though they undergo structural changes (Table I; Fig. 6). In the presence of Ca2+, the native proteins adopt a more compact structure that enhances chitinase activity but does not affect antifreeze activity (Figs. 1, 2, and 3). However, there is an interaction between freeze-thaw treatments and Ca2+. When the AFPs/PR proteins are repeatedly frozen and thawed in the presence of Ca2+, they bind Ca2+ at newly exposed sites (Fig. 5), which changes their structure (Table I; Fig. 6) and inhibits antifreeze and chitinase activities (Figs. 1 and 2). Both chitinase and antifreeze activities can be easily maintained during frozen storage in vitro by adding a chelator to minimize the interaction between AFPs and divalent cations during freezing and thawing. The free Ca2+ concentration in the leaf apoplast is low, so AFPs should be stable to freezing and thawing in planta.
Figure 7.
Hypothetical model summarizing changes observed in native apoplastic proteins exposed to Ca2+ and repeated freeze-thaw cycles. For clarity, only one native protein (NP3) is shown and is modeled with the simplest stoichiometry of one glucanase (G), one chitinase (Cht) and one thaumatin-like protein (TLP). NP3 itself has antifreeze activity (Yu and Griffith, 1999), and each of the three components exhibits antifreeze activity when separated as individual polypeptides (Hon et al., 1994, 1995). When maintained in the unfrozen state in the absence of Ca2+, NP3 exhibits both antifreeze and chitinase activities. When frozen and thawed, NP3 adopts a different structure and still exhibits both antifreeze and chitinase activities. When Ca2+ interacts with the native, unfrozen protein, the structure of NP3 becomes more compact, and the complex exhibits enhanced chitinase activity with no change in antifreeze activity. However, when NP3 is frozen and thawed repeatedly in the presence of Ca2+, NP3 binds more Ca2+ and both antifreeze and chitinase activities decrease.
MATERIALS AND METHODS
Plant Materials
Seeds of winter rye (Secale cereale L. cv Musketeer) were sown at a rate of 5 g seed per 15-cm pot of ProMix BX (Premier Horticulture, Riviere de Loup, Quebec, Canada). NA plants were grown at 20°C/16°C (day/night) with a 16-h daylength and a photosynthetic photon flux density (PPFD) of 300 μmol m−2 s−1 for 3 weeks. For cold acclimation, plants were transferred after 1 week to 5°C/2°C (day/night) with an 8-h daylength and a PPFD of 300 μmol m−2 s−1 for an additional 7 weeks. Plants were watered as needed and fertilized weekly with 0.5 g L−1 of 20-20-20 all-purpose fertilizer (Plant Products, Brampton, Ontario, Canada).
Apoplastic Protein Extraction
Apoplastic proteins were extracted as described by Hon et al. (1994). Briefly, the leaves were cut into 3-cm sections, washed with Milli-Q water, and vacuum-infiltrated with 20 mm ascorbic acid and 20 mm CaCl2. Leaves were placed into strainers within centrifuge bottles and centrifuged at 1,471g and 4°C for 30 min to recover the apoplastic contents. Protein contents were measured by the Bradford (1976) method, as modified by Bio-Rad Laboratories (Mississauga, Ontario, Canada), with bovine serum albumin as the standard protein.
Cation, Chelator, and Freeze-Thaw Treatments
Aliquots of apoplastic extracts containing 150 μg protein were dialyzed for 16 h at 4°C against several changes of 1 L of various concentrations (1, 10, 20, 50, or 200 mm) of CaCl2, and against 1 L of 20 or 200 mm of CaCl2, MgCl2, or NaCl; and against 1 L of 5 mm EDTA, sodium succinate, or EGTA (which are all carboxylic acids), at pH 5.5, using Spectra/Por dialysis membranes with a 12,000 to 14,000 MW cutoff (Spectrum Laboratories, Rancho Dominguez, CA). After dialysis, half of the samples were used as unfrozen controls, and antifreeze and chitinase activities were measured immediately. The remaining samples were subjected to freeze-thaw cycles by placing them in a freezer at −20°C for 2 h, and then thawing them rapidly (5 min) at 20°C. To examine the reversibility of cation effects, samples that were frozen and thawed in 20 or 200 mm of CaCl2 were dialyzed again against several changes of 1 L of 5 mm EGTA or 50 mm EDTA at pH 5.5 for 16 h at 4°C. Samples were assayed for antifreeze and chitinase activities immediately, and then stored at 4°C for analysis by native PAGE. All experiments were carried out using three independent extracts as replicates.
Antifreeze Activity
Antifreeze activity was assayed by examining the morphology of ice crystals grown in solution by using a thermoelectric freezing stage of a Clifton nanoliter osmometer (Hartford, NY) mounted on a phase-contrast photomicroscope (Olympus BHT, Tokyo; Hon et al., 1994). In this assay, the antifreeze activity was described as absent when ice crystals were shaped like a disc, low when ice crystals formed a hexagonal plate, and high when ice was shaped as hexagonal bipyramids (De Vries, 1986; Fig. 8). To quantify antifreeze activity, samples were diluted with Milli-Q water to determine the lowest protein concentration at which an ice crystal with a hexagonal shape could be observed. Data for antifreeze activity were expressed as the dilution volume plus 1. Therefore, a sample with no antifreeze activity was defined as 0 and a sample that only had antifreeze activity without dilution (1:0) was defined as 1. A sample that formed a hexagonal ice crystal when diluted by adding 10 volumes of Milli-Q water (1:10), but formed a circular crystal when diluted by adding 11 volumes of Milli-Q water (1:11), was calculated as 10 + 1 = 11.
Figure 8.
Antifreeze activity in apoplastic extracts determined by observing the morphology of ice crystals grown in solution. A, Circular ice crystals represent no antifreeze activity; B, hexagonal ice crystals represent low activity; C, hexagonal ice crystals with increased growth along the c axis represent moderate activity; and D, hexagonal bipyramids represent high antifreeze activity. In A and B, the basal plane of each ice crystals is parallel to the plane of the page. In C and D, the basal plane of each ice crystal is normal to the plane of the page. The magnification bar represents 10 μm.
Chitinase Activity
Chitinase activity was assayed by using 50 μm 4-methylumbelliferyl β-d-N, N′, N″-triacetylchitotrioside hydrate (4-MU (GlcNAc)3; Sigma, St. Louis) dissolved in 0.1 m phosphate buffer (pH 6.0) as the substrate (Hollis et al., 1997). Five microliters of apoplastic extract were mixed with 95 μL of substrate solution and incubated at 37°C for 30 min. The reaction was stopped by adding 2.9 mL of 1 m Gly-NaOH (pH 10.5). Release of 4-MU was measured using a spectrofluorimeter (Spectra Max GeminiXS, Molecular Devices, Sunnyvale, CA) with excitation at 360 nm and emission at 450 nm. A standard curve of free 4-MU was shown to be linear over the range of product measured (0–0.05 μm). One unit of chitinase activity was defined as the amount of enzyme releasing 1 μmol of 4-MU per min under assay conditions. Data for both antifreeze and chitinase activities were examined by two-way analysis of variance with cation, chelator, cation concentration and/or freeze-thaw cycles as the treatments. When treatment or interaction effects were significant (P < 0.05) in the ANOVA, Scheffe's post hoc comparison tests or Student's t tests were carried out.
Purification of Native Chitinase
A native apoplastic protein (NP3; Yu and Griffith, 1999) containing a class I chitinase was purified by affinity chromatography using colloidal chitin for the column matrix (Huynh et al., 1992; Hon et al., 1995). Fractions containing NP3 were obtained by elution using 20 mm acetic acid, pH 3, and assayed for protein content and chitinase activity. The polypeptide composition of NP3 was also examined by SDS-PAGE and immunoblotting.
SDS-PAGE and Native PAGE
Proteins were separated by SDS-PAGE on 12% (w/v) polyacrylamide gels according to the method of Laemmli (1970) using Bio-Rad's Mini Protean II system with prestained, broad-range Precision Protein Standards (Bio-Rad). Native proteins were separated using continuous 9% (w/v) polyacrylamide gels. Crude apoplastic extracts containing 5 to 10 μg protein, or extracts dialyzed in cation or chelator solutions, were added directly to the wells, and proteins were separated using a running buffer of pH 3.8 (30 mm β-Ala and 20 mm lactic acid) with inverted polarity for 1 h at 200 V and 4°C (Yu and Griffith, 1999). All gels were stained with Coomassie Brilliant Blue R-250.
Ruthenium Red Staining
Apoplastic extracts (30 mL) from NA and CA winter rye leaves were lyophilized and resuspended in 2 mL of Tris-EDTA buffer (20 mm Tris-HCl, pH 5.3, 20 mm EDTA). Although this step involved initially freezing the samples, we have shown that the effects of freezing in the presence of cations are reversible by adding EDTA (Figs. 1 and 2). Samples were dialyzed using Spectra/Por dialysis membranes with a 12,000 to 14,000 MW cutoff against three changes of 800 mL of Tris-EDTA buffer. Equal amounts (50 or 100 μg) of proteins from NA and CA extracts were spotted onto nitrocellulose membranes (0.45 μm pore size, Bio-Rad, preequilibrated in Tris-EDTA buffer) using a Bio Dot blot-SF apparatus (Bio-Rad). Blots were washed with the same buffer and then stained with 0.1 mg mL−1 ruthenium red (Sigma) dissolved in Tris-EDTA buffer, at room temperature for 10 min. Stained blots were washed in Tris-EDTA buffer and photographed while still damp using a digital camera (Coolpix 990, Nikon, Tokyo). Displacement of ruthenium red staining was determined by photographing membranes after washing them with solutions containing 100 mm NaCl, KCl, MgCl2, or CaCl2 dissolved in Tris-EDTA buffer for 10 min. To determine the effect of cations during freeze-thaw cycles, 40 mm NaCl, MgCl2, or CaCl2 were added to apoplastic proteins dissolved in Tris-EDTA buffer, then the samples were frozen and thawed three times as described above along with a control sample to which no salts were added. Ovalbumin was dissolved in Tris-EDTA buffer as a positive control for ruthenium red staining. All the proteins were then spotted onto a nitrocellulose membrane and stained with ruthenium red as described above.
CD and Trp Fluorescence
Apoplastic extracts were concentrated to 1 mg protein mL−1 by ultrafiltration using Microcon YM-10 spin filters (Millipore Corp., Bedford, MA) that were centrifuged at 1,500g for 2 h at 5°C. Samples of concentrated extract were dialyzed against 1 L of 5 mm EDTA (pH 5.5), 20 mm CaCl2, or 20 mm MgCl2 for 16 h at 4°C and assayed to determine the final protein content. Half of the samples were stored at 4°C as unfrozen controls. The remaining samples were subjected to three freeze-thaw cycles and then maintained at 4°C until analysis. Three near-UV (320–240 nm) and far-UV (190–250 nm) spectra were acquired and averaged for each of the six treatments at a concentration of 1 mg protein mL−1 or diluted to 50 μg protein mL−1, respectively, using a spectropolarimeter (model J-600, Jasco, Easton, MD) with a 1-cm pathlength and a 1-nm bandwidth, at a scanning rate of 20 nm min−1 at room temperature. The data correspond to the mean residue ellipticities [θ]MRW, which were calculated from the ellipticity (θ) as 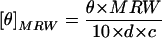 where θ is the ellipticity in degrees, MRW is the mean residue Mr for an amino acid (110 g mol−1), d is the pathlength in cm (1 cm), and c is the concentration of protein in g mL−1. Near-UV spectra are usually transformed to Δɛ, the difference in molar absorptivity between left and right circularly polarized light, but N, the total number of residues in the protein, was not available for the transformation.
where θ is the ellipticity in degrees, MRW is the mean residue Mr for an amino acid (110 g mol−1), d is the pathlength in cm (1 cm), and c is the concentration of protein in g mL−1. Near-UV spectra are usually transformed to Δɛ, the difference in molar absorptivity between left and right circularly polarized light, but N, the total number of residues in the protein, was not available for the transformation.
Independent samples of each of the treatments used for CD were also assayed for Trp fluorescence using a Shimadzu Spectrofluorophotometer RF-540 (Mandel Scientific, Guelph, Ontario, Canada) with a Shimadzu Data Recorder DR-3. Protein solutions containing 0.1 mg mL−1 were excited at 280 nm, and the emission spectra were monitored from 300 to 380 nm. The scans were performed against their salt blanks, and all analyses were performed in triplicate.
Acknowledgments
Musketeer rye seeds were obtained from Dr. Grant McLeod (Agriculture Canada, Swift Current, Saskatchewan, Canada). We are grateful to Dr. Rickey Yada and Massimo Marcone at the Department of Food Science, University of Guelph, Guelph, Ontario, Canada, and Andre Peters, University of Applied Science Berlin, Germany, for their technical assistance with the CD and Trp fluorescence experiments, and Dr. Xiao-Ming Yu, St. Jacobs, Ontario, for technical advice on native PAGE and colloidal chitin affinity chromatography. We thank Dr. Wayne Snedden, Department of Biology, Queens University, Kingston, Ontario, Canada, for advice in analyzing the properties of calcium-binding proteins. We also thank Mr. and Mrs. Stressmann for their financial support of M. Stressmann in Canada and Osaka Shoin Women's University for sabbatical support for Dr. S. Kitao. Dr. L.A. Bravo was supported by a research fellowship funded by MECESUP UCO 9906.
This work was supported by a grant from the Natural Science and Engineering Research Council of Canada (to M.G.) and by a grant from the University of Waterloo Interdisciplinary Research Program (to C.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.038158.
References
- Achenbach JC, Ewart KV (2002) Structural and functional characterization of a C-type lectin-like antifreeze protein from rainbow smelt (Osmerus mordax). Eur J Biochem 269: 1219–1226 [DOI] [PubMed] [Google Scholar]
- Antikainen M, Griffith M, Zhang J, Hon WC, Yang DSC, Pihakaski-Maunsbach K (1996) Immunolocalization of antifreeze proteins in winter rye leaves, crowns and roots by tissue printing. Plant Physiol 110: 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 341–374 [DOI] [PubMed] [Google Scholar]
- Bush DS (1995) Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol 46: 95–122 [Google Scholar]
- Campbell ID, Dwek RA (1984) Biological Spectroscopy. Benjamin/Cummings Publishing, Menlo Park, CA, pp 255–277
- Chun JU, Yu XM, Griffith M (1998) Genetic studies of antifreeze proteins and their correlation with winter survival in wheat. Euphytica 102: 219–226 [Google Scholar]
- DeVries AL (1986) Antifreeze glycopeptides and peptides: interactions with ice and water. Methods Enzymol 127: 293–303 [DOI] [PubMed] [Google Scholar]
- Duman JG, Olsen TM (1993) Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology 30: 322–328 [Google Scholar]
- Duman JG, Serianni AS (2002) The role of endogenous antifreeze protein enhancers in the hemolymph thermal hysteresis activity of the beetle Dendroides canadensis. J Insect Physiol 48: 103–111 [DOI] [PubMed] [Google Scholar]
- Ergon Å, Klemsdal SS, Tronsmo AM (1998) Interactions between cold hardening and Microdochium nivale infection on expression of pathogenesis-related proteins in winter wheat. Physiol Mol Plant Pathol 53: 301–310 [Google Scholar]
- Ewart KV, Blanchard B, Johnson SC, Bailey WL, Martin-Robichaud DJ, Buzeta MI (2000) Freeze susceptibility in haddock (Melanogrammus aeglefinus). Aquaculture 188: 91–101 [Google Scholar]
- Ewart KV, Li Z, Yang DSC, Fletcher GL, Hew CL (1998) The ice-binding site of Atlantic herring antifreeze protein corresponds to the carbohydrate-binding domain of C-type lectins. Biochemistry 37: 4080–4085 [DOI] [PubMed] [Google Scholar]
- Ewart KV, Lin Q, Hew CL (1999) Structure, function and evolution of antifreeze proteins. Cell Mol Life Sci 55: 271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart KV, Yang DSC, Ananthanarayanan VS, Fletcher GL, Hew CL (1996) Ca2+-dependent antifreeze proteins. J Biol Chem 271: 16627–16632 [DOI] [PubMed] [Google Scholar]
- Feeney RE, Yeh Y (1998) Antifreeze proteins: current status and possible food uses. Trends Food Sci Tech 9: 102–106 [Google Scholar]
- Felle HH (2001) pH: signal and messenger in plant cells. Plant Biol 3: 577–591 [Google Scholar]
- Fletcher GL, Goddard SV, Wu YL (1999) Antifreeze proteins and their genes: from basic research to business opportunity. Chemtech 29: 17–28 [Google Scholar]
- Graether SP, Gagné SM, Spyracopoulos L, Jia Z, Davies PL, Sykes BD (2003) Spruce budworm antifreeze proteins: changes in structure and dynamics at low temperature. J Mol Biol 327: 1155–1168 [DOI] [PubMed] [Google Scholar]
- Graether SP, Slupsky CM, Davies PL, Sykes BD (2001) Structure of type I antifreeze protein and mutants in supercooled water. Biophys J 81: 1677–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, Antikainen M (1996) Extracellular ice formation in freezing-tolerant plants. Adv Low-Temp Biol 3: 107–139 [Google Scholar]
- Griffith M, Ewart KV (1995) Antifreeze proteins and their potential use in frozen foods. Biotechnol Adv 13: 375–402 [DOI] [PubMed] [Google Scholar]
- Hiilovaara-Teijo M, Hannukkala A, Griffith M, Yu XM, Pihakaski-Maunsbach K (1999) Snow-mold-induced apoplastic proteins in winter rye lack antifreeze activity. Plant Physiol 121: 665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis T, Honda Y, Fukamizo T, Marcotte E, Day PJ, Robertus JD (1997) Kinetic analysis of barley chitinase. Arch Biochem Biophys 344: 335–342 [DOI] [PubMed] [Google Scholar]
- Hon WC, Griffith M, Chong P, Yang DSC (1994) Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.). Plant Physiol 104: 971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon WC, Griffith M, Mlynarz A, Kwok YC, Yang DSC (1995) Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol 109: 879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Wang L, Wusteman MC, Smallwood M, Pegg DE (2002) The stability during low-temperature storage of an antifreeze protein isolated from the roots of cold-acclimated carrots. Cryobiology 44: 307–310 [DOI] [PubMed] [Google Scholar]
- Hung T-H, Chang Y-M, Sung H-Y, Chang C-T (2002) Purification and characterization of hydrolase with chitinase and chitosanase activity from commercial stem bromelain. J Agric Food Chem 50: 4666–4673 [DOI] [PubMed] [Google Scholar]
- Huynh QK, Hironaka CM, Levine EB, Sith CE, Bormeyer JR, Shah DM (1992) Antifungal proteins from plant: purification, molecular cloning, and antifungal properties of chitinase from maize seed. J Biol Chem 267: 6635–6640 [PubMed] [Google Scholar]
- Knight CA, DeVries AL, Oolman LD (1984) Fish antifreeze protein and the freezing and recrystallization of ice. Nature 308: 295–296 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Li N, Andorfer CA, Duman JG (1998. a) Enhancement of insect antifreeze protein activity by solutes of low molecular mass. J Exp Biol 201: 2243–2251 [DOI] [PubMed] [Google Scholar]
- Li N, Kendrick BS, Manning MC, Carpenter JF, Duman JG (1998. b) Secondary structure of antifreeze proteins from overwintering larvae of the beetle Dendroides canadensis. Arch Biochem Biophys 360: 25–32 [DOI] [PubMed] [Google Scholar]
- Mühling KH, Wimmer M, Goldbach HE (1998) Apoplastic and membrane-associated Ca2+ in leaves and roots as affected by boron deficiency. Physiol Plant 102: 179–184 [Google Scholar]
- Perczel A, Fasman GD (1992) Quantitative analysis of cyclic β-turn models. Protein Sci 1: 378–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihakaski-Maunsbach K, Griffith M, Antikainen M, Maunsbach AB (1996) Immunogold localization of glucanase-like antifreeze protein in cold-acclimated winter rye. Protoplasma 191: 115–125 [Google Scholar]
- Pihakaski-Maunsbach K, Moffatt B, Testillano P, Risueño M, Yeh S, Griffith M, Maunsbach AB (2001) Genes encoding chitinase-antifreeze proteins are regulated by cold and expressed by all cell types in winter rye shoots. Physiol Plant 112: 359–371 [DOI] [PubMed] [Google Scholar]
- Reshetnyak YK, Koshevnik Y, Burstein EA (2001) Decomposition of protein tryptophan fluorescence spectra into log-normal components. III. Correlation between fluorescence and microenvironment parameters of individual tryptophan residues. Biophys J 81: 1735–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider CS, Hsiang T, Zhao G, Griffith M (2000) Role of ice nucleation and antifreeze activities in pathogenesis and growth of snow molds. Phytopathology 90: 354–361 [DOI] [PubMed] [Google Scholar]
- Strickland EH (1974) Aromatic contributions to circular dichroism spectra of proteins. CRC Crit Rev Biochem 2: 113–175 [DOI] [PubMed] [Google Scholar]
- Tsujibo H, Yoshida Y, Miyamoto M, Imada C, Okami Y, Inamori Y (1992) Purification, properties, and partial amino acid sequence of chitinase from a marine Alteromonas sp. Strain 0-7. Can J Microbiol 38: 891–897 [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Phillips BL, Krishnan VV, Feeney RE, Fink WH, Crowe JH, Risbud SH, Tablin F, Yeh Y (2002) Dynamics of antifreeze glycoproteins in the presence of ice. Biophys J 82: 464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JT, Callis PR (2001) Mechanisms of tryptophan fluorescence shifts in proteins. Biophys J 80: 2093–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman SB (2001) Cloning and characterization of a winter rye class II chitinase that is induced by abscisic acid. MSc thesis. University of Waterloo, Waterloo, Canada
- Wu DW, Duman JG, Xu L (1991. a) Enhancement of insect antifreeze protein activity by antibodies. Biochim Biophys Acta 1076: 416–420 [DOI] [PubMed] [Google Scholar]
- Wu DW, Duman JG, Xu L (1991. b) Activation of antifreeze proteins from larvae of the beetle Dendroides canadensis. J Comp Physiol [B] 161: 279–283 [DOI] [PubMed] [Google Scholar]
- Yamagami T, Funatsu G (1995) Identification of the tryptophan residue located at the substrate-binding site of rye seed chitinase-c. Biosci Biotechnol Biochem 59: 1076–1081 [DOI] [PubMed] [Google Scholar]
- Yamagami T, Funatsu G (1997) Involvement of Trp23 in the chitin-binding and Trp131 in the chitinase activity of rye seed chitinase-a. Biosci Biotechnol Biochem 61: 1819–1825 [DOI] [PubMed] [Google Scholar]
- Yeh S, Moffatt BA, Griffith M, Xiong F, Yang DSC, Wiseman SB, Sarhan F, Danyluk J, Xue YQ, Hew CL, et al. (2000) Chitinase genes responsive to cold encode antifreeze proteins in winter cereals. Plant Physiol 124: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Tang C, Kuo J (2000) A critical review on methods to measure apoplastic pH in plants. Plant Soil 219: 29–40 [Google Scholar]
- Yu XM, Griffith M (1999) Antifreeze proteins in winter rye leaves form oligomeric complexes. Plant Physiol 119: 1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Griffith M (2001) Winter rye antifreeze activity increases in response to cold and drought, but not abscisic acid. Physiol Plant 112: 78–86 [DOI] [PubMed] [Google Scholar]
- Yu XM, Griffith M, Wiseman SB (2001) Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol 126: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski RE (1998) Calmodulin and calmodulin-binding proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 49: 697–725 [DOI] [PubMed] [Google Scholar]