Abstract
INTRODUCTION
Acute pulmonary embolism (APE) is an urgent clinical condition that can progress in a wide variety of ways. Therefore, we sought to develop an easy-to-apply algorithm, to be based on readily available clinical indicators, effective in predicting unfavourable outcomes.
METHODS
This was a retrospective cohort study based on systematically collected data in a database. The study included 102 patients with APE who were admitted to a tertiary care hospital. The following outcomes were defined as unfavourable shock, the need for mechanical ventilation, the use of thrombolytics, and death. Logistic regression analysis was used to explore variables significantly associated with outcome and to calculate post-test probabilities.
RESULTS
The prevalence of unfavourable outcomes was 25.5% (26 of the 102 patients with APE). The risk of an unfavourable outcome was reduced to 7.0% for patients with APE who were aged ≤ 40 years. In patients with APE who were aged > 40 years, the presence of hypoxaemia (i.e. peripheral oxygen saturation < 90%) alone increased the risk of an unfavourable outcome to 57.0%. A recent history of trauma and the presence of pre-existing lung or heart disease were significantly associated with unfavourable outcomes. The inclusion of those variables in the logistic regression model increased the post-test risk of an unfavourable outcome to 65.0%–86.0%.
CONCLUSION
Advanced age (i.e. > 40 years), the presence of hypoxaemia, a recent history of trauma and the presence of pre-existing lung or heart disease are risk factors for unfavourable outcome in patients with APE.
Keywords: anoxia, logistic models, prognosis, pulmonary embolism, wounds and injuries
INTRODUCTION
Acute pulmonary embolism (APE) is an urgent clinical condition. Cases of APE can progress in a wide variety of ways, ranging from asymptomatic cases that resolve spontaneously (these cases are occasionally undiagnosed) to cases that progress to death. Prospective cohort studies have estimated APE-related mortality rate to range from 7% to 11%.(1) Therefore, a rapid and accurate diagnosis is of utmost importance, as is a disease severity classification system that can aid in establishing an accurate prognosis rapidly.
Prognostic models can be valuable tools, as such models can assist in medical decision-making. For example, such models can be used to determine whether a patient should be treated as an outpatient or admitted to an intensive care unit, or whether initial treatment should comprise chemical thrombolysis, mechanical thrombolysis or anticoagulants alone. With good medical decisions, the administered treatment is likely to be more appropriate and effective, potentially improving patient survival, and consequently, the expected outcome.
With the objective of establishing a more accurate prognosis for patients with APE, specific indicators (e.g. clinical findings, biological markers and imaging parameters) have been used to stratify patients. Biological markers such as brain natriuretic peptide and troponin have been used, as have echocardiographic, electrocardiographic and computed tomographic parameters, either in isolation or in combination.(2-7) However, not all of such indicators can be easily employed in clinical practice, either because the skills required to perform and interpret the results of some of these tests are not easily attained or because such tests are not always readily available. Certain prognostic models (most of which are based on clinical findings), such as the Pulmonary Embolism Severity Index (PESI), have other practical limitations such as the inclusion of numerous variables.(8) The simplified PESI was developed in an attempt to overcome this limitation.(9)
It is important to develop an APE severity classification system that: (a) is based exclusively on easily obtained clinical indicators; and (b) can be applied widely (e.g. does not require rare skills and equipment). The objective of the present study was to develop an easy-to-apply algorithm that is based on readily available clinical indicators (thereby eliminating the need for ancillary tests) and can be effective in predicting unfavourable outcomes in patients with APE.
METHODS
This was a retrospective cohort study based on systematically collected clinical data and ancillary test results available in a database from Santa Lúcia Hospital, Brasília, Brazil. All patients with APE who were admitted to the tertiary care hospital between January 2006 and February 2011 were included in the present study. The diagnosis of APE was based on clinical findings consistent with the disease. Of the 102 patients included in the study, the diagnosis of APE was confirmed by computed tomography pulmonary angiography and radionuclide imaging of the lungs in 92 (90.2) and 10 (9.8%) patients, respectively. The only inclusion criterion was a confirmed diagnosis of APE, regardless of the risk factors for APE or the presence of comorbidities. The following outcomes were defined as unfavourable: shock, the need for mechanical ventilation, the use of thrombolytics, and death.
A structured form was used to collect all relevant patient data. The following data was collected: (a) signs and symptoms at admission, i.e. subjective dyspnoea, chest pain, increased work of breathing, cough, sweating, haemoptysis, cyanosis, peripheral oxygen saturation (SpO2) < 90%, signs of deep vein thrombosis in the legs, syncope, systolic blood pressure (SBP) < 100 mmHg, and tachycardia (heart rate > 100 bpm); (b) concomitant diseases, i.e. systemic arterial hypertension, diabetes mellitus, obesity, Chagas’ heart disease, atrial fibrillation, thrombophilia, neoplasia, chronic lung disease, and chronic heart disease; and (c) risk factors for APE, i.e. smoking, impaired locomotion, recent history of trauma, recent surgery, sedentary lifestyle, oral contraceptive use, and family history of thromboembolism.
In data analysis, the variable ‘age’ was transformed into a dichotomous variable after receiver operating characteristic (ROC) curves were constructed to determine the best cutoff point. Sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were determined for each dichotomous variable. The pre-test risk of an unfavourable outcome was assumed to be the prevalence found in the study sample.
Bivariate logistic regression was used to explore any probable associations between variables and unfavourable outcome. In the analysis, some original variables were transformed to create new variables; this was permitted as long as the transformations were pathophysiologically plausible. In multivariate analysis, a model was created based on the independent variables that were found to be statistically significant in the bivariate analysis. We first included all variables simultaneously and observed the regression coefficient for each variable. The final model was obtained by forward stepwise logistic regression. After defining the variables in the model, we arranged them in sequence to set up an algorithm that would be useful to clinicians. The post-test probability of each event was calculated using the logistic equation. We evaluated the set of coefficients in the model using the log-likelihood test. The proportion of variance in the dependent variable explainable by the independent variables was expressed as Nagelkerke R2. The agreement between the predicted and observed values was evaluated using the Hosmer-Lemeshow test.
All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows version 18.0 (SPSS Inc, Chicago, IL, USA). A p-value < 0.05 was considered statistically significant. The present study was approved by the Research Ethics Committee of our institution (Protocol no. 058/2011).
RESULTS
For the 102 patients in our cohort, the mean age was 57.9 ± 20.7 (range 17–92) years, with 62 (60.8%) of them female, and 40 (39.2%), male. The prevalence of unfavourable outcome in our study cohort was 25.5% (i.e. 26 of the 102 patients) (Table I).
Table I.
Number and type of unfavourable outcome observed in the study cohort (n = 102).
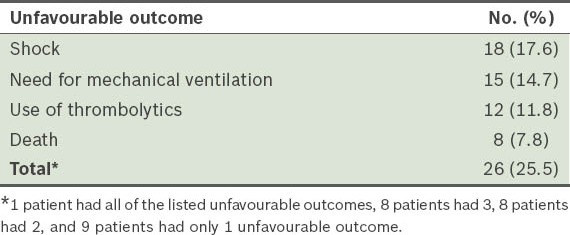
Our analysis showed that SpO2 < 90% (i.e. hypoxaemia), SBP < 100 mmHg and syncope were the only signs that had PLR > 2, with hypoxaemia having the highest PLR (i.e. 3.29) (Table II). Among the concomitant diseases included in our analysis, chronic heart disease, chronic lung disease and thrombophilia had PLR > 2 (Table III). A history of smoking and recent history of trauma were risk factors that had PLR > 2 (Table III). The remaining clinical indicators had PLR < 2.0.
Table II.
Sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) of the various signs and symptoms associated with unfavourable outcomes in patients with acute pulmonary embolism.
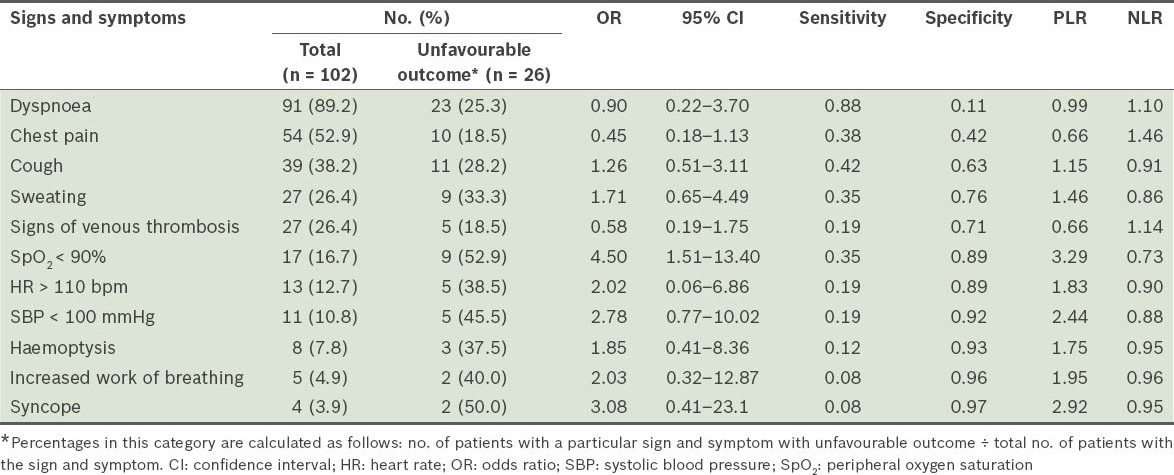
Table III.
Sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) of concomitant diseases and risk factors associated with unfavourable outcomes in patients with acute pulmonary embolism (APE).
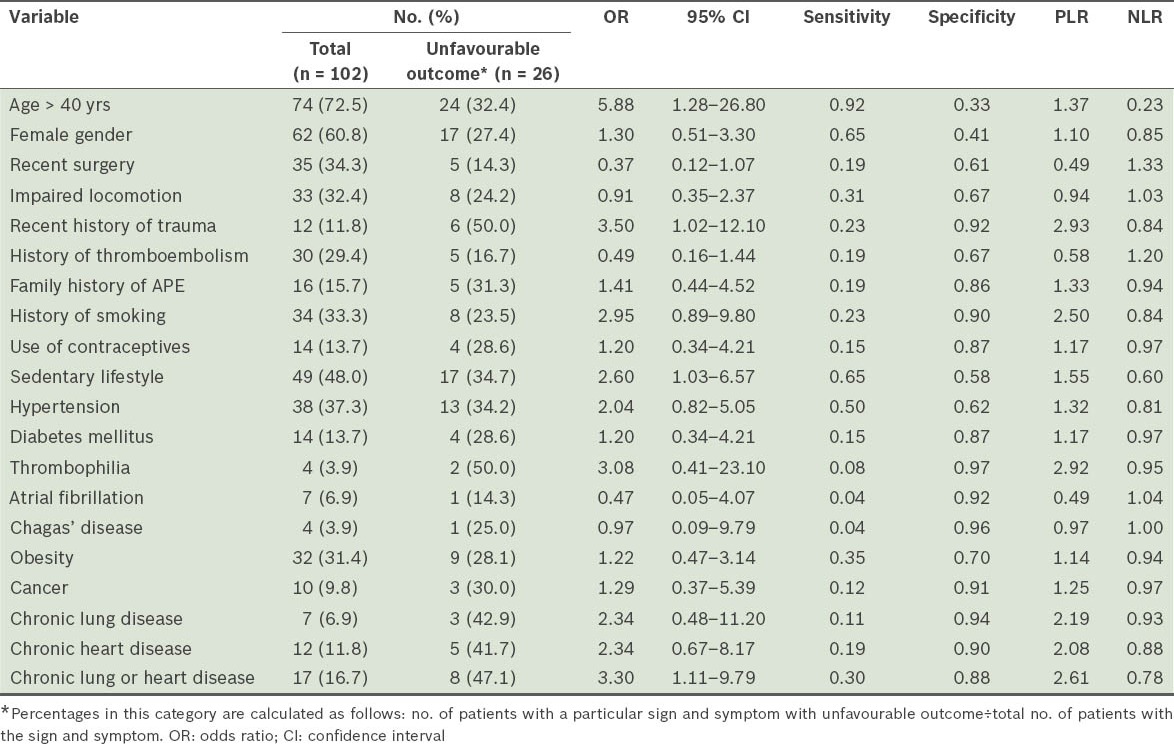
After PLR and NLR were taken into consideration, we found that the best cutoff point for age was 40 years (area under the ROC curve 0.639; 95% confidence interval [CI] 0.524–0.753). Although age > 40 years had a PLR of only 1.37, it was the variable that had the lowest NLR (i.e. 0.23). In other words, we found that age ≤ 40 years was the best clinical indicator of good prognosis. None of the remaining study variables showed NLR < 0.5. The PLR values of the other possible cutoff points for age – 65 years, 80 years and 85 years – were 1.26, 1.70 and 2.94, respectively; the respective NLR values were 0.82, 0.86 and 0.92. For the cutoff point of 85 years, sensitivity was only 11.5%.
After the variables were transformed, we found that, as variables, smoking and syncope lost their strength when combined with other variables. This is likely due to overlapping effects. When we combined smoking with the occurrence of chronic lung or heart disease, we obtained a PLR of 2.29, which was lower than that obtained for chronic lung or heart disease alone (i.e. 2.61). Similarly, when syncope was combined with hypotension and hypoxaemia, PLR was 2.20 and 2.91, respectively. Although the variables SpO2 < 90%, age > 40 years, and recent history of trauma were found to have a statistically significant association with unfavourable outcome in the analysis prior to the transformation of the variables, the occurrence of chronic lung or heart disease was the only variable that was found to have such an association after transformation of the variables.
In the forward stepwise logistic regression model, SpO2 < 90% (p = 0.01, B = 1.353) and age > 40 years (p = 0.03, B = 1.631) were selected as the most important predictors of unfavourable outcome. The model constant was −2.693 (p < 0.01). Hosmer-Lemeshow test revealed no statistically significant differences between the values observed and those predicted by the model (p = 0.70). Nagelkerke R2, which expresses the proportion of variance in the dependent variable explained by the independent variables, was 0.177.
When all significant independent variables were simultaneously included in the model (p < 0.01), their coefficients were as follows: 1.286 for SpO2 < 90%; 1.348 for age > 40 years; 1.195 for recent history of trauma; and 0.767 for chronic lung or chronic heart disease. The constant was −2.779. Hosmer-Lemeshow goodness-of-fit test was not significant (p = 0.83), and Nagelkerke R2 was 0.234. The ROC curve from the predicted probability of the whole model was also produced, with the area under the curve at 0.763 (95% CI 0.653–0.874).
The inclusion of the variable ‘age ≤ 40 years’ reduced the risk of an unfavourable outcome from 25.5% to 7.0% (pre-test vs. post-test) (Fig. 1). Even when that variable was combined with the variable ‘hypoxaemia’, the post-test risk of an unfavourable outcome remained lower than the pre-test risk (20.0% vs. 25.5%). In contrast, when the variables ‘age > 40 years’ and ‘hypoxaemia’ were combined, the post-test risk of an unfavourable outcome was high (~57.0%) (Fig. 2). The inclusion of yet another significant indicator pushed the post-test risk of an unfavourable outcome even higher, from 65.0% to 86.0%. However, in the absence of hypoxaemia, the post-test risk of an unfavourable outcome was similar to the pre-test risk of an unfavourable outcome, even among patients aged > 40 years (post-test risk 31.0% vs. pre-test risk 25.5%).
Fig. 1.
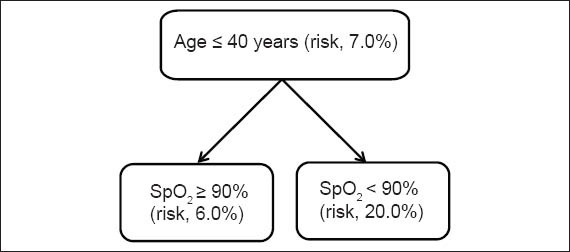
Post-test risk of an unfavourable outcome in patients aged ≤ 40 years. SpO2: peripheral oxygen saturation
Fig. 2.
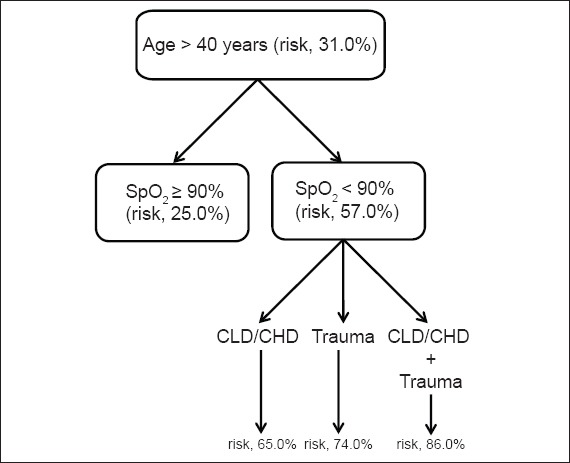
Post-test risk of an unfavourable outcome in patients aged > 40 years. CLD/CHD: the occurrence of chronic lung or heart disease
DISCUSSION
In the present study, the mortality rate was 7.8% and the overall incidence of unfavourable outcomes was 25.5%. These values are similar to those reported in other studies,(1,10,11) indicating that our study cohort was similar to those evaluated in previous studies. Although we found that the best cutoff point for age in our study cohort was 40 years, other studies have shown far higher cutoff points for age, such as 65 years,(12) 70 years,(11) and 80 years.(9) This difference is likely due to the different selection criteria used in the different studies. The cutoff point for age is usually higher when it is established for the likelihood of an unfavourable outcome but not for the unlikelihood of the unfavourable outcome. For instance, the highest PLR in our study was for 85 years of age. However, since the discriminative ability of a given model increases when the cutoff point is chosen based on both PLR and NLR, we decided to use 40 years of age as the cutoff point because it was the value that best discriminated between the likelihood and unlikelihood of an unfavourable outcome.
As SpO2 < 90% was the variable that showed the highest PLR in our study cohort, it played a central role in our prognostic model. Hypoxaemia is quite common in patients with APE, occurring via multiple pathogenic mechanisms. The pattern of distribution of either ventilation or perfusion, however, depends largely on cardiac output.(13) When such patients continue to have normal cardiac output, blood flow redistribution can result in the formation of areas of low ventilation or perfusion within the lung zone unaffected by thromboembolism. This means that there is an overperfusion of non-embolised regions, which in turn leads to hypoxaemia. In cases where cardiac output is significantly decreased, there is an increase in high ventilation or perfusion areas within the lung, including dead space. The factor observed to play an extremely important role in hypoxaemia in patients with APE is reduced mixed venous oxygen tension, which is a direct consequence of low cardiac output.(13,14) Although decreased cardiac output plays an important role in hypoxaemia, hypoxaemia can occur regardless of changes in cardiac output. Indeed, patients with massive pulmonary embolisms can present with severe hypoxaemia even if their cardiac output and blood pressure are normal.(14) Thus, hypoxaemia is a more sensitive clinical indicator than hypotension for predicting unfavourable outcome in patients with APE. However, the degree of hypoxaemia that translates to an unfavourable outcome remains unknown.
SpO2 < 90% appears to be one of the most accurate predictors of unfavourable outcome. Other studies(8,11,15) have shown that hypoxaemia plays an important role as an independent risk factor for poor prognosis, a finding that is consistent with that of the present study. In our analysis, the variable ‘hypotension’ (i.e. SBP < 100 mmHg) showed a sensitivity of 19.0% in its ability to predict unfavourable outcome; this sensitivity is lower than the 35.0% observed for SpO2 < 90%. Nevertheless, hypoxaemia and hypotension were found to be similar in terms of their specificity to predict unfavourable outcome (89.0% and 92.0%, respectively). Despite an odds ratio of 2.78, the difference was not significant for hypotension. This is probably due to the small sample size of the present study.
In patients with APE, the relationship between hypotension and progression to shock, or even death, has been well established.(16) Some studies chose to use a cutoff point of 90 mmHg,(10,16) which can increase specificity. The cutoff point of 100 mmHg has also been reported as being significantly associated with poor prognosis,(8,16) although this cutoff point did not achieve statistical significance in one report.(17)
Considering the pathophysiological impact that lung and heart disease have on cardiovascular and respiratory systems, we can assume that pre-existing lung or heart disease is an aggravating factor that increases the risk of an unfavourable outcome. In the present study, the presence of chronic lung disease and chronic heart disease was found to be significantly associated with outcome. This finding is consistent with that of some studies,(8,10,18,19) but inconsistent with others.(17,20) Such differences are probably due to differences in the characteristics of the study cohorts (e.g. some studies had study cohorts that consisted of patients with severe disease, while others had study cohorts that consisted of patients with less severe disease) or the attenuation of the strength of this variable when it is influenced by several other variables (e.g. age, hypoxaemia, hypotension and tachycardia) in multivariate analysis.
In the present study, we found that a recent history of trauma was a significant predictor of unfavourable outcome. Our finding regarding this variable is inconsistent with other studies.(4,18,20) In one study,(4) although a recent history of trauma was found to be statistically significant in the univariate analysis, it was not sufficiently significant to be included in multivariate analysis. In other studies,(18,20) it was found to have no statistical significance, even in single predictor analyses. Care should be taken when the variables ‘recent history of trauma’ and ‘recent surgery’ are studied in combination. In such cases, postoperative prophylaxis with anticoagulants might reduce the risk of death,(20) artificially reducing the negative impact of a recent history of trauma. However, the sequelae of trauma, particularly those of severe multiple trauma, can contribute to an unfavourable outcome.
In addition to the aforementioned differences in the inclusion criteria of various studies, differences in outcome criteria also decisively influence the results obtained. For example, one study showed that a history of cancer was an important predictor of poor prognosis when recurrence of thromboembolism within three months was used as an outcome measure.(15) We found no such relationship in the present study, which is consistent with the findings of Conget et al,(21) who investigated the short-term mortality of patients after APE.
Although our logistic regression model could explain the outcome in 23.4% of the cases (i.e. a reasonable proportion), we found that the ability of the model to predict unfavourable outcome in patients with APE is limited if only clinical variables are used. However, as our model uses variables that are easily understood, it can assist clinicians in making decisions regarding the initial approach for patients with APE, as well as assist them in predicting the course of the disease before confirmation of the diagnosis. Another limitation of the present study stems from its sample size, which may have some degree of inner variability. The number of patients with adverse outcomes in our study cohort was small, and thus the prediction model based on this group of patients may not be accurate.
We conclude that age > 40 years, SpO2 < 90%, recent history of trauma, and the presence of pre-existing lung or heart disease are risk factors for unfavourable outcome in patients with APE. Our prognostic model may impact the management of patients with APE, as hypoxaemia probably occurs before hypotension and shock, and is likely the key element contributing to early prognostication. Further studies are required to explore the use of hypoxaemia at admission as an indicator for early treatment or early admission to an intensive care unit.
ACKNOWLEDGEMENTS
We would like to thank Prof Verônica Moreira Amado, University of Brasília, Brazil, for providing important comments on the study, and endorsing the data and conclusion of the study. We also would like to express our gratitude to Prof Cesar Silva for his technical support.
REFERENCES
- 1.Stein PD, Kayali F, Olson RE. Estimated case fatality rate of pulmonary embolism 1979 to 1998. Am J Cardiol. 2004;93:1197–9. doi: 10.1016/j.amjcard.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 2.van der Bijl N, Klok FA, Huisman MV, et al. Measurement of right and left ventricular function by ECG-synchronized CT scanning in patients with acute pulmonary embolism: usefulness for predicting short-term outcome. Chest. 2011;140:1008–15. doi: 10.1378/chest.10-3174. [DOI] [PubMed] [Google Scholar]
- 3.Kukla P, Długopolski R, Krupa E, et al. Electrocardiography and prognosis of patients with acute pulmonary embolism. Cardiol J. 2011;18:648–53. doi: 10.5603/cj.2011.0028. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez O, Trinquart L, Caille V, et al. Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study. Am J Respir Crit Care Med. 2010;181:168–73. doi: 10.1164/rccm.200906-0970OC. [DOI] [PubMed] [Google Scholar]
- 5.Ozsu S, Karaman K, Mentese A, et al. Combined risk stratification with computerized tomography /echocardiography and biomarkers in patients with normotensive pulmonary embolism. Thromb Res. 2010;126:486–92. doi: 10.1016/j.thromres.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Vuilleumier N, Le Gal G, Verschuren F, et al. Cardiac biomarkers for risk stratification in non-massive pulmonary embolism: a multicenter prospective study. J Thromb Haemost. 2009;7:391–8. doi: 10.1111/j.1538-7836.2008.03260.x. [DOI] [PubMed] [Google Scholar]
- 7.Kjaergaard J, Schaadt BK, Lund JO, Hassager C. Prognostic importance of quantitative echocardiographic evaluation in patients suspected of first non-massive pulmonary embolism. Eur J Echocardiogr. 2009;10:89–95. doi: 10.1093/ejechocard/jen169. [DOI] [PubMed] [Google Scholar]
- 8.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–6. doi: 10.1164/rccm.200506-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383–9. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 10.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 11.Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169–75. doi: 10.1001/archinte.166.2.169. [DOI] [PubMed] [Google Scholar]
- 12.Volschan A, Albuquerque D, Tura BR, et al. Predictors of hospital mortality in hemodynamically stable patients with pulmonary embolism. Arq Bras Cardiol. 2009;93:135–40. doi: 10.1590/s0066-782x2009000800011. [DOI] [PubMed] [Google Scholar]
- 13.Manier G, Castaing Y. Influence of cardiac output on oxygen exchange in acute pulmonary embolism. Am Rev Respir Dis. 1992;145:130–6. doi: 10.1164/ajrccm/145.1.130. [DOI] [PubMed] [Google Scholar]
- 14.Manier G, Castaing Y. Contribution of multiple inert gas elimination technique to pulmonary medicine--4. Gas exchange abnormalities in pulmonary vascular and cardiac disease. Thorax. 1994;49:1169–74. doi: 10.1136/thx.49.11.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84:548–52. [PubMed] [Google Scholar]
- 16.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113:577–82. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 17.Uresandi F, Otero R, Cayuela A, et al. [A clinical prediction rule for identifying short-term risk of adverse events in patients with pulmonary thromboembolism] Arch Bronconeumol. 2007;43:617–22. doi: 10.1016/s1579-2129(07)60139-6. Spanish. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–5. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 19.Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J. 2011;37:762–6. doi: 10.1183/09031936.00070110. [DOI] [PubMed] [Google Scholar]
- 20.Laporte S, Mismetti P, Décousus H, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–6. doi: 10.1161/CIRCULATIONAHA.107.726232. [DOI] [PubMed] [Google Scholar]
- 21.Conget F, Otero R, Jiménez D, et al. Short-term clinical outcome after acute symptomatic pulmonary embolism. Thromb Haemost. 2008;100:937–42. [PubMed] [Google Scholar]


