Abstract
We report a patient with progression to a macula-off tractional retinal detachment in a fellow eye after a contralateral intraoperative intravitreal bevacizumab injection. A 32-year-old diabetic man noted decreased vision in his left eye 1 week following 25 gauge pars plana vitrectomy, gas tamponade, and intraoperative injection of bevacizumab in his right eye. Left eye visual acuity decreased from 20/80 to 20/200, and macula-off tractional retinal detachment was seen on clinical exam and imaging. Progression of tractional retinal detachment associated with proliferative diabetic retinopathy in a fellow eye after a contralateral intraoperative intravitreal bevacizumab injection may occur.
Keywords: anti-VEGF therapy, fellow eye, tractional retinal detachment, diabetes mellitus
Introduction
In patients with proliferative diabetic retinopathy (PDR) refractory to traditional treatments with panretinal photocoagulation, intravitreal injections of bevacizumab may be effective in achieving regression of retinal and iris neovascularization.1 In patients with diabetic macular edema, intravitreal injections of bevacizumab may provide stable or improved visual outcomes.2 The use of bevacizumab has been further extended to the preoperative and intraoperative settings in patients undergoing pars plana vitrectomy. Intraoperative injection of 1.25 mg/0.05 mL in these patients may be helpful in reducing the incidence of recurrent vitreous hemorrhage within the first 4 weeks of intervention.3 However, the development or progression of tractional retinal detachment (TRD) following an intravitreal delivery, has been also reported.4
The current study describes a patient with a progression of PDR to a macula-involving TRD in a fellow eye, following pars plana vitrectomy and an intravitreal bevacizumab injection to the contralateral eye.
Case report
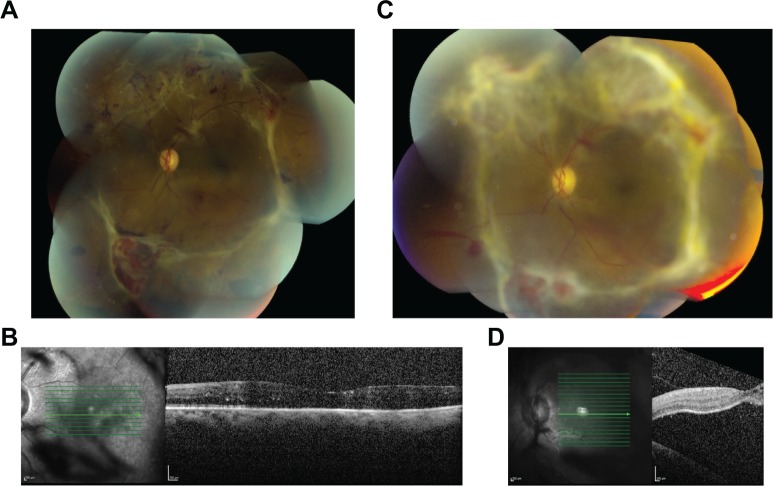
This is a 32-year-old male with insulin-dependent diabetes mellitus diagnosed at age 19. The patient stated he has had poor glycemic control for many years, with fasting blood sugars ranging from 200 to 400 mg/dL. Hemoglobin A1C was not available. His past medical history was also significant for uncontrolled hypertension. His past ocular history included PDR and panretinal photocoagulation in both eyes. On initial examination, his visual acuity was 3/200 in the right eye and 20/80 in the left eye. The right eye had macula-involving TRD, while the left eye had PDR with multiple areas of vitreoretinal adhesion but macula was attached (Figure 1A, B).
Figure 1.
Progression of diabetic tractional retinal detachment, by fundus photography and optical coherence tomography (OCT) of the left eye after a unilateral, right eye intraoperative intravitreal bevacizumab injection.
Notes: Fundus photograph (A) and OCT (B) of the left eye before right eye intraoperative intravitreal bevacizumab injection, showing vitreoretinal adhesions and attached macula. Fundus photograph (C) and OCT (D) of the left eye after right eye intraoperative intravitreal bevacizumab injection, showing decreased perfusion of the neovascular tissue and progression of tractional retinal detachment.
The patient underwent surgery for the right eye that included 25 gauge pars plana vitrectomy, membrane peel, endolaser, air-fluid exchange, intravitreal bevacizumab 1.25 mg/0.05 mL, intravitreal triamcinolone 4 mg/0.1 mL, and 16% C3F8 gas. On the postoperative week 1 follow-up examination, the right eye was healing well, but the patient reported decreased visual acuity in his left eye. The best corrected visual acuity in the left eye decreased to 20/200, and the clinical examination showed progression to macula-involving diabetic TRD with apparent decreased perfusion of the neovascular tissue (Figure 1C, D). The patient subsequently underwent surgical intervention to the left eye, with resultant visual acuity of 20/100 and 20/70 in the right and left eyes, respectively.
Discussion
Recent pharmacokinetic studies of unilateral bevacizumab injections indicate a potential bilateral effect.5,6 The pharmacokinetics of bevacizumab following a unilateral intravitreal injection in rabbits was described by Bakri et al.5 In the same study, small amounts of bevacizumab were detected in the serum and the vitreous of the fellow noninjected eye, suggesting systemic absorption and redistribution.5 Systemic pharmacokinetics after intravitreal injection of 1.25 mg of bevacizumab in humans demonstrate that during the first week after injection, plasma levels of bevacizumab are elevated above its half inhibitory concentration (IC50) for vascular endothelial growth factor (VEGF) and produce a significant reduction in plasma free-VEGF.6 Bilateral response following unilateral intravitreal injection of 1.25 mg/0.05 mL has been also reported in the treatment of PDR, diabetic macular edema, uveitic cystoid macular edema, and retinopathy of prematurity.1,7–9
The aqueous route is considered to be the primary route of vitreal bevacizumab clearance, but there also is a possible contribution of elimination via the choroidal circulation.5,10 Krohne et al studied bevacizumab pharmacokinetics in humans and concluded that the aqueous half-life of bevacizumab in nonvitrectomized eyes is 9.8 days.10
Although no corresponding study was conducted in humans, Kakinoki et al demonstrated the effect of vitrectomy on bevacizumab elimination, using a primate model. The study showed that following a 1.25 mg intravitreal injection, the mean aqueous half-life had dropped by 54%, to a mean value of 1.5±0.6 days in vitrectomized eyes in comparison with a half-life of 2.8±0.8 days in nonvitrectomized eyes.11 A similar concept has been described in rabbits with intravitreal injection of triamcinolone acetonide.12
In the current patient, the vitrectomized eye was filled with gas intraoperatively, resulting in a smaller pool of fluid in which bevacizumab was diluted and thus presumably, a higher overall drug concentration. In addition, the patient was encouraged to position “face down” postoperatively, which would facilitate aqueous clearance of this concentrated pool of bevacizumab.
Intravitreal bevacizumab produces a rapid and dramatic decreased perfusion of fibrovascular tissue of PDR, which can produce whitening of the tissue, as seen in this case.1 Furthermore, the neovascularization of PDR is quite sensitive to low doses of bevacizumab, with a previous report demonstrating effects following injections of doses 200-fold below the usual dose, or 6.25 μg.1 In addition to the vascular perfusion changes seen in this patient, the progression of the circular ring of fibrovascular adhesions to macula-involving TRD has been reported after intravitreal bevacizumab.4
Bilateral clinical effects following unilateral injection, along with accelerated elimination of bevacizumab in a setting of vitrectomy, higher concentration of medication in the gas-filled eye, and previous reports of TRD progression in eyes treated with bevacizumab, provide theoretical support for a hypothesis that contralateral progression of TRD in the fellow noninjected eye might have been a result of intraoperative bevacizumab injection.
Progression of TRD to macula-involving TRD is well-known in a setting of existing vitreoretinal adhesions in patients with PDR.13 Certainly, fellow eye involvement could be an independent event and a manifestation of PDR disease progression. In the current patient, the progression to TRD within 7 days after the injection makes it reasonable to consider the possibility of a cause and effect. Vitrectomy and gas tamponade may accelerate the elimination of bevacizumab from the injected eye, allowing for higher systemic absorption and potential drug effect in the fellow eye.
Acknowledgments
This research is funded in part by National Institutes of Health (NIH) Center Core Grant (grant number P30EY014801) and a Research to Prevent Blindness Unrestricted Grant (Department of Defense [DOD] grant number W81XWH-09-1-0675).
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the Treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695–1705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 2.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Pan-American Collaborative Retina Study Group Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114(4):743–750. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology. 2011;118(11):2218–2226. doi: 10.1016/j.ophtha.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Arevalo JF, Maia M, Flynn HW, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(2):213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 5.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114(5):855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Avery RL, Castellarin A, Steinle N, et al. Comparison of systemic pharmacokinetics post anti-vegf intravitreal injections of ranibizumab, bevacizumab and aflibercept. Abstract presented at the 2013 Annual Meeting of the American Society of Retina Specialists (ASRS); August 24–28; 2013; Toronto, ON. [Google Scholar]
- 7.Bakbak B, Ozturk BT, Gonul S, Yilmaz M, Gedik S. Comparison of the effect of unilateral intravitreal bevacizumab and ranibizumab injection on diabetic macular edema of the fellow eye. J Ocul Pharmacol Ther. 2013;29(8):728–732. doi: 10.1089/jop.2013.0049. [DOI] [PubMed] [Google Scholar]
- 8.Al-Dhibi H, Khan AO. Bilateral response following unilateral intravitreal bevacizumab injection in a child with uveitic cystoid macular edema. J AAPOS. 2009;13(4):400–402. doi: 10.1016/j.jaapos.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Karaca C, Oner AO, Mirza E, Polat OA, Sahiner M. Bilateral effect of unilateral bevacizumab injection in retinopathy of prematurity. JAMA Ophthalmol. 2013;131(8):1099–1101. doi: 10.1001/jamaophthalmol.2013.400. [DOI] [PubMed] [Google Scholar]
- 10.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146(4):508–512. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Kakinoki M, Sawada O, Sawada T, Saishin Y, Kawamura H, Ohji M. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci. 2012;53(9):5877–5880. doi: 10.1167/iovs.12-10164. [DOI] [PubMed] [Google Scholar]
- 12.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25(5):556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Danis PR, Davis MD. Proliferative diabetic retinopathy. In: Duh Elia J, editor. Diabetic Retinopathy. Totowa, NJ: Humana Press; 2008. pp. 29–65. [Google Scholar]