Abstract
Although nitrogen (N) availability is known to alter constitutive resistance against herbivores, its influence on herbivore-induced responses, including signaling pathways, transcriptional signatures, and the subsequently elicited chemical defenses is poorly understood. We used the native tobacco, Nicotiana attenuata, which germinates in the postfire environment and copes with large changes in soil N during postfire succession, to compare a suite of Manduca sexta- and elicitor-induced responses in plants grown under high- and low-N (LN) supply rates. LN supply decreased relative growth rates and biomass by 35% at 40 d compared to high-N plants; furthermore, it also attenuated (by 39 and 60%) the elicitor-induced jasmonate and salicylate bursts, two N-intensive direct defenses (nicotine and trypsin proteinase inhibitors, albeit by different mechanisms), and carbon-containing nonvolatile defenses (rutin, chlorogenic acid, and diterpene glycosides), but did not affect the induced release of volatiles (cis-α-bergamotene and germacrene A), which function as indirect defenses. M. sexta and methyl jasmonate-induced transcriptional responses measured with a microarray enriched in herbivore-induced genes were also substantially reduced in plants grown under LN supply rates. In M. sexta-attacked LN plants, only 36 (45%) up-regulated and 46 (58%) down-regulated genes showed the same regulation as those in attacked high-N plants. However, transcriptional responses frequently directly countered the observed metabolic changes. Changes in a leaf's sensitivity to elicitation, an attacked leaf's waning ability to export oxylipin wound signals, and/or resource limitations in LN plants can account for the observed results, underscoring the conclusion that defense activation is a resource-intensive response.
Ecologists have long noted that nitrogen (N) availability can dramatically influence plant resistance against herbivore attack and have proposed several nonexclusive hypotheses to explain the patterns of defense compound production under different nutrient regimes: the carbon-nutrient balance (CNB; Bryant et al., 1983); growth-differentiation balance (GDB; Hermes and Mattson, 1992); resource availability (RA; Bryant et al., 1985; Coley et al., 1985); and optimal defense (OD; Rhoades, 1979). For example, the CNB hypothesis predicts that a plant should allocate relatively more to N-containing defense metabolites when it has an abundance of N at its disposal (Muzika, 1993; Gebauer et al., 1998; Mutikainen et al., 2000). Conversely, allocation to carbon (C)-based compounds should increase under conditions of N-limited growth. The limited success of these hypotheses in explaining the patterns of environmentally induced changes in defense metabolites (Karban and Baldwin, 1997; Hamilton et al., 2001), in part, reflects the dearth of our understanding of how N availability influences primary and secondary metabolism. Not only is N an important limiting resource that influences the growth and reproduction of plants, it also functions as a signal which can reprogram N and C metabolism, resource allocation, and root development. Nitrate, for example, is the most important limiting nutrient for terrestrial plants, and, in addition, it elicits a thorough reorganization of gene expression (Wang et al., 2000, 2003).
It is now widely accepted that plants recognize attack from specific herbivore species and tailor their induced direct and indirect responses accordingly. This tailoring likely results from crosstalk among a large number of signaling pathways, including jasmonic acid (JA), ethylene, abscisic acid, and salicylate (SA) (Reymond et al., 2000; Kessler and Baldwin, 2002; Kunkel and Brooks, 2002), which can recruit a large fraction of a plant's transcriptome after herbivore attack (Reymond et al., 2000; Halitschke et al., 2001, 2003; Hermsmeier et al., 2001; Winz and Baldwin, 2001; Hui et al., 2003). Surprisingly, few studies have determined how nutrient availability influences these herbivore-induced responses and their signaling. The elicitation of volatile organic compounds (VOCs) from corn (Zea mays), which are thought to mediate an indirect defense, is the best studied in this regard. Gouinguené and Turlings (2002) reported that unfertilized corn plants release significantly lower amounts of herbivore-induced VOCs than did fertilized plants. However, in the most detailed study to date, Schmelz et al. (2003) reported a negative relationship between N availability and volicitin-induced VOC emissions and demonstrated that the response was likely mediated by N sensitivity of herbivore-induced plant signaling. Plants grown under N deficiency had higher JA accumulations and ethylene sensitivity and released more VOCs upon elicitation.
Here we examine the influence of N availability on herbivore-induced direct and indirect defenses in Nicotiana attenuata, a postfire annual native of the Great Basin desert of California, Nevada, Idaho, and Utah (Goodspeed, 1954; Wells, 1959), whose herbivore-induced responses have been intensively studied with ecological, chemical, and molecular approaches (Baldwin, 2001; Kessler and Baldwin, 2002). From this work, it is clear that N. attenuata recognizes feeding by the larvae of its specialist sphingid herbivore, Manduca sexta, as evidenced by Manduca-induced patterns of hormone signaling (JA, ethylene, and SA), secondary metabolite accumulation (responsible for both direct and indirect defenses), and gene transcript accumulation (Baldwin, 2001; Baldwin et al., 2002; A. Heidel and I. Baldwin, unpublished data). The differences between herbivore-induced responses and those induced by mechanical damage or by exogenous applications of methyl JA (MeJA) are due to the introduction of fatty acid-amino acid conjugates located in the larvae's oral secretions and regurgitants (OS) into wounds during feeding (Halitschke et al., 2000, 2001, 2003).
Variation in N supply is particularly germane to N. attenuata's life history because by synchronizing its germination from long-lived seed banks in the postfire environment, it times its growth with periods of high soil N availability (Lynds and Baldwin, 1998). The plants typically persist for three growing seasons after fires (Baldwin et al., 1994; Preston and Baldwin, 1999) and during this time, the soil N supply decreases dramatically as postfire succession and leaching depletes soil reserves (Lynds and Baldwin, 1998). One of its induced direct defenses, nicotine, is known to make large demands on the plant's N budget (for review, see Baldwin, 2001); and others, such as trypsin proteinase inhibitors (TrypPI), are also likely to require large resource investments (Glawe et al., 2003; Zavala et al., 2004). Hence N. attenuata represents an ideal system with which to examine the effects of N supply rate on herbivore-induced signaling pathways, the transcriptional reorganization, and the direct and indirect defenses these pathways elicit.
To elucidate the effects of N availability on herbivore-induced responses in N. attenuata, we grew plants in hydroponic culture to quantitatively manipulate N supply at rates similar to those observed in the immediate postfire environment (high N, or HN) and 4 years after a fire (low N, or LN) (Lynds and Baldwin, 1998). We elicited plants by treating mechanical wounds with M. sexta OS and measured the timing and magnitude of the JA and SA burst, which are reliable signatures of the plant's “recognition” that likely organize much of the tailoring of the defense responses. In addition to wounding, OS applications, and herbivore attack, JA and MeJA applications are also known to elicit the accumulation of some herbivore-induced defense metabolites in N. attenuata, such as nicotine (Winz and Baldwin, 2001), TrypPI (Van Dam et al., 2001; Glawe et al., 2003; Zavala et al., 2004), VOCs (Halitschke et al., 2000; Lou and Baldwin, 2003), caffeoylputrescine, chlorogenic acid, and diterpene glycosides (DTGs; Keinänen et al., 2001; Lou and Baldwin, 2003). We measured the elicited mixture of both systemic and local responses in two phyllotactically adjacent leaves. Since the VOC release is a whole-plant (WP) response (Halitschke et al., 2000), we measured only WP releases. To examine how herbivore-induced transcriptional responses are influenced by N supply rates, we infested plants with M. sexta larvae or elicited them with MeJA treatments and analyzed the plants' responses with a 789-oligonucleotide microarray enriched in herbivore-induced N. attentuata genes (A. Heidel and I. Baldwin, unpublished data; Voelckel and Baldwin, 2004).
RESULTS
N Deficiency Reduces Plant Growth and Wound/OS-Induced JA and SA
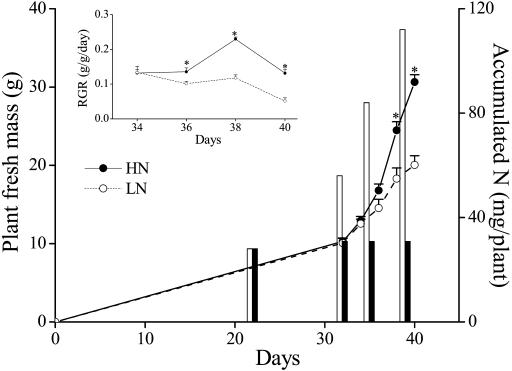
HN supply significantly increased WP fresh mass from day 36 to the end of the experiment (all values of t = 3.95, degrees of freedom (df) = 91, P < 0.05; Fig. 1). The masses of HN plants were 35% larger than those of plants grown under LN supply (overall comparison for masses from 36 to 40 d). Statistically significant differences in WP fresh mass between the two N treatments started on day 38, 6 d after the N supply treatments, but significant differences in plant relative growth rate (RGR) (Fig. 1, inset) were observed 2 d earlier.
Figure 1.
Mean (±se) WP fresh mass (lines) and accumulated nitrogen (N) (bars) per plant, and relative growth rate (RGR: inset) of N. attenuata plants grown under low nitrogen (LN) and high nitrogen (HN) supply rates. Asterisks indicate significant differences between members of a pair (P < 0.05, t test).
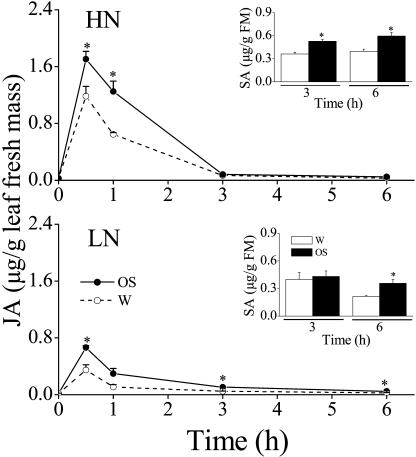
As is consistent with early studies (Halitschke et al., 2000; Schittko et al., 2000), application of M. sexta OS to puncture wounds in N. attenuata leaves caused a transient JA burst in both HN and LN treatments, which reached maximum values at 30 min (Fig. 2). Surprisingly, the JA burst was significantly attenuated in both OS and water treatments of LN plants (all t = 2.86, df = 52, P < 0.05; Fig. 2) as well as in constitutive JA levels (t = 3.32, df = 4, P < 0.05). In LN plants, the maximum values measured at 30 min in OS and water treatments were 39 and 29% of those observed in HN plants. Moreover, the JA burst in LN plants, compared to that of HN plants, did not wane as rapidly, and significant differences were still observed 6 h after elicitation (Fig. 2).
Figure 2.
Mean (±se) jasmonic acid (JA) and salicylic acid (SA, insets) concentrations in leaves of 40-d-old N. attenuata plants grown under high nitrogen (HN) and low nitrogen (LN) supply rates. Leaves were wounded with a fabric pattern wheel and the resulting puncture wounds immediately treated with either 20 μL of M. sexta oral secretions (OS) or deionized water (W) at time 0. Asterisks indicate significant differences between members of a pair (P < 0.05, t test).
Application of M. sexta OS to puncture wounds in HN and LN N. attenuata leaves also resulted in an SA burst (Fig. 2, insets). The SA burst observed in LN plants, however, was delayed compared to that in HN plants in which statistically significant differences in SA concentrations between OS and water treatments were found at 3 h after treatment. Moreover, the SA levels at 6 h in both OS (t = 3.77, df = 4, P < 0.05) and water (t = 5.99, df = 4, P < 0.05) treatments of LN plants were significantly lower than those observed in HN plants.
N Deficiency Attenuates Nicotine- and TrypPI-Induced Responses But Not Caffeoylputrescine
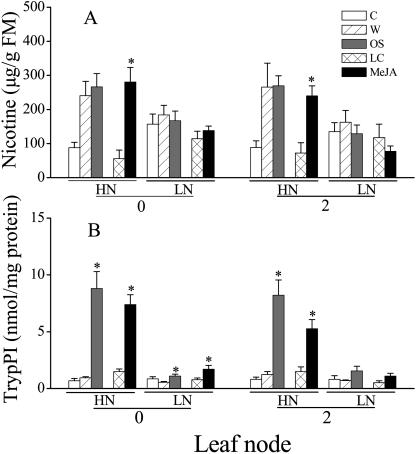
We measured the effects of N supply on three N-containing secondary metabolites: nicotine, TrypPI, and caffeoylputrescine. As previously described (Lou and Baldwin, 2003), wounding plus water (W) or OS, and MeJA significantly increased nicotine levels in locally and systemically treated leaves in HN plants but not in LN plants (Fig. 3A). Compared to HN plants, LN plants have higher constitutive nicotine levels (C treatment, t = −2.59, df = 18, P < 0.05; 1.82-fold) but lower induced levels (W, OS, and MeJA treatments, t = 5.78, df = 56, P < 0.05; 0.55-fold; Fig. 3A), reflecting the higher allometrically determined nicotine WP accumulation values of N-deficient Nicotiana plants (Ohnmeiss and Baldwin, 1994; Baldwin, 1999).
Figure 3.
Mean (±se) nicotine (A, μg/g fresh mass) and trypsin proteinase inhibitor activity (B, TrypPI) from 35-d-old N. attenuata plants grown under low nitrogen (LN) and high nitrogen (HN) supply rates. Leaves growing at nodes 0 (systemic) and 2 (local) were analyzed 3 d after leaves at nodes 2 and 3 were either treated with 20 μL of lanolin containing 150 μg MeJA (MeJA), in 20 μL of pure lanolin (LC), or wounded and treated with 40 μL of M. sexta oral secretions (OS), 40 μL of deionized water (W), or were left unwounded and untreated (C). Asterisks indicate significant differences between members of a treatment and control pair (LC versus MeJA; OS versus W; P < 0.05, t test).
Similar results were also found for TrypPI (Fig. 3B). Although OS- and MeJA-elicitation significantly increased TrypPI in both HN and LN plants from similar constitutive TrypPI levels (C treatment, t = 0.38, df = 18, P > 0.05), the increase was dramatically larger (5.48-fold) in HN plants (Fig. 3B).
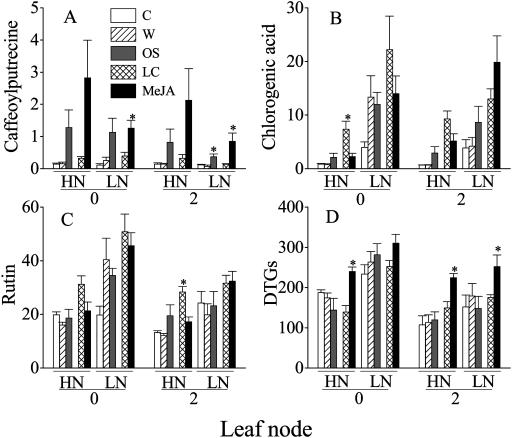
Unlike the responses observed in nicotine and TrypPI, caffeoylputrescine, another N-containing secondary metabolite, was not significantly influenced by N availability (Fig. 4A). No significant differences between the two N treatments were found in either constitutive (C treatment, t = 0.76, df = 18, P > 0.05) or elicited (OS and MeJA treatments, t = 1.92, df = 38, P > 0.05) caffeoylputrescine levels. Although MeJA and OS treatments elicited significant increases in LN plants, the response in HN plants was not significant due to high within-replicate variances (Fig. 4A).
Figure 4.
Mean (±se) caffeoylputrescine (A), chlorogenic acid (B), rutin (C) and diterpene glycosides (DTGs) from 35-d-old N. attenuata plants grown under low nitrogen (LN) and high nitrogen (HN) supply rates. Leaves growing at nodes 0 (systemic) and 2 (local) were analyzed 3 d after leaves at nodes 2 and 3 were either treated with 20 μL of lanolin containing 150 μg MeJA (MeJA) in 20 μL of pure lanolin (LC), or wounded and treated with 40 μL of M. sexta oral secretions (OS), 40 μL of deionized water (W), or left unwounded and untreated (C). Asterisks indicate significant differences between members of a treatment and control pair (LC versus MeJA; OS versus W; P < 0.05, t test).
N Deficiency Increases Constitutive C-Containing Secondary Metabolites
Three C-containing secondary metabolites (chlorogenic acid, rutin, and DTGs, which are produced in large quantities in the leaves of N. attenuata plants [Keinanen et al., 2001]) were measured in elicited and unelicited LN and HN plants. Consistent with the prediction of CNB theory (Bryant et al., 1983), LN plants had significantly higher constitutive chlorogenic acid (C, LC, and W treatments, t = 3.60, df = 58, P < 0.05), rutin (C, LC, and W treatments, t = −3.41, df = 58, P < 0.05), and DTG (C, LC, and W treatments, t = 4.51, df = 58, P < 0.05) concentrations than those observed in HN plants. MeJA elicitation significantly decreased chlorogenic acid (Fig. 4B) and rutin (Fig. 4C) levels only in HN plants, which resulted in significantly lower concentrations of these two chemicals in HN MeJA-treated plants than in LN MeJA-treated plants (chlorogenic acid, t = −4.34, df = 18, P < 0.05; rutin, t = −4.90, df = 18, P < 0.05). Although MeJA elicitation increased DTG concentrations (Fig. 4D) in both HN and LN plants, the induced DTG levels were higher in LN plants than in HN plants (t = −2.35, df = 18, P < 0.05).
N Supply Does Not Influence OS- and MeJA-Induced VOCs
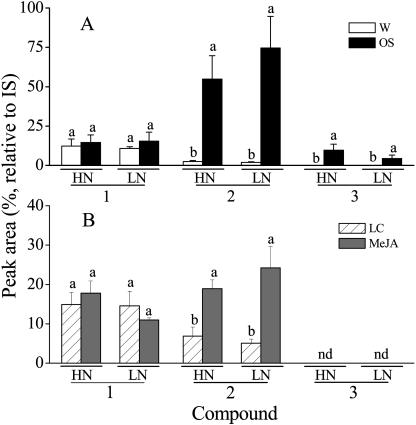
We compared the three most abundant VOCs in the headspace of HN and LN plants: limonene, cis-α-bergamotene, and germacrene A. As previously reported (Lou and Baldwin, 2003), cis-α-bergamotene was significantly increased by MeJA and OS elicitations, but germacrene A was induced only by OS treatment, and no significant differences were found between the two N supply treatments in amounts released in any of the three compounds (Fig. 5).
Figure 5.
Mean (±se) peak areas of limonene (1); cis-α-bergamotene (2); germacrene A (3) (percent, relative to internal standard [IS]) in the headspace of 36-d-old N. attenuata plants grown under low nitrogen (LN) and high nitrogen (HN) supply rates. Headspace samples were collected for 7 h, starting 24 h after treatment with 20 μL of lanolin containing 150 μg MeJA (MeJA), in 20 μL of pure lanolin (LC), or after wounding and treatment with 40 μL of M. sexta oral secretions (OS) or with 40 μL of deionized water (W) to leaves at nodes 2 and 3. For each compound, letters indicate significant differences among treatments (P < 0.05, Fisher post hoc test).
N Supply Dramatically Alters Caterpillar- and MeJA-Induced Transcriptional Responses
A complete listing of the mean (±se) expression ratios (ERs) of all spotted genes from all treatments of HN and LN plants can be found in the supplemental material (Supplemental Table I, available at www.plantphysiol.org). The observed expression patterns elicited by MeJA and caterpillar feeding in both HN and LN N. attenuata plants reflect the basic trends previously described for the herbivore-induced transcriptome (Halitschke et al., 2003; Hui et al., 2003). Overall, photosynthesis-related genes (small subunit of Rubisco, light-harvesting complex protein) were down-regulated; genes involved in defense-signaling processes (lipoxygenases LOX; ACC oxidase ACO) and secondary metabolism (e.g. a suite of PI genes; polyphenol oxidases PPO; S-adenosylmethionine decarboxylase) were strongly up-regulated in all treatments. However, N availability strongly influenced both the quantitative and qualitative expression of this basic response.
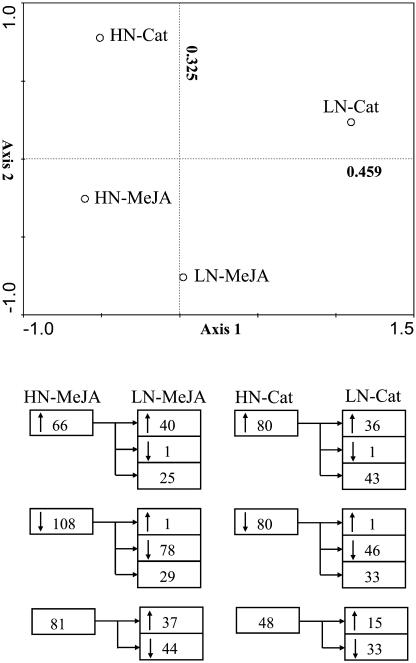
The analysis revealed MeJA- and M. sexta -specific changes in expression levels of 174 (31%) and 160 (28%), respectively, of the 568 genes spotted on the array in HN plants. MeJA elicitation significantly up-regulated the transcripts of 66 genes and significantly down-regulated 108 genes, while attack from M. sexta both up- and down-regulated transcripts each of 80 genes (Fig. 6B). These responses were largely maintained in LN plants, but differences were found, especially in M. sexta-attacked plants. Forty (61%) up-regulated genes and 78 (72%) down-regulated genes in LN plants with MeJA treatment showed the same transcriptional regulation as those in HN plants. For M. sexta-attacked LN plants, only 36 (45%) up-regulated genes and 46 (58%) down-regulated genes showed the same regulation as genes in HN plants (Fig. 6B).
Figure 6.
PCA (top) and number of up-regulated (↑), down-regulated (↓) or not-regulated ( ) genes (bottom) observed in a microarray analysis of 40-d-old N. attenuata plants grown under low nitrogen (LN) and high nitrogen (HN) supply rates, 24 h after application of 30 μL of lanolin containing 225 μg methyl jasmonate (MeJA) to leaves growing at nodes 1, 2, and 3 or attacked by eight M. sexta larvae (one second instar larva per leaf, Cat). Each array was hybridized with Cy3-labeled cDNA generated from MeJA- or Cat-treated plants and Cy5-labeled cDNA from their corresponding controls (LC or C). Principal component axis 1 accounted for 45.9% and axis 2, 32.5% of the total variation in the transcriptional responses.
We conducted a principal component analysis (PCA) with log-transformed ERs to compare the total transcriptional “signatures” that each treatment inscribes in N. attenuata's transcriptome (Fig. 6A). From this PCA, the caterpillar-feeding (Cat) treatments of HN and LN plants were clearly distinguished by their location along axes 1 and 2, which together accounted for 78% of variance in the data. The similarity between MeJA-elicited transcriptional responses of HN and LN plants was more striking than that between HN-Cat and LN-Cat responses, and higher than that observed between HN-MeJA and HN-Cat and between LN-MeJA and LN-Cat (Fig. 6A). These differences likely reflect the large differences in JA signaling between HN-Cat and LN-Cat treatments (Fig. 2). In contrast, plants in the HN-MeJA and LN-MeJA treatments both received the same amount of MeJA, which may account for their greater similarity.
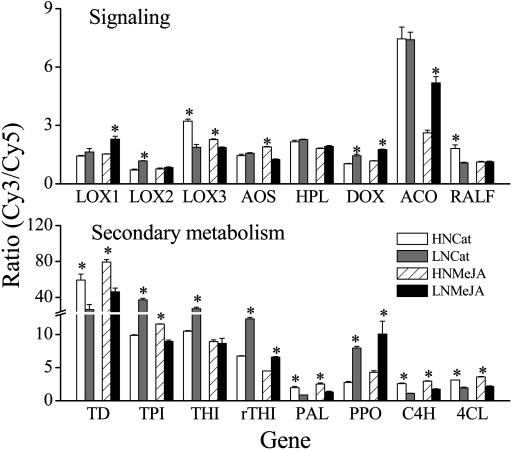
N supply clearly influenced M. sexta- and MeJA-elicited gene expression, and examples of genes involved in signaling and their responses in secondary metabolism have been selected (Fig. 7) to illustrate the two major patterns of N supply effects: those consistent with the observed responses in elicited JA and SA, namely, those in which HN supply increased elicited responses, and those with the opposite pattern of regulation. The expressions of genes involved in JA biosynthesis (lipoxygenase 3, lox3; allene oxide synthase, aos) were more up-regulated in HN plants than in LN plants, which is consistent with the measures of endogenous JA (Fig. 2). Interestingly, other N. attenuata oxylipin-related genes not directly related to JA biosynthesis and one ethylene-related gene showed the opposite pattern of regulation (lox1; lox2; α-dioxygenase, α-dox; ACC oxidase, ACO) or were not regulated (hydroperoxide lyase, hpl; Fig. 7). Genes that are known to be regulated by JA and require a functional JA cascade for their elicitation (Halitschke and Baldwin, 2003) such as threonine deaminase (TD), as well as genes not previously known to be regulated by JA (rapid alkalinization factor, RALF; flower-specific thionin, γ-thionin) but which are involved in growth responses to herbivore responses and pathogen resistance, are also more strongly elicited in HN plants. Interestingly, the pattern of regulation displayed by a group of genes (phenylalanine ammonia lyase, pal; cinnamic acid 4-hydroxylase, c4h; 4-courmarate-CoA ligase, 4cl) involved in the synthesis of phenolics was the opposite of the pattern displayed by the accumulation of the phenolic end-products. While HN supply increased the elicited transcripts, concentrations of phenolics in N. attenuata (Fig. 4) as well as in many other plant species (Dudt and Shure, 1994; Peňuelas et al., 1997; Coviella et al., 2002) tends to be higher in LN plants. Interestingly, SA, a phenolic compound involved in pathogen signaling, had the opposite pattern of regulation (Fig. 2). On the other hand, transcript levels of a polyphenol oxidase gene (PPO) that mediates the oxidation of phenolics to quinines are more strongly elicited under LN conditions. A similar disconnect between transcripts and products was found in TrypPIs. HN supply increased TrypPI elicitation (Fig. 3B) but decreased transcripts (Fig. 7, where TrypPI is labeled TPI).
Figure 7.
Comparison mean (±se) ERs of four replicate oligonucleotides of selected signaling- and secondary-metabolism-related genes of 40-d-old N. attenuata plants grown under low nitrogen (LN) and high nitrogen (HN) supply rates, 24 h after application of 30 μL of lanolin containing 225 μg methyl jasmonate (MeJA) to leaves growing at nodes 1, 2, and 3, or attacked by eight M. sexta larvae (one second instar larva per leaf, Cat). Gene names and accession numbers: LOX1, NaLOX1 (AY254347); LOX2, NaLOX2 (AY254348); LOX3, NaLOX3 (AY254349); AOS, NaAOS (AJ295274); HPL, NaHPL (AJ414400); DOX, Naα-dioxygenase (AW191821); ACO, NaACO (AY426756); RALF, NaRALF, rapid alkalinization factor (AF407278); TD, NaTD, threonine deaminase (AW191811); TPI, NaTPI, trypsin proteinase inhibitor (AY426751); THI, Na-thionin (BU494528); γTHI, Na-γ-thionin, (AY456268); PAL, Na-Phe ammonia lyase (M90692); PPO, polyphenyl oxidase (A27686); C4H, cinnamic acid 4-hydroxylase (AF212318); 4CL, 4-coumarate-CoA ligase (M62755). Asterisks indicate significant differences between members of a treatment and control pair (LC versus MeJA; OS versus W; P < 0.05, t test).
DISCUSSION
To compare the effect of N supply on herbivore-induced responses in N. attenuata, we examined the timing of the JA and SA bursts, transcriptional responses, and the subsequently elicited changes in defense chemicals in plants grown under the range of N supply rates typical of those found in the plant's native habitat. To minimize the influence of N effects on plant development, plants were grown so that they were at the same developmental stage and size but differed in their RGRs when attacked by M. sexta or treated with M. sexta OS or MeJA.
These experiments demonstrate that whereas statistically significant JA and SA bursts occurred in response to OS elicitation in both N supply treatments, the JA (OS treatment at 30 min) and SA (OS treatment at 6 h) responses in LN plants were only 39% and 60% of those observed in HN plants (Fig. 2). This decrease in these elicited phytohormone accumulations corresponded to decreases in the expression of levels of JA- and SA-related genes in LN plants (Fig. 7). The overall effects of N supply on the induced transcriptional responses measured by the microarrays resembled the effects of N supply on the OS-elicited JA and SA bursts: growth under LN supply substantially decreased the number of genes whose expression was up-regulated (Fig. 6). However, the influence of N on particular genes thought to be important in mediating herbivore-elicited responses was highly variable and defied simple categorization (Fig. 7), as was true for the effects on the elicited accumulations of secondary metabolites.
The effects of N supply on secondary metabolism differed depending on the biosynthetic pathway that produced them; whether the metabolite contained N or C did not consistently determine the patterns, as has been postulated in many treatments of the CNB theory (Bryant et al., 1983; Cipollini et al., 2002; Coviella et al., 2002). While the elicited responses of some secondary metabolites, which could be considered C- or N-intensive (constitutive levels of chlorogenic acid, rutin, and DTGs; inducible levels of nicotine and TrypPIs, respectively), followed the patterns predicted by CNB theory (with larger accumulations in plants grown under HN supply), others (inducible and constitutive levels of VOCs, caffeoylputrescine, inducible levels of DTGs, and constitutive levels of nicotine) did not. The elicited changes of the two N-intensive metabolites, nicotine and TrypPIs, which are produced in different tissues after herbivore attack to leaves, namely, roots and leaves, respectively, had patterns most consistent with CNB predictions and deserve closer scrutiny.
The attenuated JA burst in LN plants was associated with a lack of nicotine increase (Fig. 3A) and low inducibility in TrypPI (Fig. 3B), both of which require the JA burst for their activation, as has been demonstrated in N. attenuata plants transformed to silence LOX3 expression, the lipoxygenase specifically required for wound-induced JA production (Halitschke and Baldwin, 2003). However, the lack of OS-elicited nicotine and TrypPI responses in LN plants was not likely a result of attenuated JA responses in these plants (Fig. 2), because similar results were also found in MeJA-treated LN plants (Fig. 3), which were treated with the same amount of MeJA as was applied to HN plants. We propose that decreases in the plant's ability to transport the oxylipin signal to the sites of metabolite biosynthesis and/or N limitations on metabolite biosynthesis are responsible for the observed results.
Nicotine is synthesized in the roots and activated by JA (or another LOX3 produced oxylipin) imported from damaged leaves in the phloem (Zhang and Baldwin, 1997). After synthesis in the roots, nicotine is transported to the shoots in the xylem stream (Baldwin, 1999; Ohnmeiss and Baldwin, 2000). In the sibling tobacco species Nicotiana sylvestris, Ohnmeiss and Baldwin (2000) found that the cessation of significant nicotine inductions at later stages (elongation and flower stages) in ontogeny was due not to the roots' decreased ability to respond to JA but, rather, to a decline in a leaf's sensitivity to wounding and its ability to export JA from the leaves to roots. When these nonresponsive plants were supplied with extra N to their roots, their inducibility was restored, probably because increased root-sink strength facilitated the transport of the wound-induced JA from the leaves to the roots (Ohnmeiss and Baldwin, 2000). Similar mechanisms may account for the lack of induced nicotine responses in LN plants here. Given that MeJA elicitation of leaves also did not increase nicotine accumulations in LN plants, inhibited signal transport to the roots may account for the lack of elicitation; this hypothesis could be falsified by eliciting the roots of N-limited plants with MeJA. N limitations to nicotine biosynthesis may also account for the lack of response in N-limited plants. Induced nicotine accumulation requires a substantial fraction (8%) of WP N, an investment that cannot be recouped by metabolism (Baldwin, 2001). However, as has been documented in N. sylvestris (Ohnmeiss and Baldwin, 1994), N limitation increases constitutive nicotine concentrations (Fig. 3A) and increases the allometrically determined WP nicotine accumulation set-points. Hence, rather than decreasing allocation of N to nicotine biosynthesis as growth slows due to N limitation, the amount of N allocated to nicotine production increases under N stress (Ohnmeiss and Baldwin, 1994). Nicotiana plants must have a means of giving priority to the allocation of N to nicotine biosynthesis during N-limited growth. Resource limitations, on the other hand, are more likely to account for the dramatic decrease in constitutive and inducible TrypPI accumulations in LN plants (Fig. 3B).
Because TrypPIs are synthesized in leaves, the dramatic attenuation in LN plants elicited by MeJA applied to the leaves cannot be due to inhibition of transport of the wound signal. TrypPIs are likely to make large demands on a plant's N budget, but quantitative measures have not been made. However, TrypPI expression is known to be responsible for decreased growth and seed production in N. attenuata (Glawe et al., 2003; Zavala et al., 2004), which may reflect N-based tradeoffs between growth and TrypPI production. Surprisingly, the expression levels of TrypPI transcripts in OS-elicited LN plants were significantly higher than those in HN plants (Fig. 7). Higher concentrations of soluble sugars such as Glc, Fru, and Suc, in the leaves of LN plants (Rufty et al., 1988; Paul and Driscoll, 1997) are known to enhance the expression of wound-induced PI genes as well as the expression of vegetable storage proteins in other species (Johnson and Ryan, 1990; Mason et al., 1992). A similar disconnect between metabolite and transcript accumulation was found in the accumulation of phenolic metabolites (chlorogenic acid and rutin) and their associated biosynthetic transcripts (Fig. 7), which, in turn, may also be responsive to sugar signaling.
The value of classifying metabolites as emerging from N- or C-intensive metabolic pathways is challenged by metabolites such as caffeoylputrescine. These include both C-intensive phenolic as well as N-intensive amine components (Keinänen et al., 2001). Caffeoylputrescine elicitation (Fig. 4), which is coordinately expressed with nicotine in HN plants, was not attenuated in LN plants. This suggests that elicited increases in putrescine production, which supports the synthesis of the 5-membered ring of nicotine, do not limit induced nicotine accumulations in LN plants. Limitations of the flux of metabolites supporting the synthesis of the 6-membered ring of nicotine, nicotinic acid, may more significantly constrain induced nicotine production in LN plants.
Both MeJA and M. sexta-OS elicitation cause N. attenuata plants to release VOCs, which function as an indirect defense by attracting the natural enemies of the herbivores and helping them locate their prey (Kessler and Baldwin, 2002). Biotic (plant species, cultivar, developmental stage, damage tissue; herbivore species, age, feeding habitat, etc.; Loughrin et al., 1995; Gouinguené et al., 2001) and abiotic (light, temperature, humidity, nutrient, etc.; Takabayashi et al., 1994; Schmelz et al., 2001, 2003; Gouinguené and Turlings, 2002) factors are known to alter the release of herbivore-induced VOCs in many plants; and corn is perhaps the best-studied example.
The results from corn, however, are the opposite of those from N. attenuata. In corn, N deficiency significantly enhances the emission of VOCs, and this release is proportional to elicited JA and ethylene signaling (Schmelz et al., 2003). In contrast, N supply does not influence the elicited release of VOCs in N. attenuata (Fig. 5), a response that has been demonstrated to function in nature in this species (Kessler and Baldwin, 2001). This insensitivity of the VOC release to N supply suggests that elicitation is mediated by signals in addition to JA, which is reduced in LN plants (Fig. 2). The requirement for LOX3-derived signals in addition to JA for VOC elicitation in N. attenuata is consistent with previously reported results (Halitschke et al., 2000; Halitschke and Baldwin, 2003). Because relative to the resource requirements for nicotine (Baldwin, 2001) and TrypPI (Glawe et al., 2003; Zavala et al., 2004) production the resource requirements of the release of VOCs are low, with quantities of emitted compounds less than one-fifth of those released from flowers for pollinator attractions (Halitschke et al., 2000), resources are unlikely to influence VOC production.
In summary, N-limited growth significantly changes N. attenuata plants' ability to respond to M. sexta-OS or MeJA elicitations at all levels including signaling pathways, transcriptional responses, and defense chemicals. None of the current theories on resource limitations to secondary metabolism (CNB, Bryant et al., 1983; GDB, Hermes and Mattson, 1992; RA, Coley et al., 1985) account for all of the observed responses, suggesting that a more detailed physiological and biochemically informed model is required. These models should be framed in the light of evolutionary models, such as the tissue value theory (McKey, 1974), OD theory (Rhoades, 1979), and plant apparency (Feeny, 1976), which unite evolutionary predictions about the fitness value of different plant tissues growing in different environments with their defense allocation. The mechanisms that elicit these responses have clearly been subjected to natural selection, and the plant's evolutionary context will be essential for our mechanistic understanding.
MATERIALS AND METHODS
Plant Growth and RGR Measurement
An inbred genotype of Nicotiana attenuata Torr. ex Wats. (synonymous with Nicotiana torreyana Nelson and Macbr.; Solanaceae), originally collected from southwestern Utah in 1988, was used for all experiments. Seeds were sterilized and germinated on agar after soaking with a 1:50 (w/v) dilution of liquid smoke (House of Herbs, Passaic, NY). Ten-day-old seedlings were planted into soil in Teku pots (Waalwijk, The Netherlands) and 7 d later were transferred to 28-L communal hydroponic boxes with a nutrient solution consisting of 0.292 g/L of Peter's Hydrosol (W.R. Grace, Fogelsville, PA) and 0.193 g/L of Ca(NO3)2. After an adaptation period of 5 d, seedlings were transferred to individual 1-L hydroponic chambers containing a no-N hydroponic solution (Baldwin et al., 1994) with 2 mL of 1 m KNO3. Ten days later, plants (now 32-d-old) were randomly assigned to two groups and each plant was placed in a new 1-L hydroponic chamber containing no-N hydroponic solution. This period of identical growth conditions ensured that all plants were in the same developmental stage at the start of the experiment. One group received 2 mL of 1 m KNO3 to each chamber, to initiate the HN treatment, and the other received 0.2 mL of 1 m KNO3, to initiate the LN treatment. Three and 7 d later, HN plants received N supplements of 1 mL of 1 m NH4NO3, respectively, to simulate the high NH4 contents that characterize recently burned soils (Lynds and Baldwin, 1998). Total N accumulated in each plant over the experimental period is depicted in Figure 1. Plants were grown in a growth chamber with a photoperiodic cycle programmed for 16 h of light at 32°C and an 8-h dark period at 28°C with 65% constant relative humidity. To characterize plant growth under the two N supply rates, HN and LN plants were weighed every 2 d from day 32 to 40 for RGR determinations.
Plant Treatment
Plants were treated with 75 μg of MeJA in 10 μL of lanolin paste per leaf applied to two or three leaves (see detail for each experiment). Controls (lanolin) were similarly treated with 20 or 30 μL of pure lanolin. For Manduca sexta-OS-treated plants, two leaves per plant were damaged by rolling a fabric pattern wheel over the leaf surface to create a standardized mechanical wound, and 20 μL of OS [diluted 1:5 (v/v) with water] from fourth- to fifth-instar larvae were added to the puncture wounds on each leaf. Controls (water) were wounded and treated with 20 μL of deionized water. For the caterpillar feeding treatment, eight second-instar M. sexta L. (Lepidoptera: Sphingidae) larvae (eggs from North Carolina State University Insectary, Raleigh, NC) were placed on each plant (one larva per leaf). Nonmanipulated plants (controls) were included in each experiment.
Comparison of Induced Secondary Metabolites
JA and SA Burst
Thirty 40-d-old plants from each N supply treatment were selected and randomly assigned to two treatment groups: OS (20 μL of OS) or water (20 μL of deionized H2O) were added to the lamina of the second fully expanded leaf immediately after six rows of puncture wounds had been created with a fabric pattern wheel. The treated leaves were harvested at 0, 0.5, 1, 3, and 6 h after wounding and treatment, and three replicate plants for each treatment were harvested at each time. JA and SA were extracted for analysis by gas chromatography-mass spectrometry with labeled internal standards ([1,2-13C] JA and D4-SA) as described by A. Heidel and I. Baldwin (unpublished data).
TrypPI
Thirty-six-day-old plants from each N supply treatment were randomly assigned to five treatments (five plants per treatment): MeJA, lanolin, OS, water, and control. The second and third fully expanded leaves were induced and two leaves, systemic (leaf node 0, a leaf younger than the first fully expanded leaf) and local (leaf node 2, the second fully expanded leaf) were harvested (at 13:00 h) 3 d after treatment. TrypPI concentration was measured by radial diffusion assay as described in Van Dam et al. (2001) and expressed as nmol per mg of leaf protein.
Nonvolatile Secondary Metabolites
Thirty-six-day-old plants of each N supply treatment were randomly assigned to MeJA, lanolin, OS, water, and control treatments, each with five replicates, and treated and harvested as described for TrypPI measures. Leaf extracts were prepared for analysis of nicotine, rutin, caffeoylputrescine, chlorogenic acid, and DTGs by HPLC as described in Keinänen et al. (2001).
VOCs
Thirty-six-day-old plants of each N supply treatment were randomly assigned to four treatment groups (five replicates each): MeJA, lanolin, OS, and water. Plants treated as described for TrypPI measures were individually placed in 50-L glass chambers, and the VOCs released were trapped on super Q (Alltech Associates, Deerfield, IL) traps for 7 h, 24 h after elicitation (the time of maximum release after a single elicitation) and measured by gas chromatography-mass spectrometry (Lou and Baldwin, 2003). VOCs were expressed as percentages of peak areas relative to the internal standard, tetralin, per 7 h of trapping per plant.
Microarray Hybridization and Analysis
Forty-day-old plants from each N supply treatment were randomly assigned to four treatment groups (five replicates each): MeJA, lanolin, caterpillar, and control. The three youngest fully expanded leaves were treated and harvested 24 h after treatment. Pooled leaf samples were ground under liquid nitrogen, and total RNA was extracted with TRI Reagent (Sigma, St. Louis) according to the manufacturer's instructions. The MeJA- and herbivore-infested mRNA samples were labeled with Cy3 and the corresponding control (lanolin and control) mRNA samples with Cy5 according to the procedure described in Halitschke et al. (2003). The labeled samples were hybridized to the microarray (789 50-mer oligonucleotides spotted onto an epoxy-coated glass slide; Quantifoil Microtools, Jena, Germany) according to the published procedure (Halitschke et al., 2003). An Affymetrix 428 Array Scanner (Affymetrix, Santa Clara, CA) was used to scan the hybridized microarrays with sequential scanning for Cy5 cDNA and then for Cy3-labeled cDNA at a maximum resolution of 10 μm/pixel with a 16-bit depth. The images were evaluated with the AIDA Image Analyzer (Raytest Isotopenmessgeräte, Straubenhardt, Germany) software. Each image was overlaid with a grid to assess the signal strength (quantum level, QL) for both dyes from each spot. The background correction was calculated with the nonspot mode of the AIDA software package. The microarray-specific normalization factor was calculated based on the Cy5 to Cy3 total fluorescence ratio (Halitschke et al., 2003). The ratios of normalized fluorescence values for Cy3 and Cy5 of each individual spot (ER) and the mean of the four replicate spots for each cDNA were calculated. A transcript was defined as being differentially regulated, if the following three criteria were fulfilled: (1) the average ER for the four spots exceeded the thresholds (0.67 and 1.5); (2) the individual ERs were significantly different from 1 as determined by a t test; and (3) the combined signal fluorescent intensity from both Cy3 and Cy5 averaged over the four spots was greater than 1,000 QL. A complete list of all signal ratios (±se) and details on all spotted genes can be found in Supplemental Table I. To evaluate these criteria, we hybridized two microarrays with the same cDNA pools and found that 84% of the genes had the same regulation (A. Heidel and I. Baldwin, unpublished data).
Statistical Analysis
Differences in JA, SA, TrypPI, nicotine, caffeoylputrescine, chlorogenic acid, rutin, and genes ER were determined by t tests. VOCs data were log-transformed before analysis to meet requirements of normality, and then were analyzed by multivariate ANOVA (MANOVA). If the MANOVA analysis was significant (P = 0.05), univariate ANOVAs for the individual effects and Fisher lsd post hoc tests to detect significant differences between groups were conducted. Data were analyzed with STATVIEW (SAS Institute, Cary, NC). A PCA was conducted with log-transformed mean ERs of all transcripts from the four arrays (Supplemental Table I) to compare the full transcriptional response of N. attenuata to the different treatments (Canoco for Windows 4.5, Microcomputer Power, Ithaca, NY). A PCA is an unconstrained ordination technique that we used to configure microarrays in ordination space so that their distances best reflect the dissimilarities of the ERs of their transcripts.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers A27686, AF212318, AF407278, AJ295274, AJ414400, AW191811, AW191821, AY254347, AY254348, AY254349, AY426751, AY426756, AY456268, BU494528, M62755, and M90692.
Supplementary Material
Acknowledgments
We thank S. Kutschbach, W. Kröber, K. Gase, and T. Hahn for expert assistance with the microarray hybridization and reading; T. Kruegel and C. McInerney for assistance in the plant growth; E. Wheeler for editing; and the Max Planck Society for financial support.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040360.
References
- Baldwin IT (1999) Inducible nicotine production in native Nicotiana as an example of adaptive phenotypic plasticity. J Chem Ecol 25: 3–30 [Google Scholar]
- Baldwin IT (2001) An ecologically motivated analysis of plant-herbivore interactions in native tobacco. Plant Physiol 27: 1449–1458 [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Kessler A, Halitschke R (2002) Volatile signaling in plant-plant-herbivore interactions: What is real? Curr Opin Plant Biol 5: 351–354 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Staszak-Kozinski L, Davidson R (1994) Up in smoke: I. Smoke-derived germination cues for the postfire annual Nicotiana attenuata Torr. Ex Watson J Chem Ecol 20: 2345–2371 [DOI] [PubMed] [Google Scholar]
- Bryant JP, Chapin FS III, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40: 357–368 [Google Scholar]
- Bryant JP, Chapin FS III, Reichardt PB, Clausen TP (1985) Adaptation to resource availability as a determinant of chemical defense strategies in woody plants. In GA Cooper-Driver, T Swain, EE Conn, eds, Chemically Mediated Interactions between Plants and Other Organisms. Recent Advances in Phytochemistry, Vol 19. Plenum Press, New York, pp 219–237
- Cipollini ML, Paulk E, Cipollini DF (2002) Effect of nitrogen and water treatment on leaf chemistry in horsenettle (Solanum carolinense), and relationship to herbivory by flea beetles (Epitrix spp.) and tobacco hornworm (Manduca sexta). J Chem Ecol 28: 2377–2398 [DOI] [PubMed] [Google Scholar]
- Coley PD, Bryant JP, Chapin III FS (1985) Resource availability and plant antiherbivore defense. Science 230: 895–899 [DOI] [PubMed] [Google Scholar]
- Coviella CE, Stipanovic RD, Trumble JT (2002) Plant allocation to defense compounds: interactions between elevated CO2 and nitrogen in transgenic cotton plants. J Exp Bot 53: 323–331 [DOI] [PubMed] [Google Scholar]
- Dudt JF, Shure DJ (1994) The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 75: 86–98 [Google Scholar]
- Feeny P (1976) Plant apparency and chemical defense. Recent Adv Phytochem 10: 1–40 [Google Scholar]
- Gebauer R, Strain BR, Reynolds JF (1998) The effect of elevated CO2 and N availability on tissue concentrations and whole plant pools of carbon-based secondary compounds in loblolly pine (Pinus taeda). Oecologia 113: 29–36 [DOI] [PubMed] [Google Scholar]
- Glawe G, Zavala JA, Kessler A, van Dam NM, Baldwin IT (2003) Ecological costs and benefits correlated with trypsin protease inhibitor production in Nicotiana attenuata. Ecology 84: 79–90 [Google Scholar]
- Goodspeed TH (1954) The Genus Nicotiana. Chronica Botanic, Waltham, MA
- Gouinguené SP, Degen T, Turlings TCJ (2001) Variability in herbivore induced odour emissions among corn cultivars and their wild ancestors (teosinte). Chemoecology 11: 9–16 [Google Scholar]
- Gouinguené SP, Turlings TCJ (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Baldwin IT (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36: 794–807 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui D, Schmidt D, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acids. Plant Physiol 131: 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT (2000) Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124: 408–417 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125: 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR (2001) The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett 4: 86–95 [Google Scholar]
- Hermes DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67: 283–335 [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol 125: 683–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D, Iqbal J, Lehmann K, Gase K, Saluz HP, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol 131: 1877–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Ryan CA (1990) Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol 14: 527–536 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Keinänen M, Oldham NJ, Baldwin IT (2001) Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J Agric Food Chem 49: 3553–3558 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331 [DOI] [PubMed] [Google Scholar]
- Lou Y, Baldwin IT (2003) Manduca sexta recognition and resistance among allopolyploid Nicotiana host plants. Proc Natl Acad Sci USA 100: 14581–14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Tumlinson JH (1995) Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol 21: 1217–1227 [DOI] [PubMed] [Google Scholar]
- Lynds GY, Baldwin IT (1998) Fire, nitrogen, and defensive plasticity in Nicotiana attenuata. Oecologia 115: 531–540 [DOI] [PubMed] [Google Scholar]
- Mason HS, DeWald DB, Creelman RA, Mullet JE (1992) Coregulation of soybean vegetative storage protein gene expression by methyl jasmonate and soluble sugars. Plant Physiol 98: 859–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKey D (1974) Adaptive patterns in alkaloid physiology. Am Nat 108: 305–320 [Google Scholar]
- Mutikainen P, Walls M, Ovaska J, Keinanen M, Julkunen-Tiitto R, Vapaavuori E (2000) Herbivore resistance in Betula pendula: effect of fertilization, defoliation, and plant genotype. Ecology 81: 49–65 [Google Scholar]
- Muzika RM (1993) Terpenes and phenolics in response to nitrogen fertilization: a test of the carbon/nutrient balance hypothesis. Chemoecology 4: 3–7 [Google Scholar]
- Ohnmeiss TE, Baldwin IT (1994) The allometry of nitrogen allocation to growth and an inducible defense under nitrogen-limited growth. Ecology 75: 995–1002 [Google Scholar]
- Ohnmeiss TE, Baldwin IT (2000) Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology 81: 1765–1783 [Google Scholar]
- Paul MJ, Driscoll SP (1997) Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ 20: 110–116 [Google Scholar]
- Peňuelas J, Estiarte M, Llusia J (1997) Carbon-based secondary compounds at elevated CO2. Photosynthetica (Prague) 33: 313–316 [Google Scholar]
- Preston CA, Baldwin IT (1999) Positive and negative signals regulate germination in the post-fire annual, Nicotiana attenuata. Ecology 80: 481–494 [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In GA Rosenthal, DH Jansen, eds, Herbivores: Their Interactions with Plant Secondary Metabolites. Academic Press, New York, pp 3–54
- Rufty TW, Huber SC, Volk RJ (1988) Alterations in leaf carbohydrate metabolism in response to nitrogen stress. Plant Physiol 88: 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittko U, Preston CA, Baldwin IT (2000) Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta 210: 343–346 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Engelberth J, Tumlinson JH (2003) Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol 133: 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171–179 [DOI] [PubMed] [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20: 1329–1354 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27: 547–568 [DOI] [PubMed] [Google Scholar]
- Voelckel C, Baldwin IT (2004) Herbivore-induced plant vaccination: II. Array-studies reveal the transience of herbivore-specific transcriptional imprints and a distinct imprint from stress combinations. Plant J (in press) [DOI] [PubMed]
- Wang R, Guegler K, LaBrie ST, Crawford NM (2000) Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes that are induced by nitrate. Plant Cell 12: 1491–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Okamoto M, Xing X, Crawford NM (2003) Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol 132: 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PV (1959) An ecological investigation on two desert tobaccos. Ecology 40: 626–644 [Google Scholar]
- Winz R, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol 125: 2189–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT (2004) Constitutive and inducible trypsin protease inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101: 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZP, Baldwin IT (1997) Transport of [2-C-14]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203: 436–441 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.