Abstract
PHO1 has been recently identified as a protein involved in the loading of inorganic phosphate into the xylem of roots in Arabidopsis. The genome of Arabidopsis contains 11 members of the PHO1 gene family. The cDNAs of all PHO1 homologs have been cloned and sequenced. All proteins have the same topology and harbor a SPX tripartite domain in the N-terminal hydrophilic portion and an EXS domain in the C-terminal hydrophobic portion. The SPX and EXS domains have been identified in yeast (Saccharomyces cerevisiae) proteins involved in either phosphate transport or sensing or in sorting proteins to endomembranes. The Arabidopsis genome contains additional proteins of unknown function containing either a SPX or an EXS domain. Phylogenetic analysis indicated that the PHO1 family is subdivided into at least three clusters. Reverse transcription-PCR revealed a broad pattern of expression in leaves, roots, stems, and flowers for most genes, although two genes are expressed exclusively in flowers. Analysis of the activity of the promoter of all PHO1 homologs using promoter-β-glucuronidase fusions revealed a predominant expression in the vascular tissues of roots, leaves, stems, or flowers. β-Glucuronidase expression is also detected for several promoters in nonvascular tissue, including hydathodes, trichomes, root tip, root cortical/epidermal cells, and pollen grains. The expression pattern of PHO1 homologs indicates a likely role of the PHO1 proteins not only in the transfer of phosphate to the vascular cylinder of various tissues but also in the acquisition of phosphate into cells, such as pollen or root epidermal/cortical cells.
Phosphorus (P) is an essential macronutrient for all living organisms. It serves various basic biological functions as a structural element in nucleic acids and phospholipids, in energy metabolism, in the activation of metabolic intermediates, as a component in signal transduction cascades, and in the regulation of enzymes.
Of the major nutrients, P is the most dilute and the least mobile in soil. P is absorbed by plants as orthophosphate (inorganic phosphate [Pi]). Pi concentration in the soil solution hardly reaches 10 μm and may even drop to submicromolar levels at the root/soil interface. Plants have evolved a series of metabolic and developmental adaptations aimed at increasing the acquisition of this vital but poorly available nutrient from the soil, as well as to sustain plant growth and survival under low P availability (for review, see Poirier and Bucher, 2002).
Transport of Pi is a dynamic process that occurs across various membranes, between cells and tissues, as well as between organelles. For example, once imported into the root epidermal or cortical cells, Pi must be exported into the xylem vessels of the root for its transfer, via the transpiration stream, to the shoot, where Pi is imported again into leaf cells. Under Pi deficiency, Pi is redistributed from the old, fully expanded source leaves toward young, expending sink leaves, a process requiring Pi export to the phloem vessel. Pi deficiency is also known to increase the Pi import capacity of cells through, in part, increase in the level of expression of Pi transporters. Transport of Pi across the tonoplast is an important process in Pi homeostasis. In plants grown in Pi-sufficient conditions, a major portion of Pi is transported into the vacuole, while under Pi-deficient conditions, the Pi is exported out of the vacuole in order to maintain a relatively stable cytoplasmic Pi concentration.
Our knowledge of Pi transport and homeostasis in higher plants has been extended in recent years by the cloning of several genes encoding H+-Pi cotransporters involved in Pi uptake into cells (Raghothama, 1999; Rausch and Bucher, 2002). The AtPht1 gene of Arabidopsis was the first high-affinity H+-Pi cotransporter identified in higher plants (Muchhal et al., 1996). A total of nine genes form the AtPht1 family in Arabidopsis. Although a number of these homologs are primarily expressed preferentially in root epidermal or cortical cells, implicating their role in the initial step of Pi acquisition from the rhizosphere into roots, some members of the AtPht1 family are expressed in other tissues, such as pollen or cotyledons (Mudge et al., 2002). Homologs of AtPht1 have also been identified and characterized in others plants, including potato (Solanum tuberosum), tomato (Lycopersicon esculentum), tobacco (Nicotiana tabacum), wheat (Triticum aestivum), rice (Oryza sativa), Catharanthus roseus, and Medicago trunculata (for review, see Raghothama, 1999; Rausch and Bucher, 2002). These transporters share homology to the yeast (Saccharomyces cerevisiae) Pho84 H+-Pi cotransporter, and functional expression of some of them in plants or yeast has shown that they belong to the class of high-affinity phosphate transporters.
A gene homologous to the animal Na-Pi cotransporter gene has been isolated in Arabidopsis that encodes a low-affinity H+-Pi cotransporter expressed preferentially in leaves (Pht2;1; Daram et al., 1999). Pht2;1 shows no homology to the plant high-affinity H+-Pi cotransporter family. The protein is localized to the chloroplast, where it is thought to mediate Pi transport across the envelope alongside a number of translocators involved in the exchange of Pi and phosphorylated compounds (Flügge, 1999; Versaw and Harrison, 2002). Expression of Pht2;1 was shown to influence allocation of Pi within the plant as well as the expression of Pi-starvation response genes (Versaw and Harrison, 2002).
The pho1 mutant of Arabidopsis is deficient in loading root Pi into the xylem vessels, resulting in strong Pi deficiency in all the above-ground tissues (Poirier et al., 1991). The PHO1 gene has recently been cloned in Arabidopsis by a map-based approach (Hamburger et al., 2002). PHO1 shows no homology to characterized solute transporters, including the family of plant Pht1 H+-Pi cotransporters. It rather shows homology to the Rcm1 mammalian receptor for xenotropic murine leukemia retrovirus (Battini et al., 1999), as well as to the yeast Syg1 protein involved in the mating pheromone signal transduction pathway (Spain et al., 1995). PHO1 was found mainly expressed in the roots. Promoter-GUS (β-glucuronidase) fusion experiments revealed predominant expression of the PHO1 promoter in the stelar cells of the root and the lower part of the hypocotyl, consistent with the role of PHO1 in loading Pi to the xylem (Poirier et al., 1991; Hamburger et al., 2002). Although the precise mode of action of PHO1 remains to be elucidated, it is clear that the protein plays an important role in Pi homeostasis. The Arabidopsis genome contains 10 additional genes showing homology to PHO1 (Hamburger et al., 2002). In an effort to address the role of these PHO1 homologs in Pi homeostasis, we have cloned, sequenced, and characterized the expression pattern of all the PHO1 homologs.
RESULTS
Cloning and Sequencing of the PHO1 Gene Family
Analysis of the Arabidopsis genome with the PHO1 protein sequence revealed the presence of 10 homologous genes (identified as PHO1;H1 to PHO1;H10, hereafter referred as PHO1 homologs H1 to H10; Table I). Expressed sequence tags (ESTs) or full-length cDNA have only been reported for two of the potential 10 homologs, indicating that this gene family was underrepresented in the various collections of expressed genes. Based on the structure of the PHO1 gene as well as the sequence of cDNAs and ESTs for the homologs H1, H2, and H5 genes, primers have been designed to amplify and clone by reverse transcription (RT)-PCR the full coding sequence of all PHO1 homologs. Initial cloning of cDNAs in Escherichia coli using the pBluescript KS+ vector gave rise only to clones having insertions or rearrangements affecting the open reading frame. The cDNAs fragments obtained by RT-PCR have thus been ligated into the yeast-E. coli shuttle vector pYES2 and the ligation product transformed directly into yeast. Positive clones were identified by PCR and sequenced. The sequence of the H1 clone obtained by this approach was found to be identical to a full-length cDNA reported in GenBank (R14567). Out of the nine PHO1 homologs, for which only a predicted sequence was found on the Arabidopsis Information Resource (TAIR; http://www.arabidopsis.org) database, the cDNA sequences of six genes were found to be different from the TAIR prediction. In all cases, the changes involved splicing sites and modified the predicted sequences of the proteins over short stretches. The sequences of all PHO1 homologs have been submitted to GenBank.
Table I.
The PHO1 gene family
| Gene Name | Gene Identification | ESTa | FL cDNAb | Predictionc | GenBank Entry |
|---|---|---|---|---|---|
| PHO1 | At3g23430 | Yes | Yes | AF474076 | |
| PHO1;H1 | At1g68740 | No | Yes | AY507953 | |
| PHO1;H2 | At2g03260 | Yes | No | Good | AY507954 |
| PHO1;H3 | At1g14040 | No | No | Corrected | AY507955 |
| PHO1;H4 | At4g25350 | No | No | Corrected | AY507956 |
| PHO1;H5 | At2g03240 | Yes | No | Corrected | AY507957 |
| PHO1;H6 | At2g03250 | No | No | Corrected | AY507958 |
| PHO1;H7 | At1g26730 | No | No | Good | AY507959 |
| PHO1;H8 | At1g35350 | No | No | Corrected | AY507960 |
| PHO1;H9 | At3g29060 | No | No | Corrected | AY507961 |
| PHO1;H10 | At1g69480 | No | No | Good | AY507962 |
Denotes the presence or absence of ESTs in the TAIR database.
Denotes the presence or absence of full-length cDNA in the TAIR database.
Denotes whether the sequence of the newly cloned cDNA corresponds to the prediction obtained on the TAIR database.
Pairwise comparison of the PHO1 protein family revealed levels of identity or similarity ranging from 29% amino acid identity and 49% amino acid similarity between PHO1 and the H6 homolog to 86% amino acid identity and 94% amino acid similarity between the homologs H7 and H8 (Fig. 1). Analysis of the hydropathy profiles of all PHO1 homologs revealed a pattern that was very similar to PHO1, with the first half of the protein being hydrophilic and the second half being mostly hydrophobic and containing at least six potential transmembrane-spanning domains (Supplemental Fig. 1). Alignment of the proteins revealed extensive areas of similarity between the PHO1 protein family members (Fig. 2). Within the hydrophilic half of the proteins, three conserved domains are found separated by two areas of low similarity. By contrast, extensive similarities are found throughout the whole hydrophobic half of the proteins.
Figure 1.
Identity/similarity matrix for the PHO1 family. Amino acid identity and similarity are indicated by the first and second number, respectively.
Figure 2.
Alignment of the Arabidopsis PHO1 protein family. Alignment was done using the ClustalX program (Jeanmougin and Thompson, 1998; ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/). The locations of the three SPX subdomains and of the EXS domain are indicated by a black bar over the sequence. The beginning of the hydrophobic C-terminal half of the proteins is indicated by an arrowhead. The protein sequence derived from two full-length rice cDNA clones J023079I02 and J013095I12 are indicated as OsJ023 and OsJ013, respectively.
Two distinct full-length cDNAs encoding proteins having homology to PHO1 have been reported for rice (O. sativa cv japonica). Clone J023079I02 shows highest similarity to PHO1, with 49% amino acid identity and 62% amino acid similarity, while clone J013095I12 shows highest similarity to the H1 homolog with 55% amino acid identity and 66% amino acid similarity. The hydropathy profile, as well as the overall domain structure, are conserved between the rice and the Arabidopsis PHO1 genes (Fig. 2; Supplemental Fig. 1).
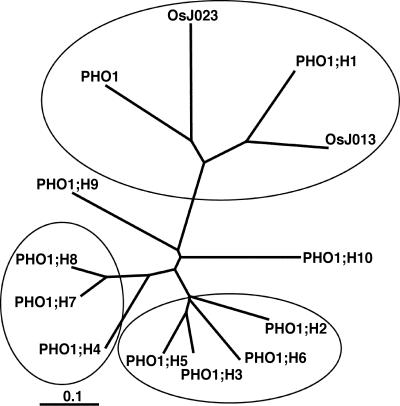
An unrooted phylogenetic tree constructed from the Arabidopsis and rice sequences revealed the presence of three clusters (Fig. 3). One cluster regroups the two rice proteins with the Arabidopsis PHO1 and the H1 homolog, a second group includes the homologs H2, H3, H5, and H6, while a third group includes the homologs H4, H7, and H8. The H9 and H10 homologs do not apparently cluster with any other PHO1 proteins. The observed clustering is the result of amino acid sequence differences spread throughout the whole proteins and not limited to a specific region.
Figure 3.
Unrooted phylogenetic tree of the PHO1 family. The tree was made using the Neighbor-Joining method available on ClustalX (Jeanmougin and Thompson, 1998; ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/).
Structure and Distribution of PHO1 Genes in the Genome
More than 60% of the Arabidopsis genome is represented within duplicated regions (The Arabidopsis Genome Initiative, 2000). The relationship between segmental duplication of the Arabidopsis genome and the PHO1 gene family has been examined. The genes of the PHO1 family are distributed over four of the five Arabidopsis chromosomes. Fours genes, namely PHO1 and the homologs H1, H8, and H9, are found in nonduplicated regions of the genome (Fig. 4). Although the H4 gene is found within a duplicated segment found on chromosome 5, no homolog is found on this later chromosome. Three genes (H2, H5, and H6) are found as direct repeats on the top arm of chromosome 2. This area of chromosome 2 is included in an inverted segmental duplication with a region on the upper arm of chromosome 1 containing the H3 gene (Fig. 4). The clustering of the H2, H3, H5, and H6 homologs on the phylogenetic tree suggests an evolutionary relationship that is reinforced by the distribution of these genes on the genome. It is thus likely that the duplication of the segment harboring H3 was followed by tandem duplication of the PHO1 homologs on chromosome 2, perhaps through unequal crossing over, giving rise to the tandem repeats. The genes H7 and H10 are also found within an inverted segmental duplication within chromosome 1. However, the higher sequence similarity found between H7 and H8 compared to H7 and H10 suggests that H8 arose later than the segmental duplication involving H7 and H10.
Figure 4.
Localization of the PHO1 gene family on the Arabidopsis chromosomes. Segmental duplications of the genome in areas containing PHO1 gene family members are indicated by links between or within chromosomes. Arrows indicate the relative orientation of the genes.
Analysis of the intron/exon structure of the PHO1 genes family revealed a greater divergence for regions encoding the N-terminal hydrophilic domains compared to the C-terminal hydrophobic domains (Fig. 5). Thus, the intron/exon structure of the C-terminal hydrophobic domains typically includes seven to eight introns, with the position and size of introns being fairly well conserved between homologs, with the exception of H6, which has distinctively larger introns. By contrast, the intron/exon structure of the N-terminal hydrophilic domains is more variable, with intron number ranging between three to six and the size of the first intron ranging from approximately 80 to 1,060 bp. Thus, the greater divergence within the N-terminal hydrophilic domains of the PHO1 protein family observed at the amino acid level is also extended at the gene structure level.
Figure 5.
Intron/exon structure of the PHO1 gene family. Exons and introns are indicated by black and white boxes, respectively. The distances in base pairs between the start and stop codons on the genomic DNA are indicated on the right, along with the numbers of introns. Asterisks denote the location of the beginning of the hydrophobic region of the proteins.
The PHO1 Proteins Harbor the SPX and EXS Domains
The PHO1 proteins show weak but significant similarities to a number of proteins found in Arabidopsis as well as in nonplant eukaryotes. These similarities are mainly confined to two domains, named SPX and EXS.
The SPX domain was named after the proteins SYG1 and PHO81 of yeast and XPR1 of human (PFAM entry PF03105; www.sanger.ac.uk). The SPX domain can typically be subdivided into three smaller domains of 35 to 47 amino acids. In most proteins, such as SYG1 and PHO81, the three subdomains are found close together, typically within a stretch of 180 amino acids. Analysis of the N-terminal hydrophilic half of the PHO1 proteins revealed the presence of the SPX domain in all proteins, with the three subdomains being found within a stretch of 300 to 360 amino acids and being separated from each other by larger regions of low similarity (Fig. 2). Yeast has at least eight proteins having a SPX domain. These include SYG1, which has been initially identified as a suppressor of the mating pheromone signal; the VTC1, VTC2, VTC3, and VTC4 proteins involved in sorting proteins to the vacuolar membrane; and the PHO81, PHO87, PHO90, and PHO91 involved in phosphate sensing and transport (Spain et al., 1995; Lenburg and O'Shea, 1996; Cohen et al., 1999; Wykoff and O'Shea, 2001; Müller et al., 2002; Auesukaree et al., 2003; Giots et al., 2003). The SPX domain is also found in the Neurospora crassa NUC2 protein involved in the control of the PHO regulon and in the mouse and human XPR1 protein, initially identified as a retrovirus receptor but for which the function in uninfected cells remains unknown (Peleg et al., 1996; Battini et al., 1999). In Arabidopsis, in addition to all members of the PHO1 family, nine other genes have been identified that harbor the SPX domains. The alignment of the three SPX subdomains within a subset of proteins found in Arabidopsis, yeast, N. crassa, and human is illustrated in Figure 6, a to c. A number of residues are well conserved across the different eukaryotes in the SPX subdomains 1 and 3, particularly Lys residues. Phylogenetic analysis of all proteins of the Arabidopsis genome containing a SPX domain revealed the presence of four gene families (Fig. 6d). One family regroups all 11 PHO1 proteins. The proteins At2g26660, At5g20150, At2g45130, and At5g15330 form a second group. These hydrophilic proteins of unknown function are homologous to the protein encoded by the Ids4 gene identified in barley (Hordeum vulgare) as a gene induced in roots under iron deficiency (Kobayashi et al., 2001). The third group encompasses the proteins At4g22990, At4g11810, and At1g63010, encoding proteins of unknown functions having several predicted transmembrane-spanning domains. The fourth group encompasses At1g02860 and At2g38920, which encode hydrophilic proteins of unknown function.
Figure 6.
Alignment of the SPX domain in Arabidopsis proteins. Alignment of the three SPX domains, referred as SPX-1 (a), SPX-2 (b), and SPX-3 (c), was done using the ClustalX program (Jeanmougin and Thompson, 1998; ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/). The proteins included are the N. crassa (Nc) NUC2; the yeast (Sc) PHO81, PHO87, PHO90, PHO91, SYG1, and VCT3; the human (Hs) XPR1; the Arabidopsis (At) PHO1 family members PHO1, PHO1;H1, PHO1;H2, and PHO1;H3; as well as nine other Arabidopsis proteins of unknown functions. d, Unrooted phylogenetic tree of the all Arabidopsis proteins containing a SPX domain. The tree was made using the Neighbor-Joining method available on ClustalX.
The EXS domain was named after the yeast ERD1, involved in the localization of endogenous endoplasmic reticulum proteins, the human XPR1, and the yeast SYG1 (PFAM entry PF03124; www.sanger.ac.uk). In contrast to the SYG1 and XPR1 proteins, which have both a SPX and EXS domain, the majority of proteins in yeast having one of these domains do not harbor the other. In Arabidopsis, only the members of the PHO1 proteins family possess both a SPX and EXS domain. In addition to all PHO1 homologs, two proteins in Arabidopsis have an EXS domain (Fig. 7). At5g35730 and At2g32295 are proteins of unknown function having several predicted transmembrane domains. At5g35730 is predicted to be localized to the chloroplast, while At2g32295 is predicted to be mitochondrial.
Figure 7.
Alignment of the EXS domain in Arabidopsis proteins. Alignment of EXS domain was done using the ClustalX program (Jeanmougin and Thompson, 1998; ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/). The proteins included are the yeast (Sc) SYG1 and ERD1, the human (Hs) XPR1, the Arabidopsis (At) PHO1 family members PHO1, PHO1;H1, PHO1;H2, and PHO1;H3, as well as two other Arabidopsis proteins of unknown functions.
Expression Profile of the PHO1 Gene Family
The expression profile of the PHO1 gene family was first examined by RT-PCR using primers that were specific to each PHO1 homolog (Fig. 8). Tissues were harvested either from plants grown in fertilized soil (leaves, stems, and flowers) or from roots growing in liquid cultures. The majority of the PHO1 homologs (H1, H2, H3, H4, H5, H7, H8, and H10) were found expressed at various levels in roots, leaves, stems, and flowers. However, the PHO1 homologs H6 and H9 showed expression only in flowers. Expression profile was also examined in plants growing under phosphate-deficient conditions. Above-ground tissues of the pho1 mutant are constantly under Pi stress due to the deficient transfer of Pi from the roots to the leaves (Poirier et al., 1991). Leaves, stems, and flowers were thus harvested from pho1 plants grown in soil, while roots were harvested from wild-type plants grown in liquid medium deprived of Pi. For the majority of the PHO1 homologs, gene expression was relatively stable in Pi-deprived tissues, with some genes showing weak down-regulation in certain tissues (e.g. H5 and H7 in stems; Fig. 7). However, expression of the H1 homolog was strongly up-regulated in leaves under Pi stress, with some weaker up-regulation also evident in roots and stems.
Figure 8.
Expression analysis of the PHO1 gene family by RT-PCR. RNA was isolated from leaves (Le), stems (St), and flowers (Fl) from either wild-type Arabidopsis (WT + Pi) or the pho1 mutant (pho1) growing in soil. RNA was also harvested from roots (Ro) of plants grown in liquid nutrient media containing either 5 mm Pi (WT + Pi) or no Pi (−Pi). DNA fragment obtained by PCR performed on genomic DNA using the same oligonucleotides used for RT-PCR is shown in the last lane (DNA). This control ensures that the lower band obtained by RT-PCR cannot be derived from the amplification of genomic DNA that could be present in the RNA preparations. See “Material and Methods” for further details.
The expression profile of the PHO1 gene family was further analyzed by the promoter-GUS approach. Fragments of 0.5 kb and 1.0 kb of each promoter were cloned in front of the GUS gene and expressed in transgenic plants. The GUS expression profile largely corroborated the RT-PCR results at the whole tissue level. For example, expression of the GUS reporter from the H6 and H9 promoters was restricted to flowers, in agreement with the RT-PCR results. For H6, GUS expression was confined to the connective tissues of the stamen, while for H9 GUS was found solely in the pollen grains (Fig. 9). In some cases, RT-PCR appeared to be more sensitive than the promoter GUS approach. For example, no GUS staining could be obtained with the H2 promoter, while RT-PCR revealed weak expression in a range of tissues (Fig. 8).
Figure 9.
Localization of GUS activity in transgenic Arabidopsis expressing PHO1 gene family promoter-GUS fusion constructs. Promoter H3 activity in root vascular tissue (A), promoter H10 activity in root epidermal/cortical cells (B), promoter H7 activity in root tip (C), promoter H1 activity in leaf vascular tissue (D), promoter H7 activity in hydathodes (E), promoter H8 activity in trichomes (F–H), promoter H5 activity in petioles (I), promoter H10 activity in leaf blade (J), promoter H1 activity in stem vascular tissue (K), promoter H7 activity in stem (L), promoter H10 activity in pollen grain (M), promoter H9 activity in pollen grain (N), promoter H8 activity in stigma apex (O), promoter H9 activity in germinating pollen grain (P), promoter H1 activity in vascular tissue of sepals, petals, and filaments (Q), close-up of promoter H1 activity in filament vascular tissue (R), and promoter H6 activity in connective tissue of the anther (S) are shown.
Several distinct GUS expression patterns have been found for various organs (Fig. 9; Table II). For roots, the H1, H3, H5, H7, and H8 were expressed in the vascular cylinder, while for H4 and H10 GUS expression was rather localized in the epidermal/cortical cells. Expression in the root tip has also been observed for H5 and H7. In leaves, GUS expression has been found in the vascular cylinder (H1 and H3), the hydathodes (H4, H7, H8, and H10), the trichomes and cells at the base of trichomes (H8), associated with the petiole (H5 and H8), or as a diffuse pattern across the leaf blade (H10). Expression in stems can be observed either associated primarily with the vascular cylinder (H1 and H8) or as a more diffuse pattern (H5, H7, and H10). Several tissues have been found to express GUS in flowers, including the pollen grains (H4, H8, H9, and H10), the vascular cylinder of sepals (H1 and H10), petals (H1) or filament (H1, H3, H5, H7, and H10), the apical end of the stigma excluding the papillae (H1, H3, H4, H5, H7, H8, and H10), the receptacle (H1, H3, H5, H7, H8, and H10), and the connective of the anthers (H1, H5, and H6). In the case of pollen grains, GUS staining could also be observed in the pollen tubes of germinating grains, indicating that GUS expression was not limited to the exine layer. Expression of the H4, H8, H9, and H10 genes in purified pollen grain was also detected by RT-PCR (data not shown).
Table II.
Expression profile of the GUS gene placed under the control of the promoter of the various PHO1 gene family members
| Promoter
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | PHO1a | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | |
| Root | Tip | + | + | |||||||||
| Vascular cylinder | + | + | + | + | + | + | ||||||
| Epidermis/cortex | + | + | ||||||||||
| Leaf | Blade | + | ||||||||||
| Vascular cylinder | + | + | ||||||||||
| Hydathode | + | + | + | + | ||||||||
| Trichome | + | |||||||||||
| Petiole | + | + | ||||||||||
| Stem | Vascular cylinder | + | + | |||||||||
| Diffuse | + | + | + | |||||||||
| Flower | Pollen | + | + | + | + | |||||||
| Sepal vasc. cyl. | + | + | ||||||||||
| Petal vasc. cyl. | + | |||||||||||
| Filament vasc. cyl. | + | + | + | + | + | |||||||
| Stigma apex | + | + | + | + | + | + | + | |||||
| Receptacle | + | + | + | + | + | + | ||||||
| Anther connective | + | + | + | |||||||||
Data reproduced from Hamburger et al. (2002).
DISCUSSION
The Arabidopsis genome database reveals the presence of 10 genes homologous to PHO1, although EST or cDNA are only described for four members of the family, namely PHO1 and the three homologs H1, H2, and H5. Our analysis of the expression pattern of all PHO1 family members by RT-PCR as well as cloning and sequencing of cDNAs revealed that all PHO1 members are expressed. It has been observed for PHO1 as well as for several PHO1 homologs that attempts to clone full-length cDNA directly into E. coli using plasmids, such as pBluescript KS+, consistently resulted in the recovery of aberrant clones representing either incomplete spliced products or having E. coli transposon inserted into the gene, resulting in the truncation of the open-reading frame (Y. Wang, D. Hamburger, and Y. Poirier, unpublished data). It is speculated that expression of mRNA from cryptic promoters on bacterial plasmids resulting in production of intact proteins of PHO1 and its homologs is toxic to E. coli. By contrast, PHO1 can be well expressed in yeast (Hamburger et al., 2002). All PHO1 homologs have thus been cloned through direct transformation into yeast, resulting in the recovery of clone encoding full-length protein. The potential toxicity of proteins belonging to the PHO1 family in E. coli is likely to be one cause of the poor representation of full-length cDNAs, despite the apparent broad expression of the corresponding genes in various tissues.
All members of the PHO1 protein family share a number of basic features. All proteins are of similar length, from 745 amino acids for H4 to 823 amino acids for H5, and are formed of two distinct parts, i.e. theN-terminal half being mainly hydrophilic, while theC-terminal half shows the presence of at least six potential membrane-spanning domains (Hamburger et al., 2002; Supplemental Fig. 1). Although the presence of membrane-spanning domains is a feature expected for ion transporters, the structure of PHO1 appears quite distinct from the Pi transporters cloned in various organisms and that are members of the major facilitator superfamily. These transporters, which include the yeast Pho84 H+-Pi cotransporter as well as all the plant H+-Pi cotransporters identified to date, are mostly hydrophobic proteins having 12 membrane-spanning domains grouped in two clusters of six domains separated by a larger hydrophilic region (Pao et al., 1998).
The level of similarity between the members of the PHO1 protein family is relatively high, with theN-terminal hydrophilic half being responsible for most of the divergence. Interestingly, the divergence of the hydrophilic domains correlates with a greater divergence in the exon/intron structure at the 5′ end of the genes. Phylogenetic analysis of the PHO1 protein family revealed the presence of three clusters (Fig. 3). In some cases, the phylogenetic relationship can be well correlated with the distribution of the genes on the genome. This is the case for the cluster regrouping the H2, H3, H5, and H6 genes, which is linked to several duplication events implicating chromosomes 1 and 2. It is too early to draw conclusions between the relevance of the phylogenetic relationship and the biological role of the PHO1 homologs belonging to a cluster, since a mutant phenotype has been described so far only for PHO1. The expression pattern of members of a cluster can be significantly divergent, with, for example, H3 being expressed in the vascular tissue of leaves and roots, while H6 is only expressed in connective of the anther (Fig. 9; Table II). It is however striking that the two rice homologs that have been identified have higher similarity to the cluster containing PHO1 and the H1 than the level of similarity between the PHO1/H1 proteins and any other PHO1 homolog within the Arabidopsis genome. This level of conservation across monocots and dicots suggests that the function of the rice homologs and the Arabidopsis PHO1 and H1 are likely to be similar.
All members of the PHO1 family have, within theN-terminal hydrophilic half, the SPX domain divided into three subdomains. While in most eukaryotic proteins the three SPX subdomains are found close together, in the PHO1 protein family these subdomains are separated by somewhat large regions (80–160 amino acids) of low similarity. Interestingly, the region of high similarity in the N-terminal hydrophilic region extends beyond the SPX domain to include the region between SPX-3 and the start of the first putative transmembrane-spanning domain (approximately 50 amino acids; Fig. 2). Although the function of the SPX domain is at present unknown, it is interesting to note that in yeast several proteins implicated in phosphate transport or sensing have a SPX domain, namely the cyclin-dependent kinase inhibitor PHO81, a key regulator of the yeast PHO operon, as well as the PHO87, PHO90, and PHO91 proteins involved in low-affinity Pi transport and potentially Pi sensing (Lenburg and O'Shea, 1996; Ogawa et al., 2000; Wykoff and O'Shea, 2001; Auesukaree et al., 2003; Giots et al., 2003). Although the function of the retrovirus receptor XPR1, the closest mammalian homolog of PHO1, is unknown, a potential link with ion transport also exists since several retrovirus receptors have been shown to encode solute transporters, including transporters for neutral and basic amino acids (Wang et al., 1991; Rasko et al., 1999) and two distinct Na+-Pi cotransporters (Kavanaugh and Kabat, 1996).
The SPX domain is also found in yeast in the VTC1, VTC2, VTC3 and VTC4 proteins forming a membrane chaperone complex involved in the sorting of proteins to the vacuolar membrane, including the V-ATPase (Cohen et al., 1999; Ogawa et al, 2000; Müller et al., 2002). It is interesting to note that the membrane protein PHO86, an essential component of the yeast Pi transport system, has been shown to be an endoplasmic reticulum resident protein required for the targeting to the plasma membrane of PHO84, the high-affinity H+-Pi transporter, while mutations in the VCT genes lead to a defect in polyphosphate accumulation in vacuoles (Lau et al., 2000; Ogawa et al., 2000). It is thus possible for proteins involved in controlling the localization of integral membrane proteins to have a role in the regulation of Pi transport and metabolism. In this context, it will be important to determine the membrane localization of each of the PHO1 proteins. One potential role of the SPX domain, revealed in a study of the yeast SYG1, is as a binding domain to the β-subunit of the heterotrimeric G protein (Spain et al., 1995). Although expression of only the N-terminal hydrophilic portion of SYG1 has been found to suppress a mutant phenotype in pheromone signal pathway, the role of the full-length protein (including the C-terminal hydrophobic domain) is unknown, and no relation with Pi transport has yet been reported, although heterotrimeric G proteins have been involved in the regulation of activity of some transporters (Logothetis et al., 1987).
In addition to all members of the PHO1 family, nine other proteins have been identified in Arabidopsis that have a SPX domain. These proteins can be grouped into three families distinct from the PHO1 family (Fig. 6d), with only one of them having features of integral membrane proteins. Unfortunately, the function of these nine proteins are presently unknown but could eventually provide some clues on the role of the SPX domain in plants.
Among proteins in eukaryotes having a SPX domain, only few of them also possess an EXS domain. In yeast, only SYG1 has both SPX and EXS, while in Arabidopsis only the members of the PHO1 family possess both domains. The EXS domain in the PHO1 proteins encompasses approximately half of the hydrophobic region. The function of the EXS domain is also unknown, although it is interesting to note that the yeast ERD1 protein, which possesses a EXS domain, is involved in the localization of endogenous endoplasmic reticulum proteins, further reinforcing the connection between SPX and EXS with sorting of protein to endomembranes (Hardwick et al., 1990). In Arabidopsis, two related hydrophobic proteins of unknown function have an EXS domain.
Analysis of the expression pattern of all PHO1 homologs reveals that a broad range of tissues can express members of the PHO1 family. However, expression of PHO1 genes in the vascular tissue appears to be predominant. For example, out of the eight genes that are expressed in roots as revealed by both GUS expression and RT-PCR, six demonstrated a GUS expression pattern in the root vascular tissue similar to the pattern initially reported for PHO1 (Fig. 9; Hamburger et al., 2002). GUS expression is also detected in the vascular tissue of several aerial organs, including leaf (H1 and H3), stem (H1 and H8), sepal (H1 and H10), petal (H1), or filament of the anther (H1, H3, H5, H7, and H10). By analogy with the function of PHO1 in loading Pi to the xylem vessels of the root, it is likely that expression of the various homologs in the vascular tissue also reflects their role in the transport of Pi either into the xylem for its distribution to the aerial organs via the transpiration stream or out of the xylem and into the cells of the aerial organs (e.g. leaves, flowers). It is also possible that expression of some homologs in the vascular tissue is associated with transport of Pi to or from the phloem vessels. Several promoters also reveal expression in the leaf hydathodes (H4, H7, H8, and H10). Hydathodes represent an extension and an exit point of the vascular tissue and are the site of water loss through guttation. Several ion transporters have been shown to be expressed in hydathodes, including transporters for sulfate, phosphate, and potassium (Lagarde et al., 1996; Mudge et al., 2002; Shibagaki et al., 2002). A role in the recovery of the ions released through guttation has been proposed for these transporters and could be extended to these PHO1 homologs.
The function of the PHO1 gene family is however clearly not limited to Pi movement to or from the vascular tissues. This is perhaps most clearly revealed by the expression of several homologs in the pollen grain (H4, H8, H9, and H10) but also indicated through the expression of the GUS protein in tissues not closely connected to the vascular tissue, such as expression of the H4 and H10 promoters in the epidermal cortical cells of the roots. In pollen, expression of PHO1 homologs could be related to the import of Pi into the maturing pollen since plasmodesmatal connections between developing pollen and the tapetum are lost early in development (Owen and Makaroff, 1995), implicating the action of Pi transporters for Pi uptake into pollen. Expression of the H+-Pi cotransporters AtPht1;6 and AtPht1;7 has also been reported for mature pollen (Mudge et al., 2002). It is also possible that expression of Pi transporters or of members of the PHO1 protein family be required for the acquisition of Pi into the pollen tube during growth through the pistil transmitting tissues.
Analysis of the pho1 mutant combined with the expression pattern of the PHO1 gene revealed a role of PHO1 in the loading of Pi to the xylem vessels, implicating that PHO1 could be mediating the exit of Pi out of the xylem parenchyma cells (Poirier et al., 1991; Hamburger et al., 2002). However, the expression profile of several PHO1 homologs, such as expression in pollen or hydathodes, suggests rather a role in Pi uptake into cells. A similar broad range of expression patterns, including vascular tissues of roots or leaves, pollen, or hydathodes, has been observed for other families of ion transporters, including the sulfate transporters and the members of the Shaker K+ channel (Lagarde et al., 1996; Gaymard et al., 1998; Takahashi et al., 2000; Mouline et al., 2002; Shibagaki et al., 2002; Yoshimoto et al., 2002). An interesting parallel exists between the Shaker and the PHO1 proteins families, since although most members of the Shaker family have been described as inwardly rectifying K+ channels involved in the entry of K+ into cells, one homolog, namely SKOR, was shown to be an outwardly rectifying channel involved in the loading of K+ to the root xylem vessels (Gaymard et al., 1998). Thus, various members of a transporter family can indeed be involved in the import or export of ions. The expression pattern of the PHO1 homologs indicates that this is likely also to be the case for the PHO1 family.
MATERIAL AND METHODS
Plant Material
Leaves, stems, flowers, or pollen were harvested from wild-type Arabidopsis, accession Columbia, or the pho1-3 mutant, grown in pots in fertilized soil under continuous illumination at 19°C (Poirier et al., 1991). For roots, plants were grown under constant agitation (100 rpm) and continuous fluorescent light at 19°C in liquid nutrient medium (10 mm KH2PO4, pH 5.5, 5 mm KNO3, 2 mm MgSO4, 1 mm CaCl2, 0.1 mm Fe-EDTA, 50 μm H3BO4, 12 μm MnSO4, 1 μm ZnCl2, 1 μm CuSO4, 0.2 μm Na2MoO4, and 2% Suc). After 14 d of culture, the aseptic roots were separated from the shoot tissue and used for RNA extraction. For roots grown under Pi-deficient conditions, plants were first grown in Pi-containing liquid nutrient media for 14 d followed by 7 d in similar media without Pi. Pollen was harvested from mature flowers as described by Mouline et al. (2002).
Cloning of the PHO1 Homologs
Total RNA from leaves, flowers, pollen, or roots was isolated by the Trizol method (Chomczynski and Sacchi, 1987) and was used for RT-PCR. RT was performed on 1 μg of total RNA using oligo(dT) and the SuperScript II reverse transcriptase (Invitrogen AG, Basel) using the manufacturer's conditions. PCR was performed with the Expand High Fidelity PCR system (Roche Diagnostics AG, Rotkreuz, Switzerland), using the manufacturer's conditions. The oligonucleotides used as primers were designed to be located 10 to 20 bases before the start codon and after the stop codon, and introduced novel restriction sites that were used for cloning the fragments into the Escherichia coli-yeast (Saccharomyces cerevisiae) shuttle vector pYES2 (Invitrogen AG). The ligation products were transformed directly into yeast strain InvSc (Invitrogen AG) using the lithium acetate procedure and the cells plated on uracil-deficient selective media (Gietz et al., 1992). Colonies were checked for the presence of the PHO1 homologs by PCR. For each gene, two clones coming from independent RT-PCR reactions and cloning events were sequenced to ensure accuracy and discriminate against mutations induced by the polymerase reaction.
Expression Analysis by RT-PCR
Analysis of the expression profile was done by semiquantitative RT-PCR based on the protocol of Burleigh (2001). Test experiments were performed on RT products of several genes to identify the number of cycles corresponding to the linear phase of amplification. Based on these results, a protocol based on 20 cycles of PCR amplifications was done for all genes. The oligonucleotides used for primers were designed to amplify a fragment of 400 to 600 bases at the 3′ end of the gene. The region amplified encompassed at least two exons, so that PCR fragments generated from contaminating genomic DNA could easily be distinguished from fragments amplified from cDNA based on the size of the products. Following RT and PCR amplification, as described above, the products were analyzed by Southern blot using probes derived from each PHO1 homolog using standard procedures (Sambrook et al., 1989).
Expression Analysis by Promoter-GUS Fusion
Genomic fragments of 0.5 and 1.0 kb located upstream of the PHO1 transcribed sequence were isolated by PCR using oligonucleotides, creating a novel HindIII site at the 5′ end and either a XbaI or BamHI site at the 3′ end. Each fragment was cloned in front of the GUS gene in the binary vector pBI121 (Malik and Wahab, 1993). Transgenic Arabidopsis (accession Columbia) plants expressing the GUS gene under the control of the various promoter fragments were obtained by the floral dip method (Clough and Bent, 1998). Leaves, stems, and flowers from soil-grown plants were stained for GUS activity according to the protocol of Lagarde et al. (1996), with the exception that tissues were not vacuum infiltrated in order to better preserve structures.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY507953 and AY507962.
Supplementary Material
Acknowledgments
We thank Aleksandra Stefanovic and Lassaad Belbahri for critical reading of the manuscript and Simon Goepfert for help with the ClustalX alignment.
This work was supported by the Fonds National Suisse de la Recherche Scientifique (grant no. 31–61731.00 to Y.P.). Contributions are also acknowledged from the University of Lausanne and the Canton de Vaud.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.037945.
References
- Auesukaree C, Homma T, Kaneko Y, Harashima S (2003) Transcriptional regulation of phosphate-responsive genes in low-affinity phosphate-transporter-defective mutants in Saccharomyces cerevisiae. Biochem Biophys Res Commun 306: 843–850 [DOI] [PubMed] [Google Scholar]
- Battini J-L, Rasko JEJ, Miller AD (1999) A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA 96: 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh SH (2001) Relative quantitative RT-PCR to study the expression of plant nutrient transporters in arbuscular mycorrhizas. Plant Sci 160: 899–904 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 6: 735–743 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Cohen A, Perzov N, Nelson H, Nelson N (1999) A novel family of yeast chaperons involved in the distribution of V-ATPase and other membrane proteins. J Biol Chem 274: 26885–26893 [DOI] [PubMed] [Google Scholar]
- Daram P, Brunner S, Rausch C, Steiner C, Amrhein N, Bucher M (1999) Pht2;1 encodes a low-affinity phosphate transporter from Arabidopsis. Plant Cell 11: 2153–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U-I (1999) Phosphate translocators in plastids. Annu Rev Plant Physiol Plant Mol Biol 50: 27–45 [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud J-B, Sentenac H (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655 [DOI] [PubMed] [Google Scholar]
- Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giots F, Donaton MCV, Thevelein JM (2003) Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol 47: 1163–1181 [DOI] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Lewis MJ, Semenza J, Dean N, Pelham HR (1990) ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J 9: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23: 403–405 [DOI] [PubMed] [Google Scholar]
- Kavanaugh MP, Kabat D (1996) Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int 49: 959–963 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nakanishi H, Takahashi M, Kawasaki S, Nishizawa N-K, Mori S (2001) In vivo evidence that Ids3 from Hordeum vulgare encodes a dioxygenase that converts 2′-deoxymugineic acid to mugineic acid in transgenic rice. Planta 212: 864–871 [DOI] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195–203 [DOI] [PubMed] [Google Scholar]
- Lau W-TW, Howson RW, Malkus P, Schekman R, O'Shea EK (2000) Pho86p, an endoplasmic reticulum (ER) resident protein in Saccharomyces cerevisiae, is required for ER exit of the high-affinity phosphate transporter Pho84p. Proc Natl Acad Sci USA 97: 1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenburg ME, O'Shea EK (1996) Signaling phosphate starvation. Trends Biochem Sci 21: 383–386 [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE (1987) The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325: 321–326 [DOI] [PubMed] [Google Scholar]
- Malik VS, Wahab SZ (1993) Versatile vectors for expressing genes in plants. J Plant Biochem Biotechnol 2: 69–70 [Google Scholar]
- Mouline K, Véry A-A, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud J-B, Sentenac H (2002) Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev 16: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 10519–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Müller O, Bayer MJ, Peters C, Andersen JS, Mann M, Mayer A (2002) The Vtc proteins in vacuole fusion: coupling NSF activity to V0 trans-complex formation. EMBO J 21: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, DeRisi J, Brown PO (2000) New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Cell Biol 11: 4309–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 185: 7–21 [Google Scholar]
- Pao SS, Paulsen IT, Saier MH (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62: 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Y, Aramayo R, Kang S, Hall JG, Metzenberg RL (1996) NUC-2, a component of the phosphate-regulated signal transduction pathway in Neurospora crassa, is an ankrin repeat protein. Mol Gen Genet 252: 709–716 [DOI] [PubMed] [Google Scholar]
- Poirier Y, Bucher M (2002) Phosphate transport and homeostasis in Arabidopsis. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0024, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J (1991) A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol 97: 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Rasko JEJ, Battini J-L, Gottschalk RJ, Mazo I, Miller AD (1999) The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc Natl Acad Sci USA 96: 2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216: 23–37 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29: 475–486 [DOI] [PubMed] [Google Scholar]
- Spain BH, Koo D, Ramakrishnan M, Dzudzor B, Colicelli J (1995) Truncated forms of a novel yeast protein suppress the lethality of a G protein α subunit deficiency by interacting with the β subunit. J Biol Chem 270: 25435–25444 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2000) The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J 23: 171–182 [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Versaw WK, Harrison MJ (2002) A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 14: 1751–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kavanaugh MP, North RA, Kabat D (1991) Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature 352: 729–731 [DOI] [PubMed] [Google Scholar]
- Wykoff DD, O'Shea EK (2001) Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159: 1491–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K (2002) Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J 29: 465–473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.