Abstract
INTRODUCTION
We evaluated reduced back pain in a multiethnic population treated with teriparatide and/or antiresorptives in real-life clinical settings over 12 months.
METHODS
This prospective observational study comprised 562 men and postmenopausal women (mean age 68.8 years) receiving either teriparatide (n = 230), antiresorptives (raloxifene or bisphosphonates; n = 322), or both (n = 10) for severe osteoporosis. The primary endpoint was the relative risk of new/worsening back pain at six months.
RESULTS
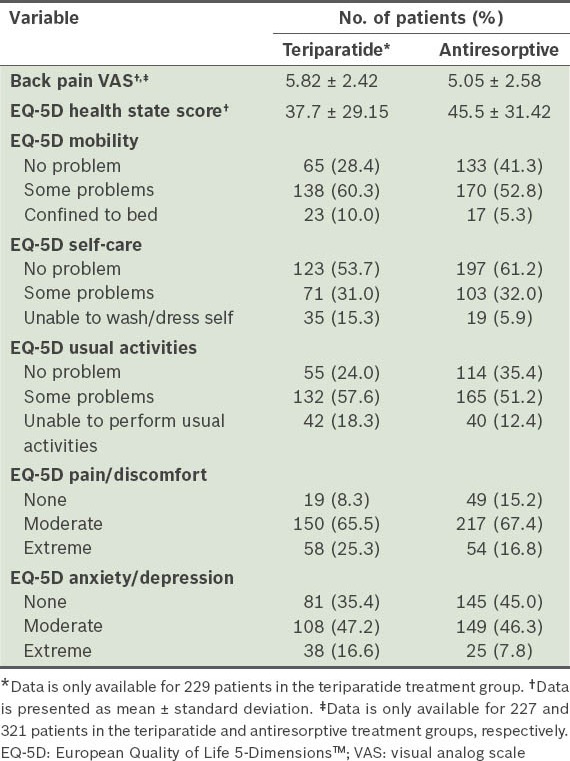
At baseline, a higher proportion of teriparatide-treated than antiresorptive-treated patients had severe back pain (30.9% vs. 17.7%), extreme pain/discomfort (25.3% vs. 16.8%), extreme anxiety/depression (16.6% vs. 7.8%) and were confined to bed (10.0% vs. 5.3%). Teriparatide-treated patients had higher visual analog scale (VAS) scores for pain (5.8 ± 2.42 vs. 5.1 ± 2.58) and lower mean European Quality of Life-5 Dimensions (EQ-5D) scores (37.7 ± 29.15 vs. 45.5 ± 31.42) than antiresorptive-treated patients. The incidence of new/worsening back pain at six months for patients on teriparatide and antiresorptives was 9.8% and 10.3% (relative risk 0.99, 95% confidence interval 0.80–1.23), respectively. The incidence of severe back pain at 12 months was 1.3% and 1.6% in the teriparatide and antiresorptive treatment groups, respectively. Teriparatide-treated patients had lower mean VAS (2.71 ± 2.21 vs. 3.30 ± 2.37) and EQ-5D (46.1 ± 33.18 vs. 55.4 ± 32.65) scores at 12 months. More teriparatide-treated patients felt better (82.7% vs. 71.0%) and were very satisfied with treatment (49.4% vs. 36.8%) compared to antiresorptive-treated patients.
CONCLUSION
Patients treated with either teriparatide or antiresorptives had similar risk of new/worsening back pain at six months.
Keywords: antiresorptives, back pain, EQ-5D, osteoporosis, teriparatide
INTRODUCTION
Chronic back pain associated with vertebral fractures is a common and serious consequence of osteoporosis that limits function and negatively affects health-related quality of life (HRQoL).(1) Osteoporosis medications improve bone strength and prevent new fractures. Antiresorptive therapies suppress bone turnover, thereby reducing bone loss and leading to an increase in bone mineral density (BMD) and a reduced risk of vertebral fractures.(2-6) As a bone anabolic agent, teriparatide stimulates bone formation by increasing the number of osteoblasts and reducing apoptosis, leading primarily to an improvement in bone quality, not just bone strength, which translates to increased BMD and a reduced risk of fractures.(7-9) Large trials evaluating antiresorptive therapies have provided little data on the effect of osteoporotic agents on back pain.(3,10-13) In contrast, data that evaluates the direct effect of teriparatide on pre-existing back pain, physical function and quality of life are only beginning to emerge.
The European Forsteo Observational Study was the first observational study to assess the effect of teriparatide treatment in routine clinical practice on clinical fracture reduction, back pain and HRQoL among 1,581 postmenopausal women with osteoporosis.(14,15) The aforementioned study reported that patients experienced rapid and significant improvements in both back pain and HRQoL with teriparatide treatment, and these effects continued for up to 18 months even after therapy was discontinued. Assessment of back pain was also the primary focus of the prospective, controlled, randomised, open-label, two-year European Study of Forsteo (EUROFORS).(16) In the EUROFORS study of 868 postmenopausal women, teriparatide treatment was associated with significant reduction in back pain, regardless of whether recent vertebral fractures were present.(16)
Nevitt et al performed two separate meta-analyses of data on back pain derived from randomised, double-blind clinical trials on patients treated with teriparatide versus an active comparator or placebo.(17,18) In their first study,(17) patients randomised to receive teriparatide treatment were found to have a significantly reduced risk of back pain (moderate and severe) compared to patients treated with either alendronate, placebo or hormone replacement therapy; without teriparatide treatment, patient outcome would have been associated with decreased functioning. The second meta-analysis(18) assessed post-treatment, observational and follow-up data from four of five initially assessed trials, and demonstrated the continuing effects of teriparatide, lasting up to 30 months post treatment.
In a randomised, double-blind, 18-month study designed to evaluate the effect of teriparatide on back pain in postmenopausal women with osteoporotic vertebral fractures and compare it against risedronate, Hadji et al found that a high proportion of patients in both treatment groups experienced a significant reduction in back pain.(19) The differences in back pain reduction between the two treatment groups were not statistically significant at either Month 6 or Month 18. Between Months 6 and 12, more patients treated with risedronate reported a worsening of the ‘worst’ back pain (p = 0.04), while between Months 6 and 18, more patients treated with risedronate reported a worsening of average back pain (p = 0.04).
A few Phase III clinical trials performed in China, Taiwan, Japan and other Asian countries (including five clinical trials – one placebo-controlled(20) and four calcitonin-comparator(21-24) studies), although short in duration (six-month course of teriparatide treatment), demonstrated increases in lumbar spine BMD and rapid increases in bone formation markers compared to the placebo or calcitonin comparator. Furthermore, another study evaluating the effect of teriparatide in Japanese patients, with an extended follow-up of up to two years, also demonstrated increased BMD and bone markers.(25)
Indeed, treatment with teriparatide has consistently resulted in early increases in bone formation markers that correlate with later changes in BMD or fracture risk.(26) The main objective of the present observational study was to compare changes in back pain that may be related to early gains of BMD and bone formation markers during an interim treatment period of 12 months with teriparatide versus antiresorptive therapies in ethnic populations that have not yet been studied.
Additionally, the present study was conducted in multiple countries (largely in Asia) to capture more data on back pain in patients from these countries treated with teriparatide compared to antiresorptives, which to our knowledge, is supported only by limited clinical studies. Indeed, it is only recently that guidelines have been published in China,(27) recommending teriparatide as an anti-osteoporotic drug with bone-forming effects. Such data would be particularly relevant, as Asian patients have additional risk factors for osteoporosis. (28) Additionally, the Asian population comprises a high population of postmenopausal women with a small body size and reduced bone strength who traditionally have an aversion to sun exposure, which results in low vitamin D levels, and who customarily avoid consumption of dairy products, which limits their dietary intake of calcium.(28)
METHODS
This was a prospective, multinational, observational study assessing the early effects of either teriparatide or antiresorptives (i.e. raloxifene, daily or weekly bisphosphonates, as representative treatments typically used in a clinical observational setting) on back pain in patients with severe osteoporosis who were treated for up to one year. Prior to this study, early improvements in back pain were reported in clinical study patients treated with teriparatide.(15-19) Therefore, this study was designed to evaluate the early efficacy of teriparatide on the reduction of back pain in a larger observational study.
Patients included in the present study were enrolled at 58 centres located in nine countries/provinces, including Hong Kong, India, Korea, Malaysia, Mexico, Pakistan, Singapore, Taiwan and Thailand. The present study was reviewed by at least one ethical review board per country. This study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki, and was consistent with good clinical practices and the applicable laws and regulations of the countries where the study was conducted, as appropriate. The study drugs were prescribed and administered according to the normal clinical practice of the physicians who participated in the study.
Baseline assessments were made upon study enrolment, and reassessed at approximately six-monthly intervals for a maximum of 12 months. It was anticipated that patients with severe osteoporosis would undergo medical evaluation approximately every six months due to their high fracture risk. The interval of visits was at the discretion of the physicians, and scheduled based on their clinical judgement and the local standard of medical care.
Men and postmenopausal women with severe osteoporosis were eligible for inclusion in the present study if they met all of the following criteria: (a) presence of a previous vertebral osteoporotic fracture sustained at least six weeks prior to joining the study; (b) free of severe or chronically disabling conditions other than osteoporosis; (c) not currently receiving and had not previously received teriparatide treatment; (d) agreed to participate in the study, return for follow-up visits and release patient information regarding treatment; and (e) deemed fit by the prescribing physician to receive the intended treatment in compliance with all the recommendations stated in the relevant product information.
Patients were excluded from the study if they had any contraindications (according to the relevant product information of the country/province in which they were being treated) or if they were simultaneously participating in a different study that included a treatment intervention and/or an investigational drug. The decision to enrol patients in the study was left to the discretion of the investigator, respecting the above criteria, and patient consent was obtained.
The primary objective of this study was to compare, in clinical practice, the effect of teriparatide versus daily or weekly antiresorptive treatment on the relative risk (RR) of new or worsening back pain at six months, assessed using the Back Pain Questionnaire.(15) Patients with severe back pain were excluded from the analysis because their pain was already of maximum severity. Secondary effectiveness measures included the severity of back pain, incidence of back pain, and changes in back pain severity, which was assessed using the visual analog scale (VAS). VAS uses a ten-point scale, where ‘0’ indicates ‘no pain’ and ‘10’ indicates ‘worst possible pain’. Safety variables included the incidence of serious and nonserious adverse events, and the incidence of adverse events possibly related to the treatment administered. The incidence of nontraumatic osteoporotic fractures at any time during the study was counted as an event (i.e. new fracture). Health outcomes included the evaluation of treatment adherence, treatment discontinuation rate at one year, reasons for discontinuation of osteoporosis treatment, and changes in HRQoL using the European Quality of Life 5-Dimensions™ (EQ-5D) questionnaire.(29) This questionnaire uses a 100-point scale, where ‘0’ indicates ‘the best health you can imagine’ and ‘100’, ‘the worst health you can imagine.’
Treatment adherence was assessed in two ways: patients were (a) asked to report the date of their first dose and the approximate date of their last dose to determine treatment persistence (i.e. number of days on therapy); and (b) asked at each visit to estimate the number of days that they missed taking their prescribed therapy. It was recommended that investigators telephone each study participant two times (at three and nine months) to evaluate these two aspects of adherence.
With an antiresorptive to teriparatide enrolment ratio of 3:2, a sample size of 478 patients was calculated to provide 80% power at a two-sided significance level of 0.05 to detect a difference in new/worsening back pain.(17) Unless otherwise stated, all tests were two-sided and conducted with type 1 error set to 0.05. No adjustments were made for multiple comparisons because there was only one primary analysis (i.e. multiple secondary analyses were considered supportive). Patients who received teriparatide or antiresorptive treatment, or both, at any time during the study were included in the safety cohort. Patients who received either teriparatide or antiresorptive treatment alone at baseline, but not both, were included in the monotherapy cohort and further classified according to the treatment received at baseline.
The covariates used in the comparisons and models were country, baseline value and propensity score. The propensity score method was used to adjust for selection bias. Propensity score was derived using logistic regression, with selected baseline characteristics such as demographics, vitals, alcohol and tobacco use, exercise, type of insurance, education, medical history (including fracture), and baseline assessments of disease and back pain as independent variables. For the calculation of propensity score, missing baseline data was imputed using treatment mean imputation for continuous measurements, and for categorical variables, by adding a ‘missing’ category to denote missing data. Six patients who had propensity scores and were in non-overlapping regions of the propensity score distributions were excluded from any comparative analysis.
The RR of new/worsening back pain at six months was estimated using a modified Poisson regression model adjusted for baseline back pain severity, country and propensity score. The point estimate and 95% confidence interval (CI) for RR were presented. Patients with severe back pain at baseline were excluded from this analysis and described separately. Severity of back pain at six months was compared using the generalised logit model adjusted for propensity score and baseline back pain severity. Odds ratios (ORs) were calculated using ‘none’ as the reference category. Descriptive analyses of actual values and changes from baseline EQ-5D health state scores were provided. Cohorts were compared with respect to EQ-5D health state score and VAS, using the analysis of covariance model, with baseline value and propensity score as covariates, and treatment cohort and country as fixed effects. For this self-rated instrument, ‘no back pain’ was rated as ‘0’ and ‘worst possible back pain’ was rated as ‘10’. Changes from baseline in back pain VAS score were analysed using mixed model repeated measure, with baseline value and propensity score as covariates; treatment cohort, country, time point and treatment cohort by time point interaction as fixed effects; and patient and error as random effects. Unstructured covariance matrix was used to model within-patient errors. Treatment adherence and satisfaction, discontinuation rate and reasons for discontinuation were summarised using descriptive statistics. Treatment discontinuation by 12 months was analysed using logistic regression, with treatment, country and propensity score included in the model. For safety, summaries were provided for serious and nonserious treatment-emergent adverse events and nontraumatic osteoporotic fractures.
RESULTS
Of the 664 patients who were screened, 647 were enrolled in the study. Among those enrolled, 562 patients took teriparatide or antiresorptive medication (Fig. 1). More patients in the teriparatide group discontinued treatment as compared to the antiresorptive group, with the most common reason for discontinuation in both groups being ‘patient decision’. The OR for treatment discontinuation by one year for the teriparatide cohort versus the antiresorptive cohort (adjusted for country and propensity score) was 1.82, with 95% CI 1.08–3.08 (p = 0.025).
Fig. 1.
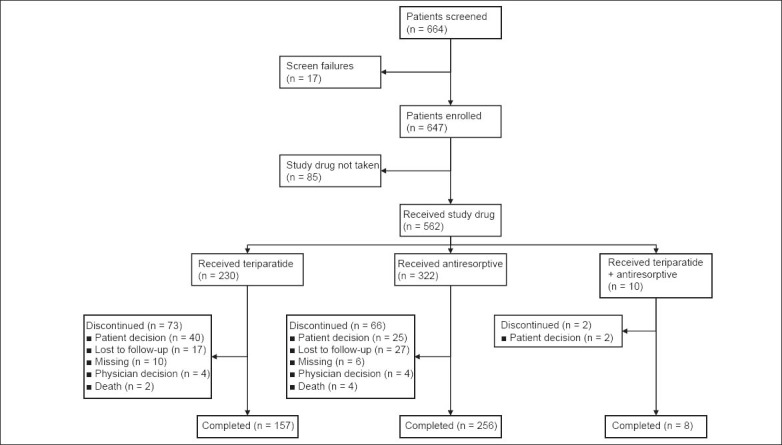
Flow chart shows the study design.
Note: ‘Enrolled cohort’ refers to patients who were prescribed study drug irrespective of whether they actually received the study drug (n = 647). ‘Safety cohort’ refers to patients who received either teriparatide or antiresorptive treatment, or both, at any time during the study (n = 562). ‘Monotherapy cohort’ refers to patients who received either teriparatide or antiresorptive treatment at baseline, but not both (n = 552).
Baseline demographics and disease characteristics for the different cohorts are shown in Tables I, II and III. Not all variables were well balanced across the groups, as could be expected due to the observational, non-randomised nature of this study. Baseline patient demographics and characteristics were similar in the teriparatide and antiresorptive treatment groups, except that disease severity generally appeared to be worse for patients in the teriparatide group than for those in the antiresorptives group. The assessment of EQ-5D showed that in all dimensions such as mobility, self-care, conduct of usual activities, pain/discomfort and anxiety/depression, patients in the teriparatide group had more severe baseline characteristics than those in the antiresorptive group.
Table I.
Baseline demographics and disease characteristics of the safety cohort.
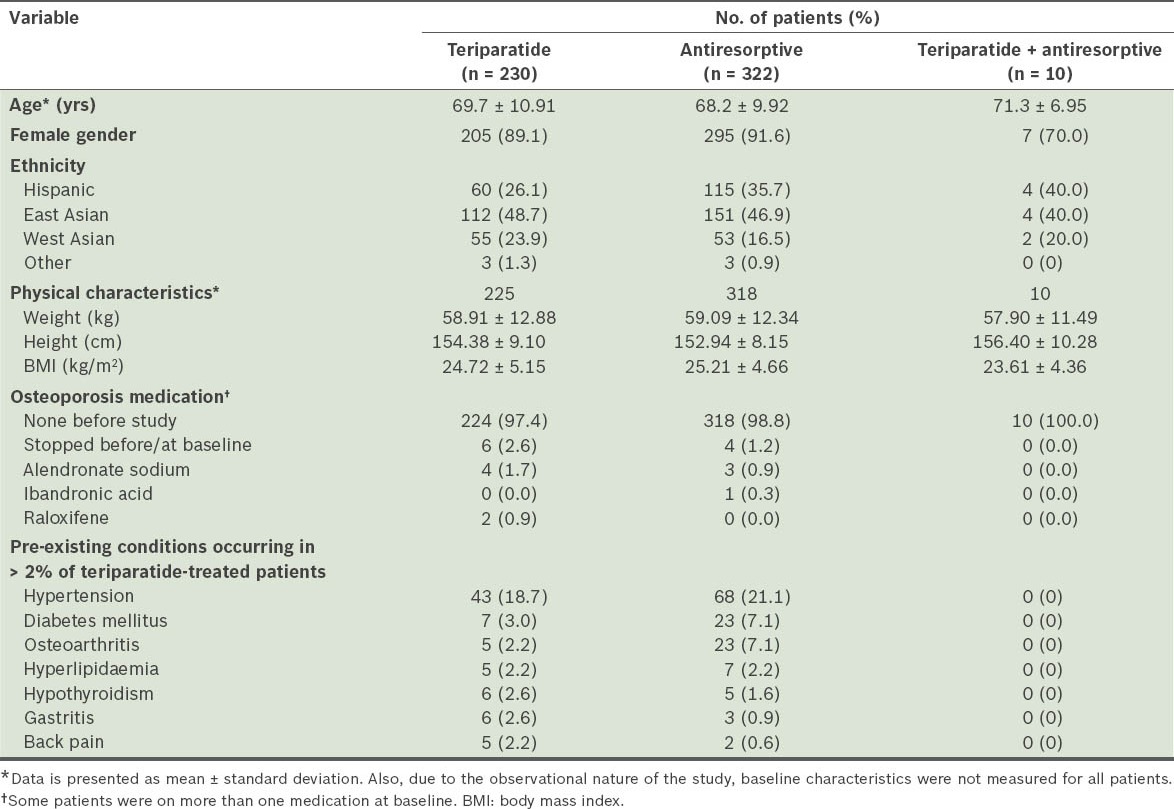
Table II.
Baseline characteristics of patients, according to the Back Pain Questionnaire.
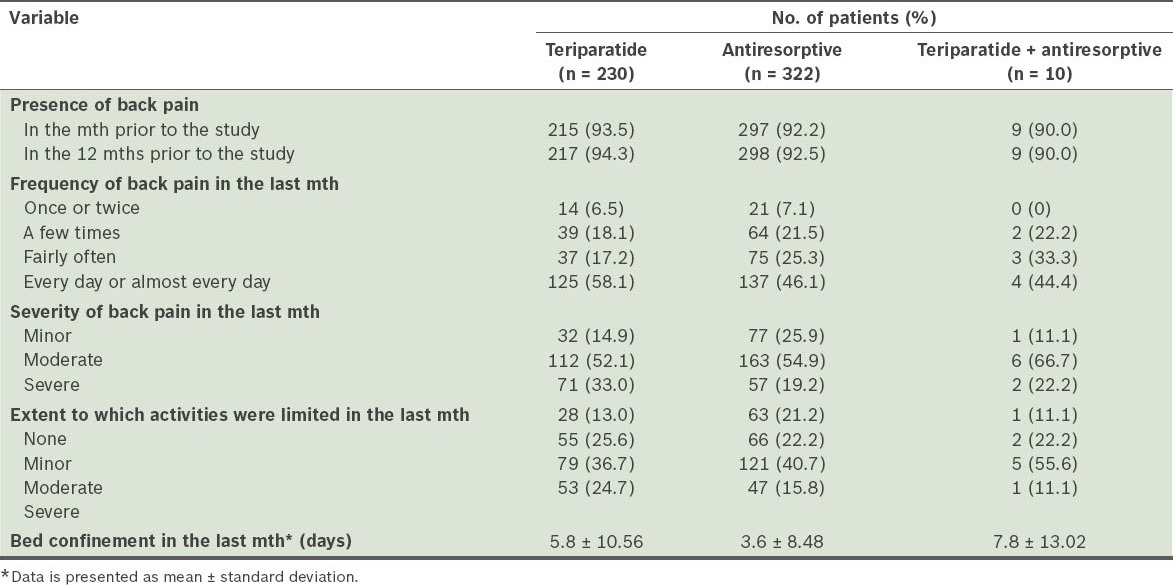
Table III.
Baseline characteristics of the monotherapy teriparatide (n = 230) and antiresorptive (n = 322) groups.
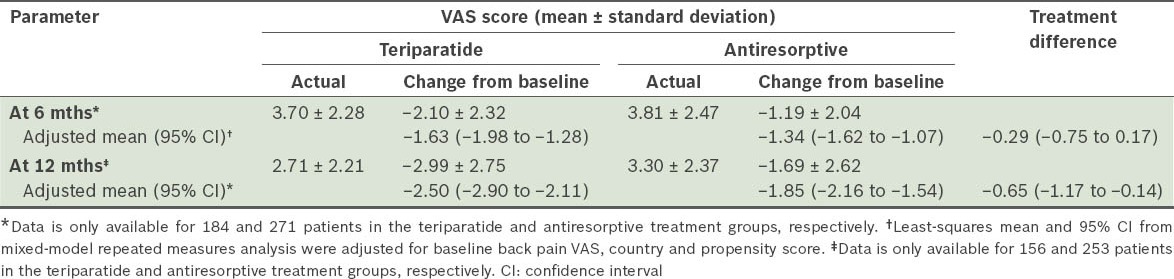
The incidences of new/worsening back pain for the monotherapy cohort (i.e. teriparatide, and antiresorptive treatment groups) at 6 and 12 months are shown in Table IV. For the primary endpoint (i.e. at six months), patients treated with teriparatide and those treated with antiresorptives both had a similar risk of new/worsening back pain. The severity of back pain recorded at each visit is shown in Table V. Notably, 30.9% of patients in the teriparatide group had severe back pain at baseline, compared to 17.7% in the antiresorptive group. The odds at six months of back pain severity, with no back pain as reference and adjusted for baseline back pain severity and propensity score, were as follows: (a) mild back pain (OR 1.64, 95% CI 0.50–5.34); (b) moderate back pain (OR 1.36, 95% CI 0.40–4.66); and (c) severe back pain (OR 0.56, 95% CI 0.10–3.09). The odds of experiencing any back pain were similar in the teriparatide and antiresorptive groups at 6 (OR 0.80, 95% CI 0.38–1.67) and 12 months (OR 0.80, 95% CI 0.38–1.70). Actual measurement and changes in back pain severity using VAS at 6 and 12 months are summarised in Table VI. Back pain severity improved for both cohorts at 6 and 12 months. However, teriparatide-treated patients reported significantly greater improvement at 12 months than those treated with antiresorptives.
Table IV.
Incidence of new/worsening back pain among patients in the monotherapy teriparatide (n = 159) and antiresorptive (n = 265) groups, excluding patients with severe back pain at baseline.
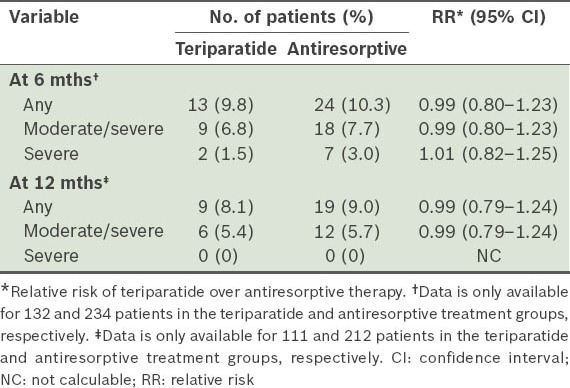
Table V.
Severity of back pain at baseline, and 6 and 12 months among patients in the monotherapy teriparatide (n = 230) and antiresorptive (n = 322) groups.
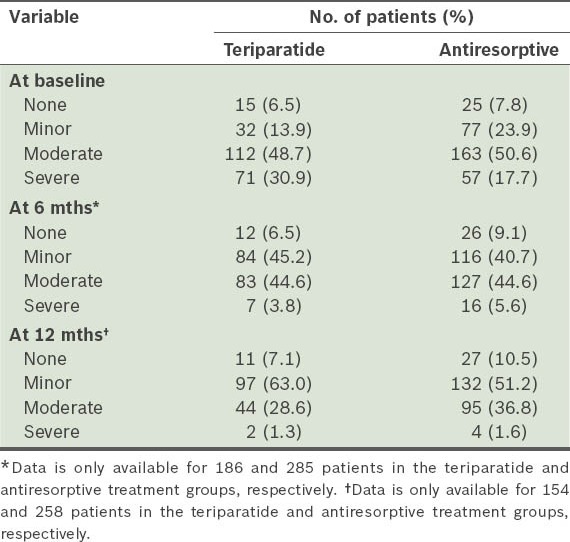
Table VI.
Change in severity of back pain according to visual analog scale (VAS) scores among patients in the monotherapy teriparatide (n = 230) and antiresorptive (n = 322) groups.
In the safety cohorts, the mean number of days on therapy was 299.8 ± 119.91 days in the teriparatide group, 329.0 ± 103.64 days in the antiresorptive group, and 329.2 ± 97.17 days for patients who received both types of treatment. An overview of adverse events is provided in Table VII. Of the deaths reported in the teriparatide (n = 3) and antiresorptive (n = 4) groups, none was related to the study drug. The most common serious adverse events (reported in ≥ 2 patients) were myocardial infarction (n = 2) in the teriparatide group and anaemia (n = 2) in the antiresorptive group. There was a low incidence of nontraumatic osteoporotic fractures in both monotherapy treatment groups.
Table VII.
Overview of adverse events.
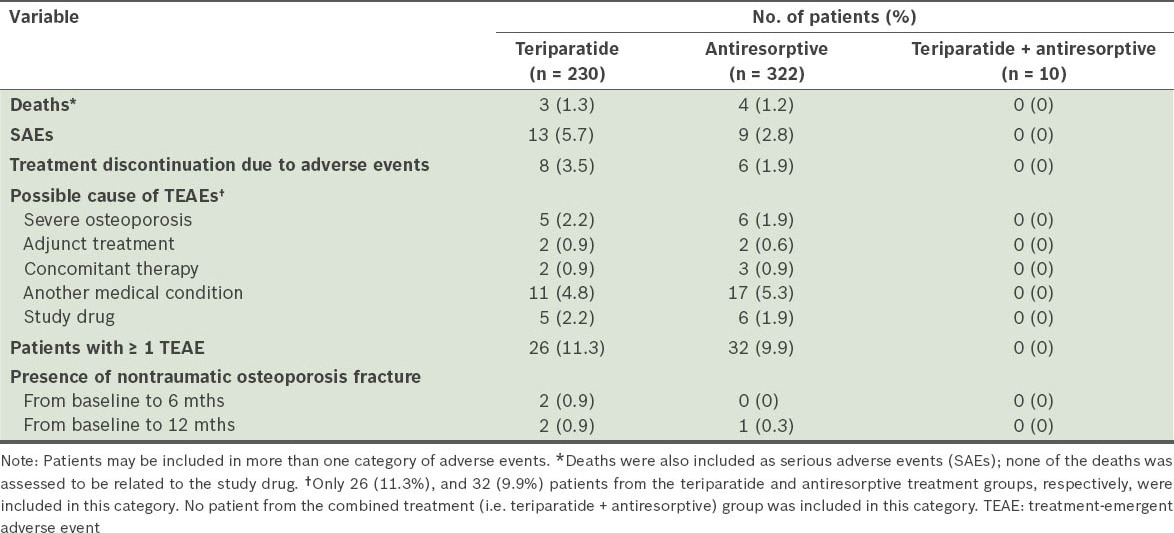
The mean EQ-5D health state VAS scores at baseline for the teriparatide and antiresorptive groups were 37.7 ± 29.15 and 45.5 ± 31.42, respectively. These scores were found to be increased for both monotherapy groups at 6 and 12 months (Table VIII). Based on responses to question 1 (i.e. “How has treatment affected you?”) on the Treatment Satisfaction Questionnaire at 6 and 12 months, a greater percentage of patients on teriparatide felt better than those on antiresorptives (Fig. 2). Based on responses to question 2 (i.e. “Please indicate how satisfied you are with the medication you took during your participation in this study”), more patients on teriparatide than those on antiresorptives were either very or extremely satisfied with the treatment.
Table VIII.
European Quality of Life-5 Dimensions (EQ-5D) Questionnaire scores among patients in the monotherapy teriparatide (n = 230) and antiresorptive (n = 322) groups.
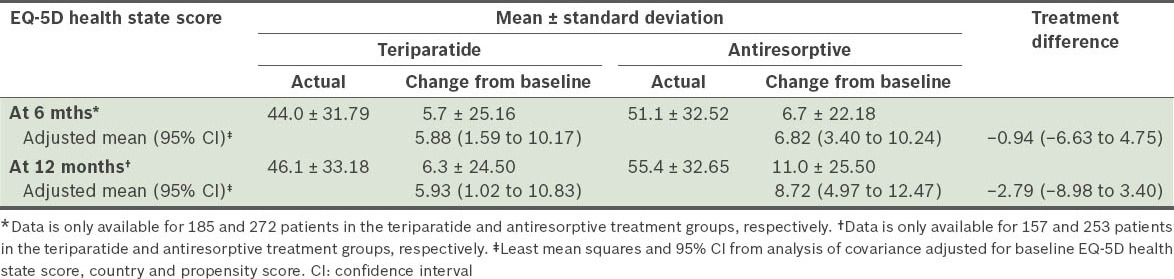
Fig. 2.
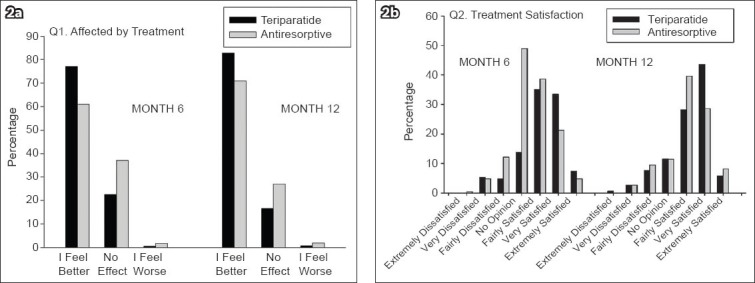
Responses to the Treatment Satisfaction Questionnaire at 6 and 12 months, with regard to the questions (a) “How has treatment affected you?”; and (b) “Please indicate how satisfied you are with the medication you took during your participation in this study”.
At six months, treatment compliance was 90.4% and 88.1% in the teriparatide and antiresorptive groups, respectively. At 12 months, treatment compliance was 91.2% and 90.7% in the teriparatide and antiresorptive groups, respectively. In the present study, we found that patients who received teriparatide discontinued treatment earlier than those who received antiresorptives. The hazard ratio for time to treatment discontinuation for the teriparatide cohort versus the antiresorptive cohort (adjusted for country and propensity score) was 1.63 (95% CI 1.07–2.49, p = 0.023).
DISCUSSION
Even without the occurrence of fractures, osteoporosis has been associated with undesirable consequences such as chronic pain, impaired physical ability, reduced social activity, poor well-being and depressed mood, which overall lead to a reduced quality of life. Therefore, studying the impact of medications on the improvement of back pain and quality of life is particularly relevant to the treatment of osteoporosis.
The results of the present study suggest that patients treated with teriparatide who presented with, at worst, moderate back pain at baseline had a similar risk of new/worsening back pain as those who were treated with antiresorptive therapy for six months. Measured using VAS, back pain severity was found to be improved in both the monotherapy treatment groups at 6 and 12 months, which corresponded to findings of early reduction of back pain in several clinical studies.(7,17,19) With regard to the EQ-5D health state score, no significant difference between the monotherapy teriparatide and antiresorptive treatment groups was observed. Compared to patients treated with antiresorptives, more patients treated with teriparatide felt better and were at least very satisfied with their treatment at 6 and 12 months. In our study, there was a low incidence of nontraumatic osteoporotic fractures and a relatively high compliance rate in both treatment groups, and both treatments were generally well tolerated.
In the present study, baseline demographics were generally worse for patients in the teriparatide treatment group; they reported more severe back pain, extreme pain/discomfort and extreme anxiety/depression, and were more often confined to bed. Although RRs were adjusted for baseline severity, country and propensity score, it was still quite difficult to fully assess whether propensity score adjustment fully addressed the selection bias. This should therefore be taken into account when interpreting the results of our study.
In the meta-analysis of randomised controlled trials by Nevitt et al,(17) the RR of any back pain was 0.66 (95% CI 0.55–0.80) for pooled teriparatide versus pooled comparator. In a study by Body et al,(30) the first trial to compare the efficacy of teriparatide and alendronate, 5.5% and 19.2% of patients treated with teriparatide and alendronate, respectively, reported new or worsened back pain (p = 0.012). Patients were treated for a median duration of 14 months in Body et al’s trial.(30) In the present study, the corresponding values were 9.8% for patients receiving teriparatide and 10.3% for patients receiving antiresorptives at six months, and 8.1% and 9.0%, respectively, at 12 months.
The results of the present study are consistent with that of the randomised controlled study by Hadji et al,(19) which compared teriparatide against risedronate. In our study, patients in both treatment groups experienced significant reductions in back pain, although there were no differences between the treatment groups with regard to the primary endpoint of proportion of patients who experienced ≥ 30% reduction in worst back pain at six months. Interestingly, Hadji et al also showed that, at 18 months, patients in the teriparatide group had a greater increase in BMD at the lumbar spine and femoral neck.(19) In their study, there were significantly fewer patients in the teriparatide group with ≥ 1 new vertebral fractures or ≥ 1 new or worsening vertebral fractures (p < 0.05) at 18 months, and patients in the teriparatide group also had significantly less height loss than those in the risedronate group.(19)
The main strength of observational studies is that they have less stringent entry criteria, and therefore, their results may be more applicable to the general population than that of randomised controlled trials. The main limitation of observational studies is the lack of randomisation, which may result in selection bias. In our study, although eligibility criteria required the occurrence of at least one osteoporotic fracture within the six weeks prior to entry into the study, we did not have a consistent evaluation of the origin of back pain to ascertain whether it was fully related to osteoporotic vertebral fracture. This may limit the assessment of the effect of the study drugs. However, other studies on back pain in patients with osteoporosis also present with similar concerns,(19) indicating that the correlation of back pain to osteoporosis remains a subjective parameter and cannot always be distinguished from the placebo. For instance, in a recent trial on the effects of vertebroplasty for osteoporosis on back pain,(31) the clinically meaningful response rate (i.e. ≥ 30% reduction in pain) in the control arm (i.e. received a sham surgery) was approximately 50% within one month after surgery. Furthermore, the design of the present study was not controlled for all confounding factors that could possibly cause back pain.
With regard to the patient population in our study, more patients were recruited into the antiresorptive therapy group than the teriparatide group due to the variability in recruitment selection in real-life clinical settings as opposed to a randomised trial. This larger number of patients in the antiresorptive treatment group may be due to physicians’ prescribing preferences and/or differences in patient access and affordability, such as the higher costs of teriparatide treatment, as the study drug was not sponsored. The cost of teriparatide treatment may have also led to its early discontinuation in some patients. Discontinuation of treatment, which occurred more in the teriparatide group, may have been related to patient demographics such as more severe osteoporosis, advanced age and more comorbidities, which would necessitate the discontinuation of treatment. Patients who received teriparatide were usually those with more severe osteoporosis, who would be expected to experience more back pain at baseline.
Vertebral fracture is the most common type of osteoporotic fracture and may result in acute or chronic back pain. Currently, treatment options addressing the underlying cause of pain from osteoporotic fractures are limited. While no osteoporotic medicine is indicated for the treatment of pain associated with vertebral fractures, data from these trials warrants further study. Future studies should focus on ensuring that enrolled patients have back pain due to vertebral fracture, with at least one moderate or severe vertebral fracture confirmed by a central reader. This will minimise the challenges faced in distinguishing back pain associated with vertebral fractures from back pain due to other aetiologies such as post-fracture postural fatigue.
Previous studies have demonstrated that teriparatide produced significant increases in BMD at the lumbar spine and femoral neck, and also reduced the risk for vertebral fractures as compared to risedronate, which is consistent with data that compared teriparatide with alendronate, another antiresorptive.(32-34) In retrospect, the methodology used in the present study was probably limited in its ability to accurately assess back pain specifically induced by osteoporotic vertebral fractures.
Data from the present observational trial complements the results of randomised clinical trials and allows clinicians to prospectively observe the early direct effects of teriparatide to reduce or prevent back pain, and address the limited function and quality of life issues common in patients with severe osteoporosis.
ACKNOWLEDGEMENTS
This study was sponsored by Eli Lilly and Company. We would like to acknowledge the study investigators, Eli Lilly staff and support at affiliates in participating countries, patients, families and healthcare workers. Writing assistance was provided by Eileen R Gallagher, a full-time employee of inVentiv Health Clinical (formerly PharmaNet/i3). Data from this trial was presented in a poster at the World Congress on Debates & Consensus in Bone, Muscle & Joint Diseases, Barcelona, Spain, 19–22 January 2012. A post hoc exploratory subgroup analysis of data from this trial conducted among Taiwanese patients was presented in a poster at the 2012 Annual Meeting of the Taiwanese Osteoporosis Association, Taiwan, 24–26 August 2012.
REFERENCES
- 1.Silverman SL, Piziak VK, Chen P, Misurski DA, Wagman RB. Relationship of health related quality of life to prevalent and new or worsening back pain in postmenopausal women with osteoporosis. J Rheumatol. 2005;32:2405–9. [PubMed] [Google Scholar]
- 2.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 3.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt GH, Cranney A, Griffith L, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am. 2002;31:659–79. doi: 10.1016/s0889-8529(02)00024-5. xii. [DOI] [PubMed] [Google Scholar]
- 5.Delmas PD, Recker RR, Chesnut CH, 3rd, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporosos Int. 2004;15:792–8. doi: 10.1007/s00198-004-1602-9. [DOI] [PubMed] [Google Scholar]
- 6.Russell RG, Rogers MJ, Frith JC, et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14(suppl 2):53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- 7.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Zhao JJ, Mitlak BH, et al. Recombinant human parathyroid hormone (1-34)[teriparatide]improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–41. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510–16. doi: 10.1007/s00198-004-1713-3. [DOI] [PubMed] [Google Scholar]
- 10.Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 12.Chesnut CH, 3rd, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 13.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 14.Jakob F, Oertel H, Langdahl B, et al. Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European Forsteo Observational Study. Eur J Endocrinol. 2012;166:87–97. doi: 10.1530/EJE-11-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahrleitner-Pammer A, Langdahl BL, Marin F, et al. Fracture rate and back pain during and after discontinuation of teriparatide: 36-month data from the European Forsteo Observational Study (EFOS) Osteoporos Int. 2011;22:2709–19. doi: 10.1007/s00198-010-1498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyritis G, Marin F, Barker C, et al. Back pain during different sequential treatment regimens of teriparatide: results from EUROFORS. Curr Med Res Opin. 2010;26:1799–807. doi: 10.1185/03007995.2010.488516. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt MC, Chen P, Dore RK, et al. Reduced risk of back pain following teriparatide treatment: a meta-analysis. Osteoporos Int. 2006;17:273–80. doi: 10.1007/s00198-005-2013-2. [DOI] [PubMed] [Google Scholar]
- 18.Nevitt MC, Chen P, Kiel DP, et al. Reduction in the risk of developing back pain persists at least 30 months after discontinuation of teriparatide treatment: a meta-analysis. Osteoporos Int. 2006;17:1630–7. doi: 10.1007/s00198-006-0177-z. [DOI] [PubMed] [Google Scholar]
- 19.Hadji P, Zanchetta JR, Russo L, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012;23:2141–50. doi: 10.1007/s00198-011-1856-y. [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi A, Matsumoto T, Shigeta H, et al. Effect of teriparatide on bone mineral density and biochemical markers in Japanese women with postmenopausal osteoporosis: a 6-month dose-response study. J Bone Miner Metab. 2008;26:624–34. doi: 10.1007/s00774-008-0871-3. [DOI] [PubMed] [Google Scholar]
- 21.Choi WH, Kim GS, Lim SK, et al. Comparison of teriparatide and calcitonin treatment in postmenopausal Korean women with osteoporosis. J Korean Soc Osteoporos. 2005;3:110–120. [Google Scholar]
- 22.Dai KR, Chen DC, Zhang ZL, et al. Comparison of the effects of subcutaneous teriparatide and intranasal calcitonin in treating established osteoporosis in postmenopausal Chinese women. Chin J Osteoporos Bone Miner Res. 2011;4:23–31. [Google Scholar]
- 23.Hwang JS, Tu ST, Yang TS, et al. Teriparatide vs. calcitonin in the treatment of Asian postmenopausal women with established osteoporosis. Osteoporos Int. 2006;17:373–8. doi: 10.1007/s00198-005-2002-5. [DOI] [PubMed] [Google Scholar]
- 24.Kung AW, Pasion EG, Sofiyan M, et al. A comparison of teriparatide and calcitonin therapy in postmenopausal Asian women with osteoporosis: a 6-month study. Curr Med Res Opin. 2006;22:929–37. doi: 10.1185/030079906X104768. [DOI] [PubMed] [Google Scholar]
- 25.Miyauchi A, Matsumoto T, Sugimoto T, et al. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone. 2010;47:493–502. doi: 10.1016/j.bone.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Reginster JY, Collette J, Neuprez A, et al. Role of biochemical markers of bone turnover as prognostic indicator of successful osteoporosis therapy. Bone. 2008;42:832–6. doi: 10.1016/j.bone.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Chinese Society of Osteoporosis and Bone Mineral Diseases branch. Diagnosis and treatment of primary osteoporosis guide (2011) Chin J Osteoporos Bone Miner Res. 2011;4:2–17. [Google Scholar]
- 28.Mithal A, Dhingra V, Lau E. Switzerland: International Osteoporosis Foundation; 2009. The Asian Audit: Epidemiology, costs and burden of osteoporosis in Asia 2009. [Google Scholar]
- 29.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 30.Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)]with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:4528–35. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 31.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–8. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 33.Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–39. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 34.Saag KG, Zanchetta JR, Devogelaer JP, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–55. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]


