Abstract
Cytochrome P450 3A4 (CYP3A4) is the most plentiful cytochrome P450 in adult human liver and small intestine and is responsible for detoxification of more than 50% of drugs in addition to the metabolic deactivation and metabolism of many carcinogens. Polymorphism of CYP3A4-A-290G considered the only allele that appears to stimulate CYP3A4 expression and has been associated with a number of clinical phenotypes, including prostate cancer, breast cancer, leukemia and the early onset of puberty. In this study, we analyzed the presence of CYP3A4-A-290G polymorphism in 77 newly diagnosed AML cases and 72 healthy control using PCR/RFLP aiming to show CYP3A4-A-290G polymorphism pattern in acute myeloid leukemia patients, and its role in disease severity and progression. A highly statistically significant difference was found between the control and AML groups as regards the heterozygous genotype (p-value = 0.002) and increases the risk of AML 11.4-fold. Also there was a highly significant difference between the control and AML patients regarding variant allele (G in AG and GG genotypes) (p-value 0.001) and increases the risk of AML 19-fold. No statistically significant association found between the CYP3A4-A-290G polymorphism and different clinical or laboratory parameters as well as an initial response to treatment, overall survival and the disease free survival. We concluded that CYP3A4-A-290G polymorphism is a genotypic factor that increases the CYP3A4 enzymatic activity and increases the risk of AML by 18.9-fold.
Keywords: CYP3A4-A, 290G, AML, Prognosis, PCR/RFLP
Introduction
Acute myeloid leukemia is the most common acute leukemia mostly affecting adults, characterized by the rapid growth of abnormal white blood cells in the bone marrow and impaired production of normal blood cells [1]. The mechanisms for AML genesis are still rarely understood. Several genetic polymorphisms have been determined as possible risk factors for leukemia [2].
Recent evidence indicates that enzymes involved in carcinogen activation or deactivation as well as their encoding genes, might play critical roles in determining individual susceptibility to cancers. Polymorphisms in these genes encoding the enzymes, possibly by altering their functions, could affect carcinogen activation (either increase or decrease) and modulating DNA repair processes [3]. Cytochrome P450 enzymes are essential role in the phase I dependent metabolism of drugs and all other xenobiotics [4].
The CYP3A enzymes are involved in the metabolism of more than 50% of all drugs presently on the market, and they contribute in the metabolic activation and metabolism of various carcinogens such as aflatoxin B and many anticancer drugs. Cytochrome P450 3A4 is found to be the most plentiful cytochrome P450 in adult human liver and small intestine [5].
Genetic polymorphisms within the drug-metabolizing enzymes are quite general and may participate to the risk of developing cancers. CYP3A4*1B is considered the only allele that appears to influence the CYP3A4 expression [6].
In this study, the presence of polymorphism was analyzed by a molecular PCR-RFLP technique in 77 newly diagnosed AML cases and 72 age and sex comparable healthy controls, to estimate the risk of AML with this polymorphism and its prognostic significance in AML patients.
Patients and methods
The study was approved by the Cairo University Hospital Research Ethics Committee (REC) and informed consent was obtained from each patient before starting the data collection. The study included 77 newly diagnosed AML patients which presented to the Adult and Pediatric Oncology Department, National Cancer Institute (NCI), Cairo University in the period between April 2010 and October 2011. Diagnosis was performed according to clinical, morphological, cytochemical and Flow Cytometric analysis. The recruited patients comprised of 43 males and 34 females between the age of 4 and 83 years with a median of 35 years and control group composed of 72 individual comprising 53 male and 19 female was randomly selected from blood donors; their ages ranged from 19 to 53 years with a median age 31.5 years.
With respect to all patients’ confidentiality, they were represented by code numbers. All patients were subjected to a precise history taking, clinical examination and routine laboratory investigations including complete blood picture, functions of both liver and kidney. For each patient, 10 ml of blood was collected in a sterile heparinized vacutainer. Response to induction therapy was assessed between days 14, and 28 after induction treatment, and follow up of cases was done for a period of at least 6 months to calculate their disease free survival and overall survival. Complete response was defined in accordance with standard criteria [7], which required an absolute neutrophils count of 1.5 × 109/L or more, a platelet count of 100 × 109/L or more, no blasts in PB, BM cellularity more than 20%, no auer rods, less than 5% BM blasts and no extra medullary leukemia.
The CYP3A4-A-290G polymorphism was determined using a PCR/RFLP method [8], and Genomic DNA used was extracted from lymphocytes using QIAamp DNA Mini isolation kit (QIAGEN).
The primers used were as follows Fwd: GGA CAG CCA TAG AGA CAA GGG CC Rev: TCA CTG ACC TCC TTT GAG TTC ATA. PCR was performed in 200 ng of genomic DNA, 0.5 μmol/L of primers Fwd and Rev, 200 μmol/L dNTPs, 10 mmol/L Tris–HCl (pH 8.3), 2.5 mmol/L MgCl2, 50 mmol/L KCl and 0.5 U Hotstart DNA polymerase (Qiagen). After denaturation for 10 min at 95 °C, 35 cycles of 45 s at 95 °C, 45 s at 59 °C, and 1 min at 72 °C, the last elongation step was extended to 7 min. Digestion of the PCR product with 10U of Msp1 restriction enzyme in a final volume of 20 μl incubated at 37 °C for overnight. The presence of the A to G polymorphism introduces the restriction site (5′CCGG3′) recognized by the Msp1 restriction enzyme (New England Biolabs Inc., Ipswich, MA). The result is either retention of a 165-base pair (bp) product or complete digestion to 142-bp and 23-bp fragments corresponding to individuals homozygous for the A (wild type) or G (variant) alleles respectively. The presence of both the 165- and 142-bp fragments corresponded to heterozygous individuals. The polymorphism was detected on a 3% agarose gel (see Fig. 1).
Fig. 1.
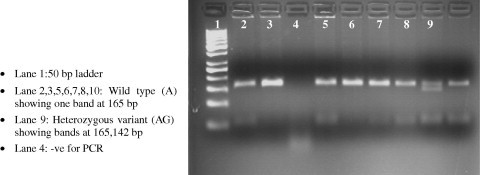
PCR-RFLP detection of CYP3A4-A-290G.
Statistical analysis
Data were analyzed with SPSSwin statistical package version 20 (SPSS Inc., Chicago, IL). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test (Fisher’s exact test) was used to examine the relationship between qualitative variables. Survival analysis was performed using the Kaplan–Meier method and comparison between two survival curves was made using the log-rank test. Logistic regression was used for calculation of odds ratio (OR) with it 95% confidence interval (CI) for risk estimation. A p-value < 0.05 was considered significant.
Results and discussion
In our study we analyzed the presence of CYP3A4-A-290G polymorphism by molecular PCR-RFLP technique in 77 newly diagnosed AML cases, and 72 age and sex comparable healthy controls (statistical analysis regarding the age and gender of studied population is shown in Table 1 and laboratory findings in AML patients are shown in Table 2). The cases were examined for the CYP3A4-A-290G polymorphism, and followed up for 6 months regarding disease outcome and overall survival (Initial response to induction therapy among AML patients at 28 days is shown in Table 3).
Table 1.
Statistical analysis regarding the age and gender of the studied population.
| Group | Age |
Sex |
||
|---|---|---|---|---|
| Mean ± SD | Median (range) | Male no. % | Female no. % | |
| Cases (no. 77) | 38.2 ± 17.5 | 35(4–83) | 43 (55.8%) | 34 (44.2%) |
| Control (no. 72) | 32 ± 9.2 | 31.5(19–53) | 53 (73.6%) | 19 (26.4%) |
Table 2.
Statistical analysis of laboratory findings in AML patients (n = 77).
| Mean ± SD | Median (range) | |
|---|---|---|
| PB Tlc × 109/L | 35.6 ± 35.356 | 27.3 (0.2–180) |
| PB Hb gm/dl | 7.4 ± 2 | 7.2 (3–13.9) |
| PB Plt × 109/L | 45.9 ± 40.64 | 37 (2–271) |
| PB Bl% | 38 ± 28 | 32 (0–90) |
| BM Bl% | 56 ± 27 | 65 (20–92) |
PB: peripheral blood, BM: bone marrow, TLC: total leucocytic count, SD: standard deviation, Plt: platelet, Bl: blast.
Table 3.
Initial response to induction therapy among AML patients at (28 days) (n = 77).
| Frequency | Percent | |
|---|---|---|
| CR | 31 | 40.3 |
| NO CR | 6 | 7.8 |
| DEAD | 40 | 51.9 |
CR: complete remission.
We found that the heterozygous genotype (AG) was more frequent in AML cases (21.6%) than the control group (2.8%), with statistically significant difference (p-value = 0.002, OR = 11.4, 95%CI = 2.513–51.974), the homozygous genotype (GG) also more frequent in AML cases (12.2%) than control group (0%), however this difference was not statistically significant (p-value = 0.999). As regards the variant allele (G in AG and GG genotypes), we found that it was more frequent in AML cases (33.8%) than in the control group (2.8%) with statistically significant difference (p-value = 0.001, OR = 18.9, 95%CI = 4.041–78.903) as shown in Table 4.
Table 4.
Comparison between the frequency of CYP3A4-A-290G types in AML vs. control groups.
| Group |
p-Value | OR | 95%CI | |||
|---|---|---|---|---|---|---|
| Control (no. = 72) | AML (no. = 74) | |||||
| Wild type (AA) | Count | 70 | 49 | Ref | ||
| % Within group | 97.2% | 66.2% | ||||
| Heterozygous (AG) | Count | 2 | 16 | 0.002 | 11.429 | 2.513–51.974 |
| % Within group | 2.8% | 21.6% | ||||
| Homozygous (GG) | Count | 0 | 9 | 0.999 | ||
| % Within group | 0% | 12.2% | ||||
| Variant allel (AG + GG) | Count | 2 | 25 | 0.001 | 18.857 | 4.041–78.903 |
| % Within group | 2.8% | 33.8% | ||||
p-Value < 0.05 was considered significant.
In agreement with our data, Voso et al. [8] in a case-control study including 160 cases of AML and 162 matched controls, reported that a significantly higher prevalence of the polymorphic variants CYP3A4-A-290G genes in AML cases, when compared with controls (9.4% vs. 3.1%, P = 0.04) increasing the risk of AML 3.2-fold, (95% CI: 1.1–11.5).
However, Bolufer et al. [9] reported in a case-control study including 443 patients with acute leukemia (302 with AML and 141 with ALL) and 454 control volunteers that the frequency of CYP3A4-A-290G variants in AML cases, compared with controls was (6.8% vs. 8.1%) without any statistically significant difference. Also, the frequency CYP3A4-A-290G variant in ALL cases, compared with controls was (5.5% vs. 8.1%), without any statistically significant difference.
Blanco et al. [10] compared the genotype frequencies for the CYP3A4-A-290G polymorphism in 224 children with ALL who did not develop t-AML (controls), and in 53 children with ALL who did develop a complication. They found no differences in the CYP3A4-A-290G allele distribution between ALL controls and t-AML patients in whites (6.6% vs. 9.8%, P = 0.339), blacks (93.8% vs. 87.5%, P = 0.498) or Hispanics (39.1% vs. 25.0%, P = 0.523). Suggesting that there is no association between the CYP3A4-A-290G polymorphism and the risk of t-AML in children treated for ALL.
Pakakasama et al. [11] also analyzed 107 children with ALL and 320 healthy controls for CYP3A4-A-290G polymorphism, and they found the very low frequency of CYP3A4-A-290G allele which was 0.8% and 0.9% in ALL and controls showing no statistically difference between patients and controls.
Kim et al. [12] analyzed 100 ALL pediatric patients for the CYP3A4-A-290G polymorphism; they found that the distribution of the variant allele was 0%.
Naoe et al. [13] analyzed 58 patients with t-AML/t-MDS and 150 Japanese healthy individuals for the CYP3A4-A-290G polymorphism; they found that all had a wild type genotype except one case only had a heterogenous genotype of the variant allele, which indicates a very low frequency or a lack of CYP3A4-A-290G polymorphism in Japanese persons.
This controversy in the results is likely due to the diverse ethnic group, the different sample size and the exposure to different carcinogens in different environments (gene environmental interaction). This could also explain by what was previously stated that the CYP3A4*1B variant has a controversial functional role, where Felix et al. [14] reported that the CYP3A4 wild genotype may increase production of potentially DNA-damaging reactive intermediates and the variant may decrease production of potentially DNA-damaging reactive intermediate. While Voso et al. [8] mentioned that CYP3A4-A-290G polymorphism is believed to cause an increase in enzymatic activity, and so increase bioactivation of several chemical carcinogens associated with cancers.
Considering the prevalence of CYP3A4-A-290G polymorphism in normal the Egyptian population, we found that the frequency of heterozygous and homozygous genotypes was 2.8% and 0% respectively in our control group, also shown in Table 4.
In our study, there was no significant value in CYP3A4-A-290G genotypes in relation to the initial response at d28 (CR or unfavorable response) (p-value = 0.757) shown in Table 5 and also no significant value in relation to the overall survival (p-value = 0.380 & for variant allele p-value = 0.459) is shown in Table 6 and Figs. 2 and 3. [Valid numbers 74 as there were 3 cases failed to get amplified in the PCR-RFLP technique].
Table 5.
CYP3A4-A-290G genotypes in relation to initial response at d28 (n = 74).
| GG no. (%) | AG no. (%) | Variant allele no. (%) | Wild type no. (%) | p-Value | |
|---|---|---|---|---|---|
| CR | 5 (55.6) | 5 (63.2) | 10 (33.3) | 20 (66.7) | 0.757 |
| NO CR | 1 (11.1) | 0 (0) | 1 (16.7) | 5 (83.3) | |
| DEAD | 3 (33.3) | 11 (36.8) | 14 (36.8) | 24 (63.2) |
p-Value > 0.05 which was considered not significant – chi-square test was used.
Table 6.
CYP3A4-A-290G genotypes in relation to the overall survival (n = 74).
| No. | Overall cumulative survival (%) | Median survival (month) | 95% Confidence interval | p-Value | |
|---|---|---|---|---|---|
| Wild AA | 49 | 42.86 | 3.52 | 0.00–8.57 | 0.380 |
| Hetero AG | 16 | 31.25 | 0.66 | 0.21–1.11 | |
| Homo GG | 9 | 44.44 | 1.51 | 1.22–1.80 | |
| Variant | 25 | 36 | 0.82 | 0.28–1.36 | 0.459 |
p-Value > 0.05 which was considered not significant log-rank test was used.
Fig. 2.
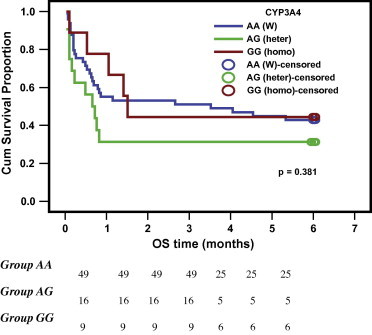
Kaplan Meier curve of overall survival for CYP3A4-290G genotypes (AA, AG, GG) groups.
Fig. 3.
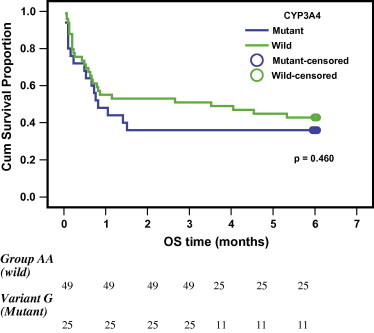
Kaplan Meier curve of overall survival as regards patients carrying the variant G allele.
In agreement with our results Barragan et al. [15] genotyped 153 patients diagnosed with de novo AML to clarify the influence of the genetic CYP3A4-A-290G polymorphism on disease outcome, and they found no relationship between the CYP3A4-A-290G polymorphism and the disease outcome.
This is nearly matched with the previously reported frequency of these genotypes in Caucasians reported by Voso et al. [8] (3.1–0%), Spanish reported by Bolufer et al. [9] (8.1–0%) and European done by Garsa et al. [16] (7.5–0%).
In contrast to our study the frequency of these genotypes was (40–39.2%) in African American reported by Zeigler-Johnson et al. [17] (36–51%) in Ghanaians by Tayeb et al. [18], and (2.9–65.1%) in Senegalese analyze by Zeigler-Johnson et al. [17], while they were not detected at all (0%) in Taiwanese, Chinese and Japanese which was reported by Walker et al. [19].
Conclusions
It could be concluded that, CYP3A4-A-290G polymorphism is a genotypic factor that increases the CYP3A4 enzymatic activity and increases the risk of AML by 18.9-fold. We need a large study sample to confirm that CYP3A4-A-290G polymorphism is a predisposing host genetic factor to AML.
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ilhan G., Karakus S., Andic N. Risk factors and primary prevention of acute leukemia. Asian Pac J Cancer Prevent (APJCP) 2006;7:515–517. [PubMed] [Google Scholar]
- 2.Yan J., Yin M., Dreyer Z.E., Scheurer M.E., Kamdar K., Wei Q. A metaanalysis of MTHFR C677T and A1298C polymorphisms and risk of acute lymphoblastic leukemia in children. Pediatr Hematol Oncol. 2012;58:513–518. doi: 10.1002/pbc.23137. [DOI] [PubMed] [Google Scholar]
- 3.Zhuo W., Zhang L., Wang Y., Zhu B., Chen Z. CYP1A1 MspI polymorphism and acute myeloid leukemia risk: meta-analyses based on 5018 subjects. J Exp Clin Cancer Res. 2012:31–62. doi: 10.1186/1756-9966-31-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFadyen M.C., Melvin W.T., Murray G.I. Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol Cancer Ther. 2004;3:363–371. [PubMed] [Google Scholar]
- 5.Antona R.C., Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 6.Antona R.C., Sayi J.G., Gustafsson L.L., Bertilsson L., Ingelman-Sundberg M. Phenotype-genotype variability in the human CYP3A locus as assessed by the probe drug quinine and analyses of variant CYP3A4 alleles. Biochem Biophys Res Commun. 2005;338:299–305. doi: 10.1016/j.bbrc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Cheson B.D., Cassileth P.A., Head D.R., Schiffer C.A., Bennett J.M., Bloomfield C.D. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8(5):813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 8.Voso MT, D’Alo F, Leone G. Detoxification enzyme polymorphisms as risk factors of t-AML. Istituto di Ematologia, Universita Cattolica del Sacro Cuore, Roma, Italy, vol. 2, No. 15; 2007. p. 46–8.
- 9.Bolufer P., Collado M., Barragán E., Cervera J., Calasanz M., Colomer D. The potential effect of gender in combination with common genetic polymorphisms of drug-metabolizing enzymes on the risk of developing acute leukemia. Haematol/Hematol J. 2007;92(03):308–314. doi: 10.3324/haematol.10752. [DOI] [PubMed] [Google Scholar]
- 10.Blanco J.G., Edick M.J., Hancock M.L., Winick N.J., Dervieux T., Amylon M.D. Genetic polymorphisms in CYP3A5, CYP3A4 and NQO1 in children who developed therapy-related myeloid malignancies. Pharmacogenetics. 2005;12(8):605–611. doi: 10.1097/00008571-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Pakakasama S., Mukda E., Sasanakul W., Kadegasem P., Udomsubpayakul U., Thithapandha A. Polymorphisms of drug-metabolizing enzymes and risk of childhood acute lymphoblastic leukemi. Am J Hematol. 2005;79:202–205. doi: 10.1002/ajh.20404. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., Kang H.J., Kim H.J., Jang M.K., Kim N.H., Oh Y. Pharmacogenetic analysis of pediatric patients with acute lymphoblastic leukemia. A possible association between survival rate and ITPA polymorphism. PLoS One. 2012;7(9):e45558. doi: 10.1371/journal.pone.0045558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naoe T., Takeyama K., Yokozawa T., Kiyoi H., Seto M., Uike N. Analysis of genetic polymorphism in NQO1, GST-M1, GST-T1, and CYP3A4 in 469 Japanese patients with therapy-related leukemia/myelodysplastic syndrome and de novo acute myeloid leukemia. Clin Cancer Res. 2000;6:4091–4095. [PubMed] [Google Scholar]
- 14.Felix C.A., Walker A.H., Lange B.J. Association of CYP3A4 genotype with treatment-related leukaemia. Proc Natl Acad Sci USA. 1998;95:13176–13181. doi: 10.1073/pnas.95.22.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barragan E., Collado M., Cervera J., Martin G., Bolufer P., Roman J. The GST deletions and NQO1*2 polymorphism confers interindividual variability of response to treatment in patients with acute myeloid leukemia. Leukemia Res. 2007;31(7):947–953. doi: 10.1016/j.leukres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Garsa A.D., McLeod H.L., Marsh S. CYP3A4 and CYP3A5 genotyping by pyrosequencing. BMC Med Genet. 2005:6–19. doi: 10.1186/1471-2350-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeigler-Johnson C.M., Walker A.H., Mancke B., Spangler E., Jalloh M., McBride S. Ethnic differences in the frequency of prostate cancer susceptibility alleles at SRD5A2 and CYP3A4. Hum Hered. 2002;54:13–21. doi: 10.1159/000066695. [DOI] [PubMed] [Google Scholar]
- 18.Tayeb M.T., Clark C., Ameyaw M.-M., Haites N.E., Evans D.A., Tariq M. CYP3A4 promoter variant in Saudi, Ghanaian and Scottish Caucasian populations. Pharmacogenetics. 2000;10:753–756. doi: 10.1097/00008571-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Walker A.H., Najarian D., White D.L., Jaffe J.M., Kanetsky P.A., Rebbeck T.R. Collection of genomic DNA by buccal swabs for polymerase chain reaction-based biomarker assays. Environ Health Perspect. 1999;107(7):517–520. doi: 10.1289/ehp.99107517. [DOI] [PMC free article] [PubMed] [Google Scholar]



