Abstract
Background and Purpose
The short biological half-life limits the therapeutic use of glucagon-like peptide-1 (GLP-1) and chemical modification to improve the interaction of peptides with serum albumin represents an effective strategy to develop long-acting peptide analogues. Coumarin, a natural product, is known to bind tightly to plasma proteins and possesses many biological activities. Therefore, we designed and synthesized a series of coumarin-modified GLP-1 derivatives, hypothesizing that conjugation with coumarin would retain the therapeutic effects and prolong the biological half-life of the conjugates.
Experimental Approach
Four cysteine-modified GLP-1 analogues (1–4) were prepared using Gly8-GLP-1(7–36)-NH2 peptide as a starting point. These analogues were conjugated with two coumarin maleimides to yield eight compounds (conjugates 6–13) for testing. Activation of human GLP-1 receptors, stability to enzymic inactivation in plasma and binding to human albumin were assessed in vitro. In vivo, effects on oral glucose tolerance tests (OGTT) in rats and on blood glucose levels in db/db mice were studied.
Key Results
Most conjugates showed well preserved receptor activation efficacy, enhanced albumin-binding properties and improved in vitro plasma stability and conjugate 7 was selected to undergo further assessment. In rats, conjugate 7 had a longer circulating t1/2 than exendin-4 or liraglutide. A prolonged antidiabetic effect of conjugate 7 was observed after OGTT in rats and a prolonged hypoglycaemic effect in db/db mice.
Conclusions and Implications
Cysteine-specific coumarin conjugation with GLP-1 offers a useful approach to the development of long-acting incretin-based antidiabetic agents. Conjugate 7 is a promising long-lasting GLP-1 derivative deserving further investigation.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| DPP IV, dipeptidyl peptidase IV | Exendin-4 |
| GLP-1 receptor | GLP-1, glucagon-like peptide 1 |
| Insulin | Liraglutide |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013a,2013b).
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance resulting in glucose intolerance and finally, hyperglycaemia. Furthermore, T2DM is a progressive disease with declining β-cell function over time and deteriorating blood glucose levels over time (Gerich, 2003; Kahn, 2003; Wild et al., 2004; Seaquist and Ibrahim, 2010). Although several hypoglycaemic agents are widely utilized for glycaemic control, there is a continuing demand for improved treatments for diabetes (Amori et al., 2007). Incretin-based diabetic therapies are considered to be one of the most effective approaches and have been intensively developed, through the administration of exogenous glucagon-like peptide-1 (GLP-1) receptor agonists (Deacon, 2007).
GLP-1 is derived from differential post-translational processing of proglucagon in L-cells and has multiple sites of action (Orskov et al., 1994; Meier and Nauck, 2005; Drucker, 2006; Baggio and Drucker, 2007). Moreover, unlike direct administration of insulin, GLP-1 receptor agonists have a low potential to induce hypoglycaemia (Werner et al., 2010). However, in vivo, GLP-1 is rapidly degraded by enzymes such as dipeptidyl peptidase IV (DPP-IV) and rapid renal filtration in vivo and therefore is of limited value for diabetes therapy (Ahren and Schmitz, 2005). To address this issue, much research effort has been focused on the development of long-acting GLP-1 derivatives. So far this development has concentrated on two distinct strategies, increasing molecular size via chemical conjugation and the introduction of functional moieties that interact physically with biological molecules, such as serum albumin or immunoglobulin (Kim et al., 2003; Green et al., 2004; Madsen et al., 2007; Soltani et al., 2007; Chi et al., 2008b). A sustained release formulation of GLP-1 based on poly(lactic-co-glycolic acid) microsphere technology is also being investigated in the context of T2DM (Kim et al., 2007). Two GLP-1 receptor agonists, exenatide and liraglutide, were approved by the FDA for the treatment of T2DM (Madsbad, 2009). Exendin-4 (exenatide is a synthetic exendin-4) is a 39-amino acid peptide produced in the salivary glands of the Gila monster (Heloderma suspectum) which shares 53% amino acid sequence similarity with GLP-1. However, the therapeutic utility of exendin-4 is limited because of the frequent injections required (twice daily) and the associated inconvenience to patients (Kolterman et al., 2003). Liraglutide is an acylated GLP-1 analogue that shares 97% amino acid sequence identity with native GLP-1 (Drucker et al., 2010; Li et al., 2011a). It has a 16-carbon fatty-acid chain with a glutamic acid spacer, which is chemically attached to the remaining lysine residue at position 26 of the peptide precursor. These modifications prolong the plasma half-life of liraglutide following s.c. injection, in part by facilitating its binding to plasma proteins (Lovshin and Drucker, 2009).
The use of chemical modification is known to improve the physical interaction of peptides with serum albumin represents an effective strategy to develop long-acting peptide analogues (Chae et al., 2010a,2010b). However, chemical modification of GLP-1 is often accompanied by a serious loss of biological activity. Our previous work has confirmed that using Cys17-Gly8-GLP-1(7–36)-NH2 (i), Cys26-Gly8-GLP-1(7–36)-NH2 (ii), Cys34-Gly8-GLP-1(7–36)-NH2 (iii), and Cys37-Gly8-GLP-1(7–37)-NH2 (iv) and further cysteine-maleimide specific conjugations could retain the biological activity of derivatives (Han et al., 2013). In this study, we have used two 7-hydroxycoumarin maleimides (Scheme 1) for conjugation with GLP-1 to yield eight coumarin GLP-1 derivatives (conjugates 6–13), because 7-hydroxycoumarin has a relatively high affinity for human serum albumin (HSA) (Joseph et al., 2009). The structural, biological and physicochemical characteristics of these eight conjugates were explored both in vitro and in vivo.
Scheme 1.
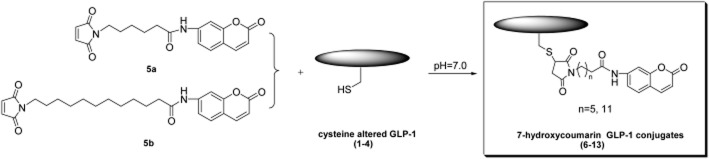
General synthetic route of coumarin GLP-1 conjugates.
Methods
Animals
All animal care and experimental protocols were approved by an ethical committee at China Pharmaceutical University and conducted according to the Laboratory Animal Management Regulations in China and adhered to the Good Practice Guide to the Administration of Substances and Removal of Blood published by the European Federation of Pharmaceutical Industries Associations and the European Centre for the Validation of Alternative Methods (Diehl et al., 2001), the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (revised 2011). The experiments were conducted in such a way that the number of animals used and their suffering was minimized. The ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010) were followed in designing the study and reporting the experiments. A total of 90 animals were used in the experiments described here.
Sprague Dawley rats (male, 250–300 g) were purchased from the Comparative Medicine Centre of Yangzhou University (Jiangsu, China). To model T2DM, diabetic C57BL/6 db/db mice (male, 6–8 weeks old, 22–25 g) were obtained from Model Animal Research Center of Nanjing University (Jiangsu, China). Animals were housed in groups of three (rat) and six (mice) in cages under controlled temperature (22 ± 2°C) and relative air humidity (set point 50%) with a reverse 12 h light: 12 h dark cycle. Tap water and standard laboratory chow were provided ad libitum throughout the study.
General synthetic route of coumarin GLP-1 conjugates 6–13
Peptides 1–4 were synthesized using the standard solid-phase peptide synthesis protocol with N-Fmoc/tBu chemistry (Chi et al., 2008a; Zhou et al., 2010; Han et al., 2013). Cysteine altered peptides (1–4, 5 μmol) were conjugated with 7-hydroxycoumarin maleimides (5a, 5b, 10 μmol) in 5 mL of 0.05 mol·L−1 sodium phosphate buffer (pH 7.0). The reaction mixture was stirred at 20°C under N2 for 1.5 h until the reaction was complete (as shown by HPLC).
GLP-1 receptor activation assay
cAMP is the primary effector of GLP-1-induced insulin secretion. After activation of the GLP-1 receptor, an increase of intracellular Ca2+ and cAMP-dependent closure of KATP channels leads to glucose-dependent secretion of insulin. Therefore, a GLP-1 receptor activation assay was employed to evaluate the in vitro receptor activation capacities of the eight conjugates (6–13). HEK293 cells stably expressing human GLP-1 receptor were used for measurement of intracellular cAMP (Sloop et al., 2010). Cells were grown at 37°C in 5% CO2 in DMEM-31053 (Invitrogen, Carlsbad, CA, USA) supplemented with 0.5% FBS, 2 mmol·L−1 L-glutamine, 50 units·mL−1 penicillin, 50 μg·mL−1 streptomycin and 20 mmol·L−1 HEPES. Two hours before testing the compounds, cells were resuspended in this medium and plated in 96-well half area, solid black microplates. Conjugates were dissolved in DMSO, diluted in medium containing 0.1% BSA fraction V substituted for 0.5% FBS, and added to cells. Following a 20 min incubation, cells were assayed for cAMP using the cAMP dynamic 2 kit with homogenous time resolved fluorescence technology (Cisbio). Fluorescence was measured according to the manufacturer's instructions using an Envision 2,104 Multilabel Reader (Perkin Elmer, Baesweiler, Germany). The potency and efficiency of the conjugates (EC50 values) were determined by sigmoidal curve fitting using GraphPad Prism version 5.0 (GraphPad, San Diego, CA, USA).
Plasma stability test in rat plasma
The resistance to proteolysis by rat plasma of the eight conjugates was evaluated by incubating samples at 37°C in rat plasma and following their degradation profiles by LC-MS/MS (Miranda et al., 2008). Rat plasma was collected from adult male Sprague Dawley rats. The plasma was stored at −20°C until use. In vitro stability of Gly8-GLP-1(7–36)-NH2, exendin-4, liraglutide and conjugates 6–13 were carried out with initial concentration of 1000 ng mL-1 of each peptide in rat plasma at 37°C. A sample (100 μL) was taken from the incubation solution at 0, 1, 2, 4, 6, 8, 12, 24, 36, 48 and 72 h time points followed by solid-phase extraction on an Oasis HLB 96-well plate (Waters). Then 20 μL of the extract was injected into the LC-MS/MS system under identical analytical conditions to those previously described (Han et al., 2013).
Physicochemical characterization of derivatives 6–13
Coumarin conjugation alters the physicochemical characteristics of peptides, especially their specific albumin-binding property. The albumin-binding characteristics of Gly8-GLP-1(7–36)-NH2, exendin-4, liraglutide and conjugates 6–13 were investigated using an albumin-binding assay with albumin-conjugated Sepharose™ (Sigma-Aldrich, St. Louis, MO, USA) resin according to the manufacturer's instructions. Briefly, Gly8-GLP-1(7–36)-NH2, liraglutide and conjugates 6–13 (100 μg·mL−1 in PBS, 50 μL) were mixed with HSA resin and incubated for 3 h at 25°C. The resin and supernatant were separated by centrifugation (1853× g, 12 min) and unbound peptide contents were determined using a Mico BCA Protein assay kit (Pierce, Rockford, IL, USA). The non-specific absorptions of the peptides on albumin-free resin were determined using N-hydroxysuccinimide-inactivated resin in a similar manner.
Insulin secretion assay in Sprague Dawley rats
The insulin secretion assays were carried out by injecting i.p. the vehicle, exendin-4 (25 nmol·kg−1), liraglutide (25 nmol·kg−1) or conjugate 7 (25 nmol·kg−1) into male Sprague Dawley rats (n = 3per group) (Han et al., 2013; Li et al., 2011b). The vehicle was sterilized 5% (v/v) 1, 2 – propylene glycol/saline solution and the concentration of test compounds was 25 nmol·mL−1. Briefly, the Sprague Dawley rats were fasted overnight (12 h) and randomly allocated to four groups. Half an hour prior to oral glucose load (10 g·kg−1, 0 min), Sprague Dawley rats were injected with the compounds. Blood samples (0.1 mL) were collected in EDTA-containing microcentrifuge tubes from the lateral tail vein at −30, 0, 5, 10, 15, 30, 45, 60, 90, 120 and 180 min respectively. Vasodilatation may be necessary to promote bleeding and can be caused by exposing an animal to 37°C for 5–8 min or by local warming of the tail. There appear to be few disadvantages that affect animal well-being, but animals must be closely monitored for signs of distress if heat exposure is used. The plasma samples were then obtained by centrifugation (1235× g, 15 min) and assayed for insulin levels using a rat insulin elisa kit (Millipore).
Insulin secretion assay in db/db mice
As db/db mice are chronically hyperglycaemic, plasma insulin levels of db/db mice were measured after administration of vehicle, exendin-4, liraglutide and conjugate 7. Briefly, the db/db mice (n = 6 per group) were fasted overnight (18 h). Half an hour before the oral glucose load (2.0 g·kg−1, 0 h), db/db mice were injected i.p. with vehicle, exendin-4 (25 nmol·kg−1), liraglutide (25 nmol·kg−1) or conjugate 7 (2.5, 25 or 250 nmol·kg−1) respectively. The vehicle was sterilized 5% (v/v) 1, 2 – propylene glycol/saline solution and the concentration of test compounds was 2.5 nmol·mL−1. Blood samples (approximately 0.1 mL) were collected in EDTA-containing microcentrifuge tubes from the lateral tail vein 15 min after the glucose load. The plasma samples were then obtained by centrifugation (1235× g, 15 min) and assayed for insulin levels using a mouse insulin elisa kit (Millipore).
Pharmacokinetic assessments in Sprague Dawley rats
The pharmacokinetic parameters of exendin-4, liraglutide and conjugate 7 were determined in male Sprague Dawley rats (n = 3 per group) using a modification of a previously described method (Han et al., 2013). Following an overnight fast (12 h), the animals were randomly divided into four groups and each animal received a test compound by s.c. injection (15 nmol per rat). The dosing vehicle was sterilized 5% (v/v) 1, 2 – propylene glycol/saline solution and the concentration of test articles was 25 nmol·mL−1. Serial blood samples were collected in EDTA-containing microcentrifuge tubes at pre-dose, 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 48 h post-dose after s.c. administration. Approximately 0.1 mL of blood was collected from the lateral tail vein at each time point. Blood samples were immediately centrifuged at 4°C. The obtained plasma was frozen with dry ice and stored at −20°C. Plasma drug levels were determined using a LC-MS/MS assay. Plasma samples were prepared for analysis by precipitating plasma proteins with two volumes of acetonitrile containing an internal standard. The samples were vortex mixed and the precipitated proteins were removed by centrifugation (17 295× g, 14 min). The resulting supernatants (10 μL) were used for analysis.
Multiple oral glucose tolerance tests (OGTT) in db/db mice
In order to simulate the human diet of three meals a day, a modified multiple OGTT in db/db mice was used to assess the duration of the reduction of glucose levels (Chen et al., 2012; Han et al., 2013). Briefly, the db/db mice (n = 6 per group) were fasted overnight (18 h). Half an hour before the first oral glucose load (2.0 g·kg−1, 0 h), male db/db mice were injected i.p. with vehicle, exendin-4 (25 nmol·kg−1), liraglutide (25 nmol·kg−1) or conjugate 7 (25 nmol·kg−1) respectively. The vehicle was sterilized 5% (v/v) 1, 2 – propylene glycol/saline solution and the concentration of test articles was 2.5 nmol·mL−1. Blood (1 μL) was collected from the cut tip of the tail vein at 0, 0.25, 0.5, 1 and 3 h after oral administration of glucose and the blood glucose levels were measured using a blood glucose monitoring system (FreeStyle Freedom, Abbott Diabetes Care, Alameda, NC, USA). After the first OGTT, the next glucose loads were administered at 6 and 12h respectively. The time intervals between blood collections were the same after each glucose load. Each time the blood collection was finished (3h after loading), the animals were rested with free access to water. The mice were re-fed immediately after the experiment was finished.
Hypoglycaemic efficacies test in db/db mice
The hypoglycaemic effects of conjugate 7 were evaluated using a modification of a previously described method, using male db/db mice (7 weeks, male, 22–25 g) (Kim et al., 2011; Han et al., 2013). Under non-fasting conditions with free access to food and water, mice (n = 6 per group) received a single injection (i.p.) of vehicle, exendin-4 (25 nmol·kg−1), liraglutide (25 nmol·kg−1) or conjugate 7 (25 or 250 nmol·kg−1). The vehicle was sterilized 5% (v/v) 1, 2 – propylene glycol/saline solution and the concentration of test compounds was 2.5 nmol·mL−1. A drop of blood was drawn from a tail vein of each animal at different times (0, 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 36 and 48 h) and blood glucose levels were determined using a blood glucose monitor (FreeStyle Freedom, Abbott Diabetes Care). In addition, we measured the length of time over which a blood glucose level of <8.35 nmol·L−1 was maintained.
Data analysis
All data are presented as means ± SD, n referring to the number of mice in each group. Statistical evaluation of the results was made with GraphPad Prism version 5.0 (GraphPad). The data obtained from different genotypes were analysed by Student's t-test or anova, as appropriate. The homogeneity of variances was assessed with the Levene test. In case of sphericity violations, the Greenhouse–Geisser correction was applied. Post-anova analysis of group differences was performed with the Tukey's honestly significant difference test, when the variances were homogeneous, and with the Games–Howell test, when the variances were unequal. Probability values P ≤ 0.05 were regarded as statistically significant.
Materials
Fmoc Rink Amide-MBHA resin and Fmoc-protected amino acids were obtained from GL Biochem (Shanghai, China). Two 7-hydroxycoumarin maleimides (5a, 5b) were purchased from Aladdin Reagent (Shanghai, China). HPLC grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Gly8-GLP-1(7–36)-NH2, exendin-4 and liraglutide were purchased from GL Biochem. cAMP dynamic kit was purchased from Cisbio (Bedford, MA, USA). Rat/Mouse insulin elisa kit was purchased from Millipore (Billerica, MA, USA). HbA1c kit was purchased from Glycosal (Deeside, UK). All other reagents, unless indicated, were purchased from Sigma-Aldrich Co. (Saint Louis, MO, USA) and were used as received. Microwave irradiation procedures were performed in a discover-focused, single-mode microwave synthesis system (CEM, Matthews, NC, USA), which produced continuous irradiation at 2450 MHz. The HPLC analysis was performed on a Shimadzu 2010C HPLC system (Shimadzu, Kyoto, Japan). For purification, Shimadzu LC-10 preparative reverse phase (RP)-HPLC system (Shimadzu) was used. The electrospray ionization mass spectrometry of the compounds were obtained with Waters ACQUITY UPLC Systems with mass (Waters, Milford, MA, USA). Plasticware was rinsed with solvent containing 0.1% v/v rat plasma for three times and used after drying to minimise adsorption of peptides.
Results
Synthesis and characterization of coumarin GLP-1 conjugates
Four cysteine altered peptides Cys17-Gly8-GLP-1(7–36)-NH2 (i), Cys26-Gly8-GLP-1(7–36)-NH2 (ii), Cys34-Gly8-GLP-1(7–37)-NH2 (iii), Cys37-Gly8-GLP-1(7–37)-NH2 (iv) were synthesized by standard solid-phase peptide synthesis protocol with N-Fmoc/tBu chemistry. Microwave irradiation was performed in coupling steps and deprotection steps. Peptides were further identified by LC-MS after purification by HPLC. Two 7-hydroxycoumarin maleimides (5a, 5b) were synthesized as maleimide is a highly chemoselective Michael-acceptor to thiol groups at neutral pH. Finally, the cysteine-altered peptides (1–4) were reacted with 7-hydroxycoumarin maleimides (5a, 5b) to give the final compounds (Scheme 9). Eight coumarin GLP-1 conjugates (Figure 1) were synthesized and purified by preparative RP-HPLC and further identified by HPLC and LC-MS.
Figure 1.
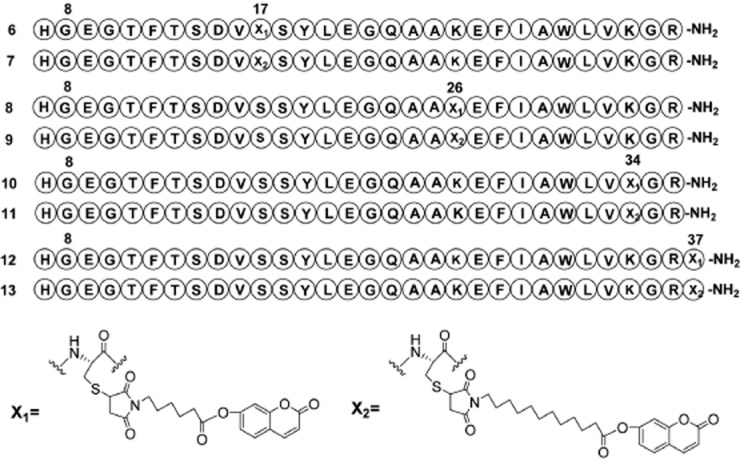
Structures of the eight coumarin-modified GLP-1 derivatives investigated here.
GLP-1 receptor activation assay
HEK293 cells overexpressing human GLP-1 receptors were used to determine the receptor activation potency of Gly8-GLP-1(7–36)-NH2, GLP-1(7–36)-NH2, exendin-4, liraglutide and conjugates 6–13. As summarized in Table 1, most coumarin GLP-1 conjugates preserved their receptor activation potency. Notably, conjugates 8 and 9 were more potent than other derivatives, indicating that conjugation on position 26 with 7-hydroxycoumarin maleimides (5a, 5b) could avoid the loss of potency that often follows chemical modification of peptides. The chemical conjugation of 7-hydroxycoumarin maleimides (5a, 5b) to Cys17-Gly8-GLP-1(7–36)-NH2 resulted in a reduction of receptor activation potency of conjugates 6 and 7 (2.1–2.2-fold reduction vs. that of Gly8-GLP-1(7–36)-NH2), while the potency reduction of conjugates 8 and 9 were not significant. Interestingly, the effects of these conjugates were similar to that of GLP-1(7–36)-NH2 (at 10 nM, Table 1).
Table 1.
The potency and efficacy of the coumarin modified GLP-1 conjugates 6–13 on the activation of cloned human GLP-1 receptors in HEK293 cells
| Compound | Potency (EC50, pM) | Efficacy (at 10 nM, 100%) | Compound | Potencya (EC50, pM) | Efficacyb (at 10 nM, 100%) |
|---|---|---|---|---|---|
| Gly8-GLP-1(7–36)-NH2 | 8.3 ± 0.4 | 97.3 ± 0.8 | GLP-1(7–36)-NH2 | 3.4 ± 0.7 | 98.5 ± 1.6 |
| Exendin-4 | 1.6 ± 0.4 | 99.4 ± 0.9 | Liraglutide | 9.5 ± 0.8 | 97.3 ± 1.3 |
| Conjugate 6 | 18.1 ± 0.9**** | 95.9 ± 1.5 | Conjugate 7 | 18.6 ± 1.3**** | 96.1 ± 0.6 |
| Conjugate 8 | 7.1 ± 0.9*** | 97.9 ± 1.3 | Conjugate 9 | 8.1 ± 0.6**** | 98.3 ± 1.2 |
| Conjugate 10 | 12.1 ± 0.5**** | 96.3 ± 1.4 | Conjugate 11 | 19.9 ± 1.4**** | 97.4 ± 1.0 |
| Conjugate 12 | 11.3 ± 0.6**** | 96.6 ± 1.3 | Conjugate 13 | 16.8 ± 0.7**** | 96.7 ± 0.7 |
The receptor potency data are given as means ± SD. All experiments were performed in triplicate and repeated three times (n = 3).
The efficacy data were normalized with respect to the maximal response attained by GLP-1(7–36)-NH2.
P < 0.001,
P < 0.0001, compared with GLP-1(7–36)-NH2.
Plasma stability in rat plasma
The in vitro stability of the eight derivatives (6–13), compared with Gly8-GLP-1(7–36)-NH2, exendin-4 and liraglutide, was evaluated in the plasma stability test. As shown in Figure 2, Gly8-GLP-1(7–36)-NH2 had an approximate half-life of 0.6 h in rat plasma at 37°C, the long-acting GLP-1 agonist exendin-4, had a nearly 10-fold longer half-life (t1/2 = 4.8 h). Once-daily human GLP-1 analogue liraglutide had a longer half-life (t1/2 = 17.1 h) than exendin-4. As expected, all coumarin-modified compounds were more stable than exendin-4 except conjugate 12 (P < 0.05). Particularly, conjugates 7 (P < 0.0001) and 9 (P < 0.05) were more stable than liraglutide. The half-life of most compounds were longer than 6 h, and the longest half-life was shown by conjugate 7 (t1/2 = 52.2 h) (Figure 2A).
Figure 2.
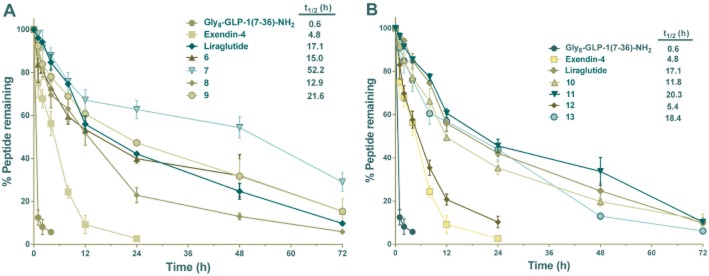
Degradation of the coumarin-modified GLP-1 derivatives 6–13, Gly8-GLP-1(7–36)-NH2, exendin-4 and liraglutide by rat plasma. A total of 1000 ng mL−1 Gly8-GLP-1(7–36)-NH2, exendin-4, liraglutide and conjugates 6–13 were incubated with rat plasma at 37°C and 100 μL samples were taken from the incubation solution at 0, 1, 2, 4, 6, 8, 12, 24, 36, 48 and 72 h time points. Means ± SD, n = 3.
Human albumin-binding assay
The increased in vitro metabolic stability observed earlier might be attributed to the increased albumin-binding affinities of our conjugates. To test this possibility, albumin binding was tested for conjugates 6–13, Gly8-GLP-1(7–36)-NH2, exendin-4 and liraglutide. As illustrated in Figure 3, 6.8 ± 0.7% of Gly8-GLP-1(7–36)-NH2 and 19.2 ± 2.2% of exendin-4 were found to associate with albumin resin, respectively under physiological conditions (in PBS pH 7.4). All of the coumarin GLP-1 conjugates showed moderately to highly increased albumin binding, compared with Gly8-GLP-1(7–36)-NH2 and exendin-4 (P < 0.0001). Particularly, the albumin binding of conjugate 7 was higher than that of liraglutide (P < 0.001). Interestingly, the binding correlated well with stability (Figure 3). In this study, conjugate 7 possessed exceptionally high plasma stability, which was important for the achievement of long-acting in vivo activity. Thus, the in vivo biological activity of conjugate 7 was evaluated in the subsequent insulin secretion assay.
Figure 3.
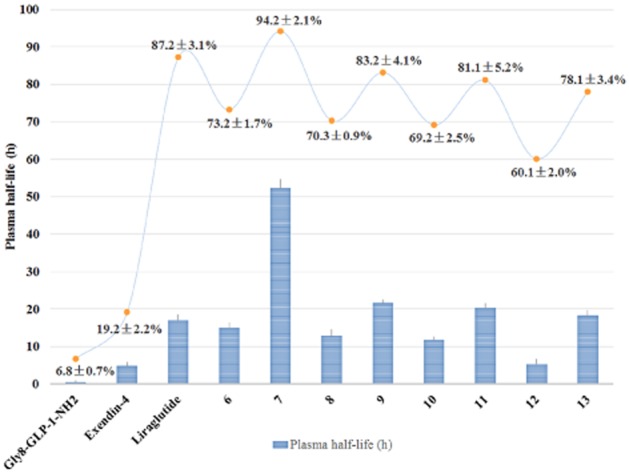
The plasma stability and albumin-binding test of the coumarin-modified GLP-1 derivatives 6–13, Gly8-GLP-1(7–36)-NH2, exendin-4 and liraglutide. Means ± SD, n = 3. *P < 0.05, ****P < 0.0001, compared with liraglutide.
Insulin secretion assay in Sprague Dawley rats
The in vivo antidiabetic activities of conjugate 7 was examined by OGTT in Sprague Dawley rats after observing the excellent in vitro stability of this compound in the previous stability assays. As expected, after treatment with either conjugate 7, liraglutide or exendin-4, plasma insulin concentrations were significantly higher than those of the control group at 15 min and 45 min (Figure 4). The time course of increased insulin for all three stimuli (exendin-4, liraglutide or conjugate 7) were very similar, with a peak at 15 min and a subsequent fall to near normal levels by 120 min. Furthermore, calculation of the the AUCinsulin indicated that this dose of conjugate7 induced slightly greater, but not significant, insulin secretion than exendin-4 or liraglutide.
Figure 4.
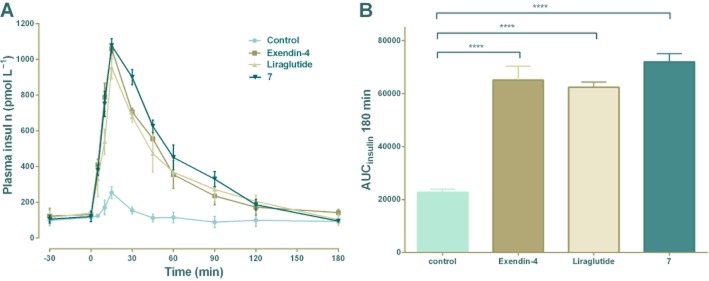
In vivo biological activity tests of 7. Exendin-4, liraglutide and conjugate 7 (25 nmol·kg−1) were injected i.p. into Sprague Dawley rats; glucose was given orally (10 g·kg−1). Plasma insulin levels were measured by a rat insulin detection kit. (A) Insulinotropic activities of exendin-4, liraglutide and conjugate 7 (25 nmol·kg−1) in Sprague Dawley rats. Means ± SD, n = 3. (B) AUCinsulin after oral glucose administration, ****P <0.0001.
Insulin secretion assay in db/db mice
The effects of conjugate 7 on insulin secretion were further explored in db/db mice by OGTT. As shown in Figure 5, after oral administration of 2 g·kg−1 glucose, the secreted insulin levels were increased by exendin-4 or liraglutide at 15 min. The conjugate 7 also induced a significant and dose-dependent elevation of insulin secretion after an oral glucose challenge.
Figure 5.
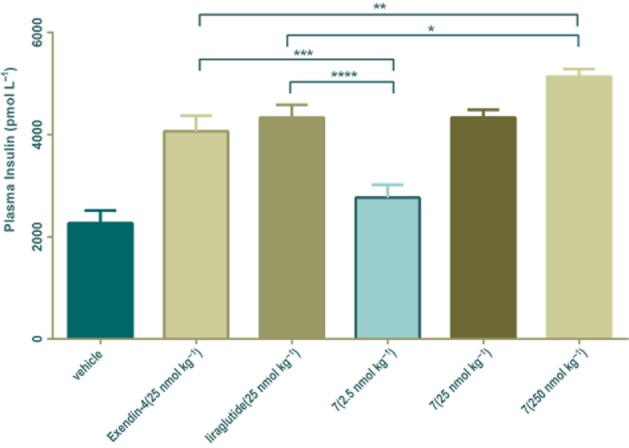
Effects of conjugate 7 on plasma insulin levels in db/db mice. Vehicle, exendin-4 (25 nmol·kg−1), liraglutide (25 nmol·kg−1) and conjugate 7 (2.5 nmol·kg−1, 25 nmol·kg−1, 250 nmol·kg−1) were injected i.p. in db/db mice; glucose was given orally (2 g·kg−1). Blood insulin levels were measured by a mouse insulin detection kit. Means ± SD, n = 6. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
In vivo pharmacokinetics in Sprague Dawley rats
The pharmacokinetics of conjugate 7 was compared with those of exendin-4 and liraglutide in rats, as illustrated in Figure 6 and Table 2. The plasma concentration of exendin-4 rapidly increased after s.c. injections and peaked within 1 h (tmax = 0.4 ± 0.1 h), after which it rapidly declined to baseline at 6 h post-injection with a calculated elimination half-life (t1/2) of 2.5 ± 1.2 h. As expected, liraglutide had longer elimination half-life (t1/2 = 14.9 ± 0.9 h) than exendin-4 and showed delayed absorption patterns (tmax = 1.9 ± 0.2 h). Interestingly, conjugate 7 showed similar delayed absorption patterns to liraglutide following the injection and tmax increased to 3.1 ± 1.3 h. Furthermore, conjugate 7 was found in plasma for much longer after injection with almost a 50% increase in half-life (t1/2 = 22.9 ± 7.2 h), compared with liraglutide. Detailed pharmacokinetic analysis revealed that coumarin conjugation not only extended plasma circulation time but also markedly increased drug utilizations, as represented by AUCinf values. The AUCinf value of conjugate 7 was approximately 9.3-fold greater than that of exendin-4 (P < 0.0001) and 1.7-fold greater than that of liraglutide (P < 0.01).
Figure 6.
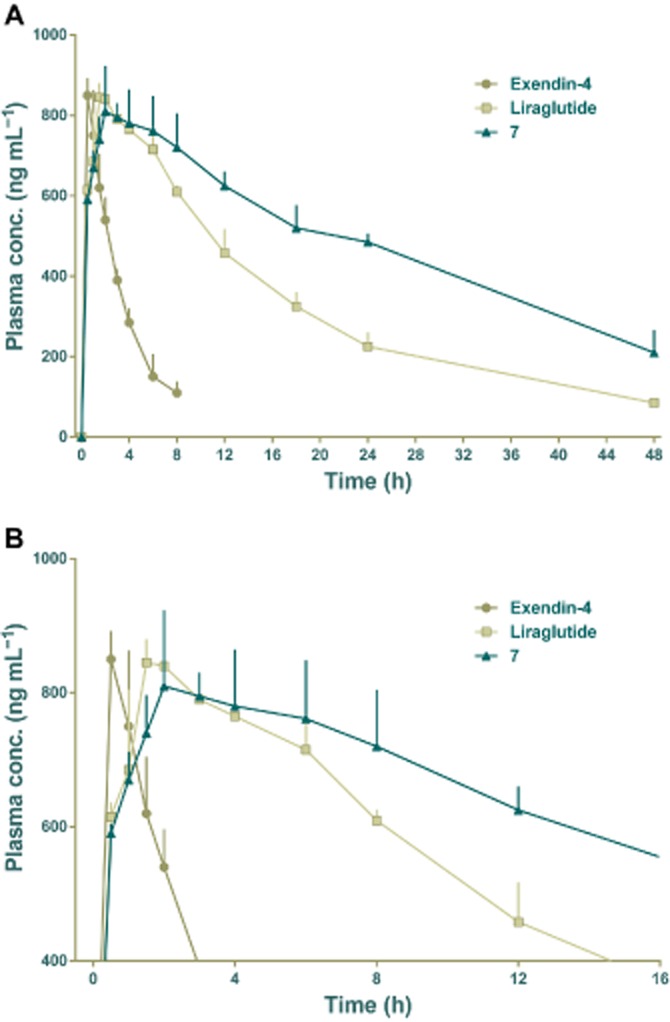
Pharmacokinetic profiles of exendin-4, liraglutide and 7 after s.c. administration (15 nmol per rat), over 48 h (A). In (B), the same data is shown over 16h, to clarify the early results. Each animal was given a compound by s.c. injection and serial blood samples were collected in EDTA-containing microcentrifuge tubes. Collections were made before dosing and at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 48 h after treatment. Means ± SD, n = 3.
Table 2.
Pharmacokinetic parameters of exendin-4, liraglutide and conjugate 7 in Sprague Dawley rats
| Samples | Tmax (h) | Cmax (ng·mL−1) | AUCinf (ng·h·mL−1) | t1/2 (h) | MRT (h) |
|---|---|---|---|---|---|
| Exendin-4 | 0.4 ± 0.1* | 850 ± 25.6 | 3 196 ± 981**** | 2.5 ± 1.2** | 3.9 ± 1.3** |
| Liraglutide | 1.9 ± 0.2 | 875 ± 21.4 | 17 702 ± 870** | 14.9 ± 0.9 | 20.7 ± 1.1 |
| Conjugate 7 | 3.1 ± 1.3 | 865 ± 62.3 | 29 615 ± 4 155 | 22.9 ± 7.2 | 32.9 ± 10.1 |
Data shown are the means ± SD (n = 3).
P < 0.05,
P < 0.01,
P < 0.0001,
compared with conjugate 7. AUCinf, area under the curve from zero to infinity; Cmax, maximum plasma concentration; MRT, mean residence time; Tmax, time to reach maximum plasma concentration; t1/2, elimination half-life.
Multiple OGTT in db/db mice
Because of the in vitro stability and the in vivo pharmacokinetic profile of conjugate 7, we tested its ability to reduce glucose levels was assessed in a modified multiple OGTT in db/db mice, a model of T2DM. Conjugate 7, exendin-4, liraglutide (25 nmol·kg−1) and control (saline) were administered 0.5 h before the first glucose load, which was given at time 0. Two further glucose loads were administered at 6 and 12 h. As illustrated in Figure 7, blood glucose levels in the control group treated with saline rapidly increased, to over 30 mmol·L−1, 0.5 h after every glucose load. The glucose-lowering ability of treatment with exendin-4 was maintained during 0–6 h but decreased dramatically after 6 h and was almost ineffective after 12 h (Figure 7). Liraglutide and conjugate 7 showed comparable glucose-lowering ability during the whole experimental period (0–15 h). These results indicated that a single dose of conjugate 7 (25 nmol·kg−1) could exert glucoregulatory abilities over 15 h.
Figure 7.
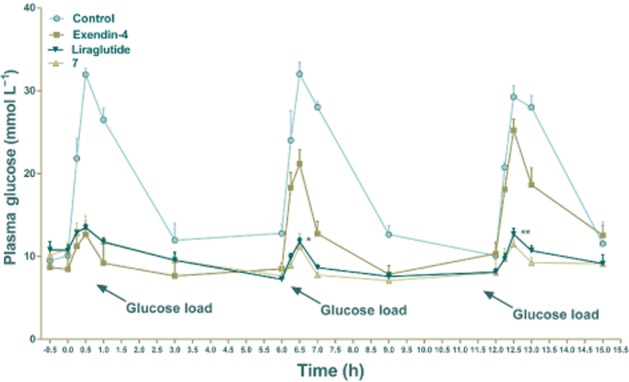
Long-term hypoglycaemic effects of conjugate 7 was studied using multiple OGTT in db/db mice. Plasma glucose levels are shown in panels A and B. Exendin-4, liraglutide and conjugate 7 (25 nmol·kg−1) and control (vehicle) were given 0.5 h before the first glucose load, and the glucose loads were administered orally at time points 0, 6 and 12 h. Blood was collected at 0, 0.25, 0.5, 1 and 3 h after the glucose load and the plasma glucose was measured using a blood glucose monitor. The time intervals between collections in each OGTT were the same. The time of the glucose load is indicated by arrows. Means ± SD, n = 6. *P < 0.05, **P < 0.01, compared with exendin-4.
Hypoglycaemic duration test in db/db mice
The hypoglycaemic effect of conjugate 7 was examined at two doses (25 and 250 nmol·kg−1, i.p.) in db/db mice. In this study, a blood glucose level of below 8.35 mmol·L−1 was regarded as normal and the length of time blood glucose remained below this value provided a practical indication of potential for antidiabetic treatment (Kim et al., 2011). As illustrated in Figure 8, blood glucose levels in control mice (saline injected) maintained a hyperglycaemic state (average > 25 mmol·L−1) and exendin-4 injections (25 nmol·kg−1) rapidly normalized blood glucose for 4.5 h following injection, with a later increase in blood glucose. The hypoglycaemic effects of conjugate 7, at a dose of 25 nmol·kg−1, lasted much longer than those of exendin-4 and slightly longer than liraglutide. The time required to return to a glucose level of 8.35 mmol·L−1 was ∼21.5 h in mice given conjugate 7, compared with ∼4.1 h in exendin-4 and ∼17.0 h in liraglutide (Figure 8A). In particular, the t∼8.35 mmol·L−1 value of conjugate 7 in the highest dose administered mice (250 nmol·kg−1) was 47.1 h, which means that a single injection of conjugate 7 provided ‘normal’ blood glucose levels for 2 days in db/db mice. Calculated glucose AUC0–48h values also revealed that conjugate 7 had greater antidiabetic effect than exendin-4 at the dose of 25 nmol·kg−1 (Figure 8B, P < 0.001 for 7 vs. exendin-4). Furthermore, conjugate 7 at the higher dose, also had better antidiabetic effect than liraglutide (Figure 8B, P < 0.05 for 7 and P < 0.001 for 7 vs. liraglutide).
Figure 8.
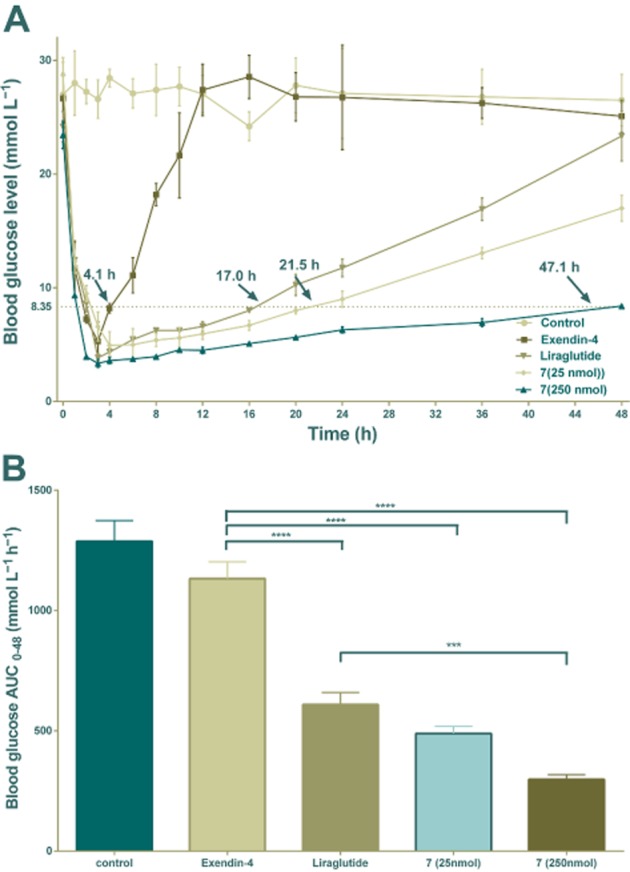
Glucose-lowering and stabilizing effect of exendin-4, liraglutide and conjugate 7 as shown by the duration of hypoglycaemia in non-fasted db/db mice. (A) Time-course average blood glucose levels of db/db mice after an i.p. injection of exendin-4 (25 nmol·kg−1), liraglutide (25 nmol·kg−1) or 7 (25/250 nmol·kg−1). Times (with arrow) represent the duration of blood glucose levels below 8.35 mmol·L−1. (B) Hypoglycaemic effects of exendin-4, liraglutide and 7 based on the calculated glucose AUC0–48 h values. Means ± SD, n = 6. ***P < 0.001, ****P < 0.0001.
Discussion and conclusions
In the present study, with the specific aims of developing long-acting incretin mimetics, we designed and prepared a series of novel coumarin GLP-1 conjugates. All conjugates were obtained in high purity. The initial GLP-1 receptor activation experiments provided direct evidence of the relevant structure-activity relationships. As illustrated in Table 1, conjugates 6–13 exhibited well preserved receptor activation, with conjugates 6 and 7 being less potent than the other conjugates. The main reason for this loss of potency was that the position of conjugation with the 7-hydroxycoumarin maleimide was near the N-terminal, which interfered with the activation of the GLP-1 receptor by conjugates 6 and 7. Notably, chemical conjugation at position 26 of GLP-1 hardly affected the receptor activation potency, as in liraglutide, and the corresponding Cys26 7-hydroxycoumarin conjugates (8 and 9) were more potent. The in vitro stability test and albumin-binding test revealed that the albumin-binding ability and plasma stability of all conjugates (6–13) were improved to different degrees (Figure 3). Importantly, the plasma stabilities of test compounds correlated well with their albumin-binding abilities. These results showed that through alterations in their physicochemical characteristics, the biological and pharmaceutical features of peptides can be transformed. Interestingly, the in vitro stability profiles of conjugates 9, 11 and 13 were comparable with liraglutide while their albumin-binding abilities were lower than that of liraglutide. Moreover, conjugates 7, 9, 11, 13 were more stable than conjugates 6, 8, 10, 12. One possible explanation for this difference is that these compounds have altered the DPP-IV cleavage site through the change of Ala8 to Gly8, thus preventing the rapid cleavage by DPP-IV, and further coumarin modification with various lengths of fatty chain linker.
Conjugate 7 which exhibited the best albumin-binding ability and the longest plasma half-life was further evaluated for in vivo insulinotropic activity. The insulinotropic activity of conjugate 7 was comparable with that of liraglutide and exendin-4 (Figure 4), indicating that the in vivo biological action of conjugate 7 was exerted via a glucose-dependent mode. This might offer the advantage of greater clinical safety, compared with agents that increased insulin secretion via glucose-independent mechanisms, such as the sulfonylureas, implying that conjugate 7 could be used without the risk of hypoglycaemia. Moreover, the insulinotropic activity was further confirmed in db/db mice and the results indicated that conjugate 7 induced a significant and dose-dependent elevation of insulin secretion after an oral glucose challenge and achieved a higher insulin level than exendin-4 (25 nmol·kg−1) and liraglutide (25 nmol·kg−1), at the dose level of 250 nmol·kg−1. Evaluation of the in vivo pharmacokinetic profile of 7 showed that the elimination half-life of conjugate 7 was significantly improved, probably because of the reduced enzymic metabolism and renal clearance resulting from its strong binding to albumin. The absorption of conjugate 7 into the systemic circulation was delayed similar to liraglutide probably because of the formation of self-assembled colloidal structures in aqueous environment (Kim et al., 2005; Jonassen et al., 2006). The albumin binding of conjugate 7 and its pharmacokinetic profiles were consistent with previous reports of the prolonged in vivo circulation half-life values of fatty acid-modified GLP-1 analogues, which were attributed to their physical interactions with serum albumin (Madsen et al., 2007; Buse et al., 2009). The long-acting glucose-lowering effects of conjugate 7 were further confirmed by multiple OGTT, as well as the hypoglycaemic duration test in db/db mice. The duration of hypoglycaemia induced by conjugate 7 in db/db mice was much greater than that of exendin-4 and better than that of liraglutide, regardless of the dose administered. In addition, the administration of conjugate 7 (250 nmol·kg−1) sustained normal glycaemia for 11.5 and 2.8-fold longer than exendin-4 and liraglutide, respectively, and drug clearance in humans is known to be much slower than in rodents. Thus, the antidiabetic duration of 7 may be substantially longer in humans than the ∼47 h observed in db/db mice in our study. Furthermore, the glucose-lowering effect of a single i.p. injection of 25 nmol·kg−1 of conjugate 7 was better than that of the other long-acting incretin mimetics, such as albumin-conjugated GLP-1 (CJC 1131, ∼10 h glucose normalization by 100 nmol·kg−1 s.c. injection) (Kim et al., 2003).
In summary, the present study demonstrates that the substitution of GLP-1 residues by cysteine and further coumarin modification with a fatty chain linker offers a useful approach to the development of long-acting incretin-based antidiabetic drugs. This study also shows that conjugate 7, which has a well-preserved biological activity, prolonged pharmacokinetics and long-acting antidiabetic characteristics, is a long-acting GLP-1 derivative deserving further investigation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81172932 and 81273376), the Natural Science Foundation of Jiangsu Province (No. BK2012356) and the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. JKGZ201103).
Glossary
- DPP-IV
dipeptidyl peptidase IV
- GLP-1
glucagon-like peptide-1
- HSA
human serum albumin
- OGTT
oral glucose tolerance test
- T2DM
type 2 diabetes mellitus
Author contributions
J. H., L. S., X. H., H. Q. and W. H. designed the research study. Moreover, J. H., L. S., X. H. performed the research, analysed the data and wrote the paper. Z. L. and C. Z. contributed essential reagents and performed some part of the research.
Conflict of interest
The authors confirm that this article content has no conflicts of interest.
References
- Ahren B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 2005;36:867–876. doi: 10.1055/s-2004-826178. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- Chae SY, Choi YG, Son S, Jung SY, Lee DS, Lee KC. The fatty acid conjugated exendin-4 analogs for type 2 antidiabetic therapeutics. J Control Release. 2010a;144:10–16. doi: 10.1016/j.jconrel.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Chae SY, Jin C-H, Shin JH, Sona S, Kima TH, Lee S, et al. Biochemical, pharmaceutical and therapeutic properties of long-acting lithocholic acid derivatized exendin-4 analogs. J Control Release. 2010b;142:206–213. doi: 10.1016/j.jconrel.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou Y, Zhang H. Stability and bioactivity studies on dipeptidyl peptidase IV resistant glucogan-like peptide-1 analogues. Protein Pept Lett. 2012;19:203–211. doi: 10.2174/092986612799080194. [DOI] [PubMed] [Google Scholar]
- Chi Y, Zhang H, Huang W, Zhou J, Zhou Y, Qian H, et al. Microwave-assisted solid phase synthesis, PEGylation, and biological activity studies of glucagon-like peptide-1(7–36) amide. Bioorg Med Chem. 2008a;16:7607–7614. doi: 10.1016/j.bmc.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Chi Y, Zhang H, Zhou J, Huang W, Ni S. Microwave-assisted solid phase synthesis of glp-1 analogues. Lett Org Chem. 2008b;5:399–402. [Google Scholar]
- Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2007;9:23–31. doi: 10.1111/j.1463-1326.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Dritselis A, Kirkpatrick P. Liraglutide. Nat Rev Drug Discov. 2010;9:267–268. doi: 10.1038/nrd3148. [DOI] [PubMed] [Google Scholar]
- Gerich JE. Contributions of insulin-resistance and insulin-secretory defects to the pathogenesis of type 2 diabetes mellitus. Mayo Clin Proc. 2003;78:447–456. doi: 10.4065/78.4.447. [DOI] [PubMed] [Google Scholar]
- Green BD, Liu HK, McCluskey JT, Duffy NA, O'Harte FPM, McClenaghan NH, et al. Function of a long-term, GLP-1-treated, insulin-secreting cell line is improved by preventing DPP IV-mediated degradation of GLP-1. Diabetes Obes Metab. 2004;7:563–569. doi: 10.1111/j.1463-1326.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- Han J, Huang X, Sun L, Li Z, Qian H, Huang W. Novel fatty chain-modified glucagon-like peptide-1 conjugates with enhanced stability and prolonged in vivo activity. Biochem Pharmacol. 2013;86:297–308. doi: 10.1016/j.bcp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Jonassen I, Havelund S, Ribel U, Plum A, Loftager M, Hoeg-Jensen T, et al. Biochemical and physiological properties of a novel series of long-acting insulin analogs obtained by acylation with cholic acid derivatives. Pharm Res. 2006;23:49–55. doi: 10.1007/s11095-005-9047-1. [DOI] [PubMed] [Google Scholar]
- Joseph K, Moser AC, Basiaga SB, Schiel JE, Hage DS. Evaluation of alternatives to warfarin as probes for Sudlow site I of human serum albumin: characterization by high-performance affinity chromatography. J Chromatogr A. 2009;1216:3492–3500. doi: 10.1016/j.chroma.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jetté L, et al. Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003;52:751–759. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- Kim K, Kwon S, Park JH, Chung H, Jeong SY, Kwon IC, et al. Physicochemical characterizations of self-assembled nanoparticles of glycol chitosan-deoxycholic acid conjugates. Biomacromolecules. 2005;6:1154–1158. doi: 10.1021/bm049305m. [DOI] [PubMed] [Google Scholar]
- Kim TH, Jiang HH, Lee S, Youn YS, Park CW, Byun Y, et al. Mono-PEGylated dimeric exendin-4 as high receptor binding and long-acting conjugates for type 2 anti-diabetes therapeutics. Bioconjug Chem. 2011;22:625–632. doi: 10.1021/bc100404x. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Zheng X, Tang L, Xu W, Gong M. Disulfide bond prolongs the half-life of therapeutic peptide-GLP-1. Peptides. 2011a;32:1400–1407. doi: 10.1016/j.peptides.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng X, Tang L, Xu W, Gong M. GLP-1 analogs containing disulfide bond exhibited prolonged half-life in vivo than GLP-1. Peptides. 2011b;32:1303–1312. doi: 10.1016/j.peptides.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)-preclinical and clinical results. Best Pract Res Clin Endocrinol Metab. 2009;23:463–477. doi: 10.1016/j.beem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thøgersen H, Wilken M, et al. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007;50:6126–6132. doi: 10.1021/jm070861j. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21:91–117. doi: 10.1002/dmrr.538. [DOI] [PubMed] [Google Scholar]
- Miranda LP, Winters KA, Gegg CV, Patel A, Aral J, Long J, et al. Design and synthesis of conformationally constrained glucagon-like peptide-1 derivatives with increased plasma stability and prolonged in vivo activity. J Med Chem. 2008;51:2758–2765. doi: 10.1021/jm701522b. [DOI] [PubMed] [Google Scholar]
- Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaquist ER, Ibrahim HN. Approach to the patient with type 2 diabetes and progressive kidney disease. J Clin Endocrinol Metab. 2010;95:3103–3110. doi: 10.1210/jc.2010-0504. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Willard FS, Brenner MB, Ficorilli1 J, Valasek K, Showalter AD, et al. Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes. 2010;59:3099–3107. doi: 10.2337/db10-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani N, Kumar M, Glinka Y, Prud'Homme G, Wang Q. In vivo expression of GLP-1/IgG-Fc fusion protein enhances beta-cell mass and protects against streptozotocin-induced diabetes. Gene Ther. 2007;14:981–988. doi: 10.1038/sj.gt.3302944. [DOI] [PubMed] [Google Scholar]
- Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept. 2010;164:58–64. doi: 10.1016/j.regpep.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ni S, Zhang H, Qian H, Chi Y, Huang W, et al. Synthesis and bioactivity evaluation of dipeptidyl peptidase IV resistant glucagon-like peptide-1 analogues. Protein Pept Lett. 2010;17:1290–1295. doi: 10.2174/092986610792231546. [DOI] [PubMed] [Google Scholar]


