Abstract
Plants defend themselves against attack from insects and pathogens with various resistance strategies. The jasmonate and salicylate signaling pathways are two induced responses that protect plants against these attackers. Knowledge of the range of organisms that are affected by each response is important for understanding how plants coordinate their defenses against multiple attackers and the generality of effect of different resistance mechanisms. The jasmonate response is known to protect plants against a wide range of insect herbivores; in this study, we examined the role of the jasmonate response in susceptibility to eight pathogens with diverse lifestyles in the laboratory and field. Recent biochemical models suggest that the lifestyle of the pathogen (necrotroph versus biotroph) should predict whether the jasmonate response will be involved in resistance. We tested this by examining the susceptibility of wild-type (cv Castlemart with no known genes for resistance to the pathogens used) and jasmonate-deficient mutant tomato (Lycopersicon esculentum) plants (def1) and by employing rescue treatments of the mutant. Plant susceptibility to five of the eight pathogens we examined was reduced by the jasmonate response, including two bacteria (Pseudomonas syringae and Xanthomonas campestris), two fungi (Verticillium dahliae and Fusarium oxysporum f. sp. lycopersici), and an oomycete (Phytophthora infestans). Susceptibility to three fungi was unaffected (Cladosporium fulvum, Oidium neolycopersici, and Septoria lycopersici). Our results indicate that the jasmonate response reduces damage by a wide range of pathogens from different lifestyles, a result that contrasts with the emerging picture of diseases on Arabidopsis. Thus, the generality of jasmonate-based resistance of tomato challenges the view that ecologically distinct plant parasites are resisted via different mechanisms.
Plants employ defensive strategies that protect them against a diversity of attackers. It is well established that the jasmonate response provides resistance against a wide range of insects that feed on plants, including chewers, suckers, and cell-content feeders (Walling, 2000; Thaler et al., 2002b). The salicylate response is known to protect plants against many pathogens, including fungi, bacteria, and viruses (Kuć, 1982; Ryals et al., 1996). Although the jasmonate and salicylate responses were initially considered to be dichotomous strategies, independent of each other, we now know that they interact (Bostock et al., 2001). In some cases, the expression of these two pathways is negatively correlated with each other phenotypically and genetically (Niki et al., 1998; Felton et al., 1999; Thaler et al., 1999; Spoel et al., 2003). When the jasmonate and salicylate pathways attenuate each other, this is termed a negative signal interaction (Bostock et al., 2001). Such negative signal interactions have been demonstrated in plant defenses of many plants, including Arabidopsis (Gupta et al., 2000), tobacco (Nicotiana tabacum; Preston et al., 1999), and tomato (Lycopersicon esculentum; Peña-Cortes et al., 1993). The ecological consequences of this signal interaction depend, in part, on the range of insects and pathogens that are affected by the jasmonate and salicylate responses. The greater the overlap in the organisms that are negatively affected by both the jasmonate and salicylate responses, the smaller the negative consequence of a trade-off in jasmonate and salicylate expression.
Each pathway may not only provide resistance against its proposed target group but also to species in the other group (Dong, 1998). For example, the role of the jasmonate response in resistance to some pathogens has been demonstrated in several plants, including Arabidopsis (Thomma et al., 1998), tomato (Diaz et al., 2002), Norway spruce (Kozlowski et al., 1999), and barley (Mitchell and Walters, 1995). Penninckx et al. (1996) and Pieterse et al. (1998) described two signaling networks involving jasmonate that could protect plants against pathogens: (1) the jasmonate-ethylene network and (2) the induced systemic resistance network (see also Diaz et al., 2002). The mechanism by which the jasmonate responses affect pathogens is not fully understood, but it can induce pathogenesis-related genes and defensins in Arabidopsis (Penninckx et al., 1996; Thomma et al., 1998). Jasmonate itself can decrease sporangial germination and mycelial growth of Phytophthora infestans (Cohen et al., 1993) and inhibit appressoria differentiation of Erysiphe graminis (Schweizer et al., 1993; Mitchell, 1998). In addition, Ser proteases, regulated by the jasmonate response, have been shown to suppress spore germination and germ tube elongation in two pathogenic fungi (Lorito et al., 1998).
From studies on Arabidopsis and an intuitive model, McDowell and Dangl (2000) proposed that the lifestyle of the pathogen might be a predictor of whether the pathogen will be affected by the jasmonate response or not. This model classifies pathogens into biotrophs and necrotrophs (Parbery, 1996). Biotrophs are pathogens that live and feed in living tissue. Necrotrophs are defined as organisms that live and feed in dead tissue. McDowell and Dangl argued that the salicylate response, frequently deployed to protect plants against pathogens, is associated with production of a hypersensitive response, a response generally considered a form of programmed cell death. This cell death may restrict the growth of a pathogen that has invaded a living cell but can fail to restrict necrotrophic pathogens (Govrin and Levine, 2000). The association between the salicylate response and the hypersensitive response may limit the utility of this set of responses against necrotrophic pathogens, which draw nutrients from host cells they have killed in advance of colonizing (Parbery, 1996; Cohn et al., 2001; Thomma et al., 2001a). The salicylate response is involved in resistance to many biotrophs (Kuć, 1982; Parbery, 1996) and some necrotrophs (Murphy et al., 2000) but apparently fails more often to protect against necrotrophic pathogens (Thomma et al., 2000, 2001a). For example, in Arabidopsis, studies found that salicylate deficiency resulted in increased susceptibility to three out of three of the biotrophic pathogens, whereas salicylate deficiency only resulted in increased susceptibility to one out of three necrotrophic pathogens (for review, see Thomma et al., 2001b). Therefore, the jasmonate response, not associated with cell death, has been proposed as an alternative form of defense against necrotrophic pathogens (McDowell and Dangl, 2000; Thomma et al., 2001a).
In this study, we examined the role of the jasmonate response in susceptibility to eight pathogen species with a range of lifestyles by inoculation of wild-type tomato cv Castlemart and the jasmonate-deficient mutant def1. We tested plant resistance to two bacterial, five fungal, and one oomycete pathogen of tomato plants. Two of these species are unambiguously biotrophic fungi (Cladosporium fulvum and Oidium neolycopersici), sporulating from living tissue. Like many pathogens, the six other species have a less clear-cut lifestyle. P. infestans is a hemibiotrophic oomycete, with an initial biotrophic phase (3–5 d) and a later necrotrophic stage (Smart et al., 2003). By contrast, Septoria lycopersici, while often classified as a necrotroph, has a brief biotrophic phase in tomato, as indicated by intercellular growth between living cells at the lesion margin (Bouarab et al., 2002; V.J. Higgins, unpublished data). The vascular wilt fungi Fusarium oxysporum f. sp. lycopersici and Verticillium dahliae typically colonize xylem vessels, which no longer have a protoplast, and only invade other tissues after the wilting and death caused by the plugging of the vessels by fungal and degraded cell wall material (Bishop and Cooper, 1983). For this study, we consider the wilts closer to biotrophs than necrotrophs because when they initially invade the plant, before reaching the vascular tissue, they have a biotrophic lifestyle (E.J. Robb, personal communication). The apoplastic colonizing bacteria Pseudomonas syringae and Xanthomonas campestris pv vesicatoria are classified by some as biotrophs but, by the definitions used for fungi, are probably best classified as hemibiotrophs because when they initially invade the plant, they have a biotrophic lifestyle but later are necrogenic (Alfano and Collmer, 1996).
To directly test jasmonate deficiency as a factor in the differences between the two plant types, we treated the wild-type and jasmonate-deficient def1 plants with jasmonate to restore the jasmonate response and tested whether this would reduce susceptibility to several of the pathogens. In addition, the jasmonate-insensitive mutant jai1 was tested for susceptibility to disease under field conditions.
RESULTS
Susceptibility of Field-Grown Plants
To examine the role of jasmonate in plant defense against pathogens, 150 wild-type (var Castlemart) and jasmonate-insensitive (jai1) plants were planted in a plowed field at the Koffler Scientific Reserve at Joker's Hill. The jai1 plants are null mutants that lack the ability to respond to jasmonic acid (Li et al., 2004). jai1 plants in the field had higher mortality due to stem wilting than wild-type plants (27% compared to 1.4%; P < 0.001). We isolated a Fusarium species from several of the wilting plants, indicating that this was the likely cause of death.
Comparison of Symptom Development
In laboratory experiments, we examined the role of jasmonate in the development of disease symptoms, using several different techniques depending on the biology of the pathogen. We measured the number of spores produced by the pathogen, the leaf area of the plant that was killed by the pathogen, or the influence of infection on plant growth/biomass. We employ a broad definition of susceptibility here, implying that fewer spores, less plant damage, or greater plant growth indicate decreased susceptibility. In these experiments, we compared the susceptibility of wild-type and jasmonate-deficient mutants (def1). The def1 plants are reduced in their ability to induce jasmonate or proteinase inhibitor activity following herbivore damage (Li et al., 2002) but have the advantage that they can be rescued by exogenous application of jasmonate.
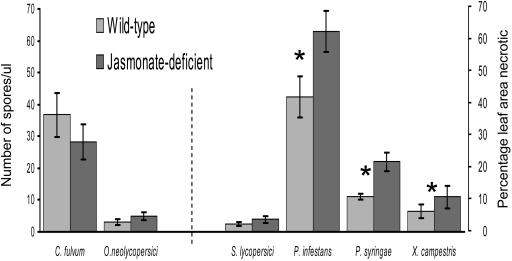
Reduced jasmonate did not result in increased plant susceptibility to the two biotrophs examined. Because these true biotrophs do not produce distinct lesion margins, the degree of susceptibility was measured by the reproduction of the fungus, i.e. spore production. There was no difference in the number of C. fulvum spores produced on jasmonate-deficient (def1) or wild-type plants (Fig. 1; P = 0.294). Similarly, there was no difference in the number of O. neolycopersici spores produced on def1 or wild-type plants (Fig. 1; P = 0.698).
Figure 1.
Comparison of disease production on wild-type and jasmonate-deficient (def1) tomato plants: Extent of C. fulvum and O. neolycopersici infection is given as spores per microliter of extract. Lesion area as a percentage of total leaf area is given for S. lycopersici, P. infestans, P. syringae (×10), and X. campestris (×10) infection. Asterisk indicates a significant difference (P < 0.05) between wild-type and def1 plants. Bars indicate mean ± se.
Contrary to the proposed models, reduced jasmonate had no effect on susceptibility to the most necrotrophic pathogen tested. def1 plants infected with S. lycopersici had the same percentage of necrotic leaf area compared to wild-type plants (Fig. 1; P = 0.420).
In contrast to the effects on the pathogens at the biotrophic and necrotrophic ends of the spectrum, the reduced jasmonate in def1 plants resulted in increased susceptibility to each of the five intermediate species we examined. In a detached leaf assay used routinely in screening P. infestans isolates, def1 plants infected with P. infestans had 50% more necrotic leaflets compared to wild-type plants (Fig. 1; P = 0.033). def1 plants infected with a coronatine-producing strain of P. syringae had twice the necrotic leaf area compared to infected wild-type plants (Fig. 1; P = 0.002), and those infected with X. campestris had three times the necrotic leaf area compared to infected wild-type plants (Fig. 1; P < 0.001).
Plant growth and mortality were measured to indicate plant susceptibility to F. oxysporum and V. dahliae because infection by these pathogens lacks external symptoms. We found that jasmonate was involved in reducing susceptibility to both F. oxysporum and V. dahliae. There was high mortality of F. oxysporum-infected def1 plants (88%) compared to zero mortality of uninfected def1 plants or infected/uninfected wild-type plants (P < 0.001). Infection by F. oxysporum and V. dahliae reduced the growth of def1 but not wild-type plants (Fig. 2; Supplemental Table I). Jasmonate-deficient plants were 40% shorter than the controls when inoculated with V. dahliae and 93% shorter when inoculated with F. oxysporum. By contrast, the height of wild-type plants was reduced by 4% and 14% when inoculated with V. dahliae and F. oxysporum, respectively, compared to controls. The reduced growth of def1 plants when infected was also seen in our final measure of plant biomass (46% reduction by V. dahliae, 77% reduction by F. oxysporum), while the biomass of wild-type plants was not affected (data not shown). Culturing of stem slices confirmed that the wild-type plants were infected with F. oxysporum and V. dahliae.
Figure 2.
Comparison of height (mm) of wild-type and jasmonate-deficient (def1) plants that were not inoculated or inoculated with F. oxysporum f. sp. lycopersici or V. dahliae. Letters indicate significant differences between treatments (P < 0.05) using Fisher's least significant differences method. Bars indicate mean ± se.
Jasmonate Recovery of Mutant Plants
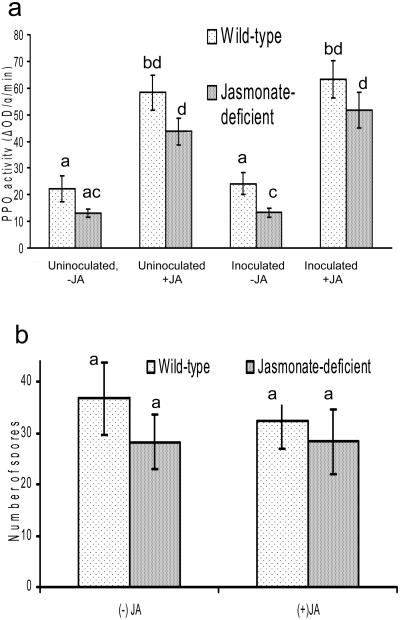
To directly implicate jasmonate deficiency as a causal factor of the differences between the two plant types, we treated the def1 plants with jasmonate to restore the jasmonate response and tested whether this would decrease the plants' susceptibility to two of the pathogens. The def1 mutant can induce the jasmonate response if the plant is treated with jasmonic acid (Howe et al., 1996; Thaler et al., 2002a). We tested the effect of jasmonate treatment on susceptibility to one pathogen (F. oxysporum) where the def1 plants were more susceptible to disease than the wild-type plant and one pathogen (C. fulvum) where there was no difference in susceptibility between def1 and wild-type plants. We measured the effect of jasmonate application on chemical expression of the jasmonate response using polyphenol oxidase (PPO) as our marker (Thaler et al., 2002a) and on plant susceptibility to the pathogens.
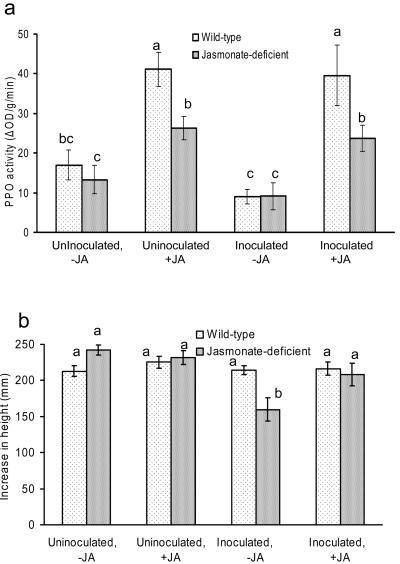
Jasmonate-treatment increased PPO activity in both uninoculated and inoculated wild-type and def1 plants (Fig. 3a; Supplemental Table II). C. fulvum inoculation had no effect on PPO activity. Jasmonate treatment did not affect the number of C. fulvum spores produced on either wild-type or def1 plants (Fig. 3b; P = 0.723). This is consistent with the lack of difference in spore production on wild-type and def1 plants reported above. In the experiment with F. oxysporum, jasmonate treatment again increased PPO activity in both uninoculated and inoculated wild-type and def1 plants (Fig. 4a; Supplemental Table II). F. oxysporum inoculation had no effect on PPO activity. Although F. oxysporum inoculation decreased growth of the untreated def1 plants, plant growth was fully restored when def1 plants were treated with jasmonic acid (Fig. 4b). F. oxysporum inoculation did not influence the growth of the wild-type plants, regardless of jasmonate treatment. The jasmonate treatment itself did not affect the growth of the wild type or def1 (Fig. 4b; Supplemental Table III). These results are consistent with our finding that def1 plants are more susceptible to F. oxysporum and directly implicate the jasmonate response in decreasing susceptibility to several, but not all, pathogens.
Figure 3.
Effect of exogenous jasmonate application on infection of wild-type and jasmonate-deficient (def1) tomato plants by C. fulvum. a, PPO activity in wild-type and def1 plants that are infected with C. fulvum and/or treated with 0.5 mm jasmonic acid. b, Number of C. fulvum spores produced by wild-type and def1 plants that are either treated with 0.5 mm jasmonic acid or controls. Letters indicate significant differences between treatments (P < 0.05) using Fisher's least significant differences method. Bars indicate mean ± se.
Figure 4.
Effect of exogenous jasmonate application on infection of wild-type and jasmonate-deficient (def1) tomato plants by F. oxysporum f. sp. lycopersici. a, PPO activity in wild-type and def1 plants that are infected with F. oxysporum and/or treated with 0.5 mm jasmonic acid. b, Growth of wild-type and def1 plants that are infected with F. oxysporum and/or treated with 0.5 mm jasmonic acid. Letters indicate significant differences between treatments (P < 0.05) using Fisher's least significant differences method. Bars indicate mean ± se.
DISCUSSION
Overall, we found that jasmonate deficiency increased the susceptibility of tomato plants to five of the eight pathogens examined. All of the pathogens were virulent on the wild-type plants. This increased susceptibility was found for both of the bacteria (P. syringae and X. campestris), the two vascular wilt fungi (F. oxysporum and V. dahliae), and for the oomycete (P. infestans). Susceptibility to three other fungi, S. lycopersici, C. fulvum, and O. neolycopersici, was not affected. Thus, the jasmonate response is involved in limiting susceptibility to pathogens from a wide range of taxonomic groupings and lifestyles.
Perhaps biotrophy and necrotrophy are best viewed as a continuum. Although there are numerous unambiguous examples of biotrophs, e.g. the rust and powdery mildew fungi, based on the current microscopical and ultrastructural standards (Parbery, 1996), absolute necrotrophy is perhaps less frequent. Some fungi such as Sclerotinia, which require an external food base such as dead petals to colonize a host plant, appear to be true necrotrophs. On close examination, especially via microscopy, numerous other fungi that were once assumed to be necrotrophs have been reclassified as hemibiotrophs because colonization involves a brief or extended period before dead cells appear. Parbery (1996) classified pathogens into those predominantly biotrophic hemibiotrophs, in which, for tomato, we would include P. infestans, V. dahliae (also the bacteria P. syringae and X. campestris), and those predominantly necrotrophic hemibiotrophs, in which we would include S. lycopersici. Based on the degree of necrotic symptoms seen in Fusarium-inoculated plants, F. oxysporum f. sp. lycopersici might also best fit this latter classification. We propose that the pathogens used in this study would thus line up along this gradient, starting with the most biotrophic, as follows: Oidium > Cladosporium > Phytophthora > Verticillium > Pseudomonas > Xanthomonas > Fusarium > Septoria.
As predicted, the two clear biotrophs were not affected by the jasmonate response (C. fulvum and O. neolycopersici). However, the most necrotrophic of the group, S. lycopersici, was also not affected by the jasmonate deficiency. The jasmonate-deficient plants exhibited increased susceptibility to all of the intermediate and/or difficult-to-classify species. We predicted that those hemibiotrophs closest to the biotrophic end of the gradient would not be affected by the deficiency in jasmonate. The late blight pathogen P. infestans, particularly under the high humidity conditions used in our experiments, is only marginally down the biotroph gradient from C. fulvum, with both distinguished from Oidium by their ease of growth in culture. These results do not support the McDowell and Dangl (2000) prediction that the jasmonate response will only affect necrotrophic pathogens, not biotrophs. Thus, overall our results do not support a dichotomy between the lifestyles of pathogens and the effects of the jasmonate pathway.
Several other studies on tomato plants have examined the role of the jasmonate response in susceptibility to pathogens. An interesting picture emerges from comparing our results with P. syringae to those of Zhao et al. (2003). They find that coronatine-producing strains of P. syringae induce the jasmonate response of tomato plants, resulting in reduced expression of the salicylate response and increased susceptibility of the plant to the bacteria (compared to strains that do not produce coronatine). In our study, we find that the jasmonate response reduces the susceptibility of the plant to our strain of P. syringae, also a producer of coronatine (Cuppels and Ainsworth, 1995). This suggests that while both the salicylate and jasmonate responses can reduce susceptibility to P. syringae, the jasmonate response may be less effective. Bacterial induction of the jasmonate response and the resulting reduction of the salicylate response increase the plant's susceptibility. This interpretation is consistent with previous results demonstrating that while both the jasmonate and salicylate response can reduce disease caused by P. syringae, the salicylate response is more effective (Thaler et al., 1999).
In addition, Cohen et al. (1993) found that induction of the jasmonate response, using methyl jasmonate, resulted in increased resistance to P. infestans, whereas Smart et al. (2003) failed to find an effect of jasmonate-deficient plants on resistance to P. infestans. In our experiments on P. infestans, we found results similar to Cohen. The discrepancy between Smart et al. (2003) and the findings of Cohen et al. (1993) and this study are difficult to resolve because none of the data is reported by Smart et al. (2003). Thus, where quantitative data have been presented, jasmonate appears to be effective at reducing susceptibility of tomato to P. infestans. Diaz et al. (2002) found that jasmonate-deficient plants were more susceptible to Botrytis cinerea, a necrotroph; however, Audenaert et al. (2002) found no effect on B. cinerea. Note that Parbery (1996) describes at least one Botrytis-host interaction, in which Botrytis would be classified as a hemibiotroph, suggesting that close examination of the early colonization process in tomato foliage is needed. These inconsistencies point to the importance of variation in the particular microbial strains used and/or methods employed in experiments.
The most comprehensive examination of the effect of the jasmonate response on pathogens has been in Arabidopsis (Thomma et al., 2001b), where resistance of wild-type and jasmonate-deficient Arabidopsis plants to 10 pathogens has been studied. The pattern in Arabidopsis appears to be different from what we find in tomato. In Arabidopsis, a reduced jasmonate response resulted in increased susceptibility to five necrotrophs: Alternaria brassicicola (Penninckx et al., 1996; Thomma et al., 1998), Pythium irregulare (Staswick et al., 1998), Pythium mastophorum (Vijayan et al., 1998), B. cinerea (Thomma et al., 1998), and Erwinia carotovora (Norman-Setterblad et al., 2000). Three biotrophs were unaffected: Erysiphe orontii (Reuber et al., 1998), Peronospora parasitica (Thomma et al., 1998), and Phytophthora porri (a hemibiotroph; Roetschi et al., 2001). One biotroph, Erysiphe cichoracearum, was negatively affected by the jasmonate response (Ellis et al., 2002). P. syringae, a species whose lifestyle is difficult to classify but has been called a biotroph in this literature, was negatively affected by the jasmonate response (Pieterse et al., 1998; Ellis and Turner, 2001). Thus, from Arabidopsis, the evidence is reasonably strong, although not without exception, that the jasmonate response protects plants against necrotrophic pathogens but not most biotrophic pathogens. There is nothing obviously different about the lifestyles of pathogens that we employed in this study except that we also examined two vascular wilts. The differences between the Arabidopsis and tomato interactions with pathogens highlight the importance of testing patterns of resistance in more than one system before generalizations can be made (Diaz et al., 2002).
The effect of the jasmonate and salicylate response on a large of number of species that attack tomato plants has been examined (Table I). In particular, our knowledge of the herbivores affected by the jasmonate and salicylate response in tomato is more complete than our knowledge in Arabidopsis. The jasmonate response increases plant resistance to a wide range of insects and pathogens (12 out of 16 species). Conversely, the salicylate response increases plant resistance to many but not all pathogens (7 out of 8 species). Although the salicylate response also increases resistance to phloem feeding insects (2 out of 2 species; Table I; Ellis et al., 2002), it also consistently induces susceptibility to chewing insects (3 of 4 species; Felton et al., 1999; Thaler et al., 2002b) and had no effect on cell-content feeders (2 out of 2 species).
Table I.
Organisms for which resistance mediated by both the jasmonate and salicylate response have been tested in tomato plants
| Species | Lifestyle | Jasmonate | Salicylate |
|---|---|---|---|
| Insects | |||
| Spodoptera exigua | Chewer | Protection (1, E) | Susceptibility (4, E) |
| Trichoplusia ni | Chewer | Protection (2, E) | Susceptibility (4, E) |
| Helicoverpa zea | Chewer | Protection (1, E) | Susceptibility (12, E) |
| Manduca sexta | Chewer | Protection (3, G), No effect (2, E) | No effect (4, E) |
| Frankliniella occidentalis | Cell-content feeder | Protection (4, E) | No effect (4, E) |
| Tetranychus urticae | Cell-content feeder | Protection (4, E) (16, G) | No effect (4, E) |
| Macrosiphum euphorbiae | Phloem feeder | Protection, No effect (4, E) | Protection (13, G and E) |
| Liriomyza spp. | Palisade mesophyll | Protection (1, E) | Protection (9, E) |
| Pathogens | |||
| Pseudomonas syringae | ?/Biotroph | Protection (5, G; 13, E) | Protection (11, E) |
| Xanthomonas campestris | ?/Biotroph | Protection (5, G) | Protection (9, E) |
| Fusarium oxysporum | Vascular wilt | Protection (5, G) | Protection (10, E) |
| Oidium spp. | Biotroph | No effect (5, G) | No effect, Protection (9, E) |
| Cladosporium fulvum | Biotroph | No effect (5, G) | No effect (8, G) |
| Phytophthora infestans | Hemibiotroph | Protection (5, G; 6, E), No effect (7, G; 13, E) | No effect (7, G) |
| Botrytis cinerea | Necrotroph | Protection (15, G), No effect (15, G) | Protection (14, G and E; 15, E) |
| Tomato spotted wilt virus | ? | No effect (4, E) | Protection (17, E) |
References are given in the parentheses as follows: 1, Stout and Duffey (1996); 2, Thaler et al. (1996); 3, Orozco-Cardenas et al. (1993); 4, Thaler et al. (2002b); 5, this study; 6, Cohen et al. (1993); 7, Smart et al. (2003); 8, Brading et al. (2000); 9, Inbar et al. (1998); 10, Benhamou and Belanger (1998); 11, Louws et al. (2001); 12, Stout et al. (1999); 13, J. Thaler, unpublished data; 14, Audenaert et al. (2002); 15, Diaz et al. (2002); 16, Li et al. (2002); 17, Tally et al. (1999). Methods of evaluation (given in parentheses) include natural elicitation with an organism known to induce the jasmonate or salicylate response (N), elicitation with a chemical elicitor of the jasmonate or salicylate response (E), or use of a plant with a genetically modified jasmonate or salicylate response (G).
In conclusion, the large degree of overlap in the organisms affected by the jasmonate response indicates that the ecological consequences of inducing this response will be great, influencing not only interactions with other species of herbivorous insects but also many pathogens and higher trophic levels (Thaler, 1999; Thaler and Bostock, 2004). The greater the overlap in the organisms that are affected by both the jasmonate and salicylate responses, the less likely there will be negative consequences for the plant of a trade-off in their expression. This study shows that when the jasmonate response is activated, even to the detriment of activation of the salicylate response, the plant will still induce resistance to taxonomically diverse pathogens with varying lifestyles.
MATERIALS AND METHODS
Susceptibility of Field-Grown Plants
Wild-type and jai1 plants were grown in 200-mL mesh pots in a greenhouse until the three-leaf stage, when they were planted in a tilled field (June 6, 2001) and fertilized with 10:52:10 N:P:K liquid fertilizer. Between June 14 and 17, the aboveground parts of many plants wilted and died. This mortality was scored. The severely wilted plants were brought to the lab and tested for colonization by standard surface sterilization methods and plating on V-8 juice agar.
Comparison of Symptom Development
Plants
Jasmonate-deficient plants (def1) were originally produced from mutagenized seed (var Castlemart) and identified based on their reduced ability to induce proteinase inhibitor II activity following mechanical damage (Lightner et al., 1993). def1 plants receive more herbivore damage compared to wild-type plants (Howe et al., 1996; Li et al., 2002; Thaler et al., 2002a). The mutant has a similar growth form to the wild type in at least several traits, including height, number of leaves, and dry mass (J. Thaler, unpublished data). The mutant plants used in this experiment were homozygous and backcrossed five times in the Castlemart variety.
Cladosporium fulvum
Wild-type and def1 plants were grown in 3-inch pots with Promix BX soil (Redhill, PA) augmented with 5 mL of Nutricote 13:13:13 N:P:K (Vicksburg Chemical, Vicksburg, MS) and fertilized weekly with water-soluble 20:20:20 N:P:K. C. fulvum race 2.3 culture was grown at 22°C on V-8 juice agar with a 16-h photoperiod. Spore suspensions, in a 0.01% (v/v) aqueous Tween 20 solution, were adjusted to contain 1 × 105 spores/mL of water. The control solution was water with 0.01% (v/v) Tween 20. Three weeks after planting (three-leaf stage), C. fulvum was inoculated by spraying the entire plant until it was wet. Plants were then moved to an incubator at 23°C, 14 h light, and high humidity maintained by covering in plastic bags supported by bamboo stakes. For the first 7 d, the bags were tied at the bottom to increase the humidity, and following this the bags were opened at the bottom. Fourteen days postinoculation, a leaf disc was taken from leaf 2 with a number 7 cork borer and placed in a tube containing 0.5 mL of a 0.01% (v/v) Tween 20 solution. This was vortexed, and three 2-μL drops were removed and the spores counted using a hemocytometer. Counts for the three drops were averaged to get a mean per microliter for each plant. Two trials of the experiment were performed with 13 to 20 replicates per treatment per trial. The number of spores was analyzed by two-way ANOVA, with plant and trial as the main effects.
Oidium neolycopersici
Wild-type and def1 plants were grown in 3-inch pots as described above. Conidia of O. neolycopersici were collected from visibly sporulating leaf areas immediately before inoculation from infected plants growing in a greenhouse. Three weeks after planting (three-leaf stage), the spores were wiped onto the four lateral leaflets of leaf 3. The plants were placed inside a clear plastic tent in the greenhouse (16-h-light/8-h-dark cycle). Eight days postinoculation, leaf discs were taken and spores counted as for C. fulvum. Two trials were conducted with 11 to 17 replicates per treatment per trial. The number of spores was analyzed by two-way ANOVA, with plant and trial as the main effects.
Septoria lycopersici
Wild-type and def1 plants were grown in 4-inch pots as described above. S. lycopersici was grown on V-8 agar at 22°C 16 h light for approximately 2 weeks prior to inoculation. Four weeks after planting (six-leaf stage), the four lateral leaflets of leaf 4 were treated as above (1 × 105 spores/mL in 0.01% [v/v] Tween 20) for the Cladosporium experiments. After inoculation, plants were placed in the greenhouse in a humidified clear plastic tent. Fourteen days postinoculation, the percentage leaf area infected was quantified using ImagePro Plus (Media Cybernetics, Silver Springs, MD). Two trials of the experiment were performed with 16 to 20 replicates per treatment per trial. The percentage area infected was analyzed using two-way ANOVA, with plant and trial as the main effects.
Phytophthora infestans
Wild-type and def1 plants were grown in 4-inch pots as described above. P. infestans (P1151, A1 mating type collection, virulent on both tomato [Lycopersicon esculentum] and potato [Solanum tuberosum]) was grown for 2 weeks on rye media at 18°C. A suspension of 1.3 × 106 spores/mL was used. Three weeks after planting, four lateral leaflets plus the terminal leaflet of leaf 4 were excised and floated abaxial side in tap water, and one droplet of the spore suspension was placed on either side of the midvein. The five leaflets per plant were placed in a 15°C, 12-h-light/12-h-dark incubator for the first 6 h, then 18°C for 7 d. Seven days postinoculation, the number of leaflets from each plant that were sporulating was scored to give a percentage leaflet area infected per plant. Three trials were conducted with 5 to 12 replicates per treatment per trial. The number of leaflets infected was analyzed using two-way ANOVA, with plant and trial as the main effects.
Fusarium oxysporum f. sp. lycopersici and Verticillium dahliae
Wild-type and def1 tomato seeds were planted in growth chambers in plug trays (1.6 × 3.6 cm; Plant Products, Leamington, Canada) containing Promix soil with an 18-h photoperiod at 23°C and approximately 70 μmol light. F. oxysporum (UT22 race) and V. dahliae (race 1 isolate no. 122) were grown on V-8 agar for 1 week. When the plants were 3 weeks old, they were divided into three groups: group 1 inoculated with F. oxysporum, group 2 inoculated with V. dahliae, and group 3 an uninoculated control. All plants were removed from their pots, washed in tap water to extend the roots, and cut so that each plant had 1.5 inches of root left. The cut roots were either dipped into spore suspensions of the F. oxysporum or V. dahliae inoculum (1.0 × 105 spores/mL) or dipped into water as a control. Each plant was transferred to a styrofoam cup (42 mL) with a drain hole in the bottom and grown for an additional 3 weeks. Plants were fertilized with 20:20:20 N:P:K at week 4.
Weekly measurements of plant height, mortality, and number of leaves (data not shown) were taken. On the day of the final harvest (3 weeks postinoculation), aboveground biomass was measured, and a section of the stem was removed and cultured on V-8 agar to confirm that the wild-type and jasmonate-deficient plants were indeed infected with the appropriate fungus. Three trials of this experiment were performed with 30 replicates per treatment per trial (n = 270). The height and biomass were analyzed separately for F. oxysporum- and V. dahliae-inoculated plants using three-way ANOVA, with plant type, inoculation, and trial as the main effects. Mortality caused by each pathogen was analyzed using a chi-square test.
Pseudomonas syringae and Xanthomonas campestris
Wild-type and def1 plants were grown in 4-inch pots as described above. P. syringae pv tomato (DCT6D1) and X. campestris pv versicatoria (DC93-1) were grown at room temperature on King's B agar (King et al., 1954) for 3 d before use. Four weeks after planting (six-leaf stage), the four lateral leaflets of leaf 4 were lightly dusted with carborundum and about 1 mL of inoculum (1 × 107 bacteria/mL in aqueous 0.01% [v/v] Tween 20 solutions) or control solution (0.01% [v/v] Tween 20) was divided between the four leaflets on each leaf and gently rubbed. After inoculation, plants were placed in a greenhouse in a humidified clear plastic tent. The percentage leaf area infected was measured 12 d postinoculation by removing the inoculated leaf, scanning leaves on a flat bed scanner, and then using ImagePro Plus to determine lesion and total leaf areas. The P. syringae experiment was repeated two times with 19 to 20 replicates per treatment per trial, and the X. campestris experiment was repeated twice with 11 to 28 replicates per treatment per trial. The percentage area infected was analyzed by two-way ANOVA, with plant and trial as the main effects. For the X. campestris experiments, the residuals were not normally distributed so a Mann-Whitney U test was performed (P < 0.001) to verify the main effect of plant in the ANOVA.
Jasmonate Rescue of Jasmonate-Deficient Plants
Plants were grown and infected using the same protocols as in the above experiments. Two days prior to inoculation, the wild-type and def1 plants were divided into two groups, for one group the entire plant was misted with 0.5 mm jasmonic acid in 0.5 mL of acetone/liter of water, and the control group was misted with 0.5 mL of acetone/liter of water. The jasmonic acid was synthesized from methyl jasmonate according to the methods of Farmer and Ryan (1992). Two days following jasmonate treatment, half of the jasmonate-treated plants and half of the control plants were inoculated with either F. oxysporum or C. fulvum and the resistance assay conducted in the same manner as described above.
For C. fulvum treatments the terminal leaflet of leaf 2 was collected for the PPO measurement, and for F. oxysporum treatments the terminal leaflet of leaf 4 was collected for the PPO measurement. The leaflets were frozen until the chemical assay was performed. To determine PPO activity, weighed leaflets were homogenized in ice-cold buffer, and the homogenate was centrifuged to obtain a clarified extract for enzyme analyses. The supernatant was added to a caffeic acid solution and absorbance read at 470 nm (Thaler et al., 1999).
For the F. oxysporum experiment, 30 replicates per treatment were employed for the measurement of plant height and 12 to 24 replicates per treatment for the PPO activity measurement. For the C. fulvum experiments, 13 to 18 replicates per treatment per trial were employed for the measurement of spore production and 8 to 14 replicates per treatment per trial for the PPO activity measurement. Plant height (F. oxysporum) or the number of spores (C. fulvum) was analyzed using three-way ANOVA, with plant type, infection, and trial as the main effects. PPO activity (C. fulvum experiment) was analyzed using four-way ANOVA, with plant type, jasmonate treatment, C. fulvum infection, and trial as the main effects; in the F. oxysporum experiment, PPO activity was analyzed with three-way ANOVA, with plant type, jasmonate treatment, and F. oxysporum infection as the main effects.
Supplementary Material
Acknowledgments
We thank Lisa Plane, Alice Cheung, and Forzana Nadeem, without whom this study would not have been possible. We thank Gregg Howe for providing seeds of the jasmonate-insensitive plants, D. Cuppels (Agriculture and Agri-Food Canada [AAFC]) for the bacterial isolates, H. Platt (AAFC) for the P. infestans isolate, and J. Robb (University of Guelph, Canada) for the V. dahlia isolate. Anurag Agrawal, Bart Thomma, and two anonymous reviewers provided useful comments on the manuscript.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grants to J.S.T. and V.J.H.), the Canadian Foundation for Innovation, and a Premier's Research Excellence Awards program (award to J.S.T.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041566.
References
- Alfano JR, Collmer A (1996) Bacterial pathogens in plants: life up against the wall. Plant Cell 8: 1683–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Hofte MM (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128: 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou N, Belanger RR (1998) Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato. Plant Physiol 118: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CD, Cooper RM (1983) An ultrastructural study of root invasion in 3 vascular wilt diseases. Physiol Plant Pathol 22: 15–27 [Google Scholar]
- Bostock RM, Karban R, Thaler JS, Weyman PD, Gilchrist D (2001) Signal interactions in induced resistance to pathogens and insect herbivores. Eur J Plant Pathol 107: 103–111 [Google Scholar]
- Bouarab K, Melton R, Peart J, Baulcombe D, Osbourn A (2002) A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418: 889–892 [DOI] [PubMed] [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JDG (2000) Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J 23: 305–318 [DOI] [PubMed] [Google Scholar]
- Cohen Y, Gisi U, Niderman T (1993) Local and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid and jasmonic acid methyl ester. Phytopathology 83: 1054–1062 [Google Scholar]
- Cohn J, Sessa G, Martin GB (2001) Innate immunity in plants. Curr Opin Immunol 13: 55–62 [DOI] [PubMed] [Google Scholar]
- Cuppels DA, Ainsworth T (1995) Molecular and physiological characterization of Pseudomonas syringae pv tomato and Pseudomonas syringae pv maculicola strains that produce phytotoxin. Appl Environ Microbiol 61: 3530–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, ten Have A, van Kan JAL (2002) The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea. Plant Physiol 129: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X (1998) SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1: 316–323 [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis L, Turner JG (2002) Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact 15: 1025–1030 [DOI] [PubMed] [Google Scholar]
- Ellis C, Turner JG (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Korth KL, Wesley SV, Huhman DV, Mathews MC, Murphy JB, Lamb C, Dixon RA (1999) Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr Biol 9: 317–320 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10: 751–757 [DOI] [PubMed] [Google Scholar]
- Gupta V, Willits MG, Glazebrook J (2000) Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of defense responses: Evidence for inhibition of jasmonic acid signaling by SA. Mol Plant Microbe Interact 13: 503–511 [DOI] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signalling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M, Doostdar H, Sonoda RM, Leibee GL, Mayer RT (1998) Elicitors of plant defensive systems reduce insect densities and disease incidence. J Chem Ecol 24: 135–149 [Google Scholar]
- King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–307 [PubMed] [Google Scholar]
- Kozlowski G, Buchala A, Metraux JP (1999) Methyl jasmonate protects Norway spruce [Picea abies (L.) Karst.] seedlings against Pythium ultimum Trow. Physiol Mol Plant Pathol 55: 53–58 [Google Scholar]
- Kuć J (1982) Induced immunity to plant disease. Bioscience 32: 854–860 [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130: 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao YF, McCaig BC, Wingerd BA, Wang JH, Whalon ME, Pichersky E, Howe GA (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16: 126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J, Pearce G, Ryan CA, Browse J (1993) Isolation of signaling mutants of tomato (Lycopersicon esculentum). Mol Gen Genet 241: 595–601 [DOI] [PubMed] [Google Scholar]
- Lorito M, Woo SL, Fernandez IG, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zonia A, et al. (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95: 7860–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louws FJ, Wilson M, Campbell HL, Cuppels DA, Jones JB, Shoemaker PB, Sahin F, Miller SA (2001) Field control of bacterial spot and bacterial speck of tomato using a plant activator. Plant Dis 85: 481–488 [DOI] [PubMed] [Google Scholar]
- McDowell JM, Dangl JL (2000) Signal transduction in the plant immune response. Trends Biochem Sci 25: 79–82 [DOI] [PubMed] [Google Scholar]
- Mitchell A (1998) Expression of systemic resistance in Hordeum vulgare against Erysiphe graminis by treatment with abiotic elicitors. PhD thesis. University of Glasgow, Glasgow, Scotland
- Mitchell AF, Walters DR (1995) Systemic protection in barley against powdery mildew infection using methyl jasmonate. Asp Appl Biol 42: 323–326 [Google Scholar]
- Murphy AM, Holcombe LJ, Carr JP (2000) Characteristics of salicylic acid-induced delay in disease caused by a necrotrophic fungal pathogen in tobacco. Physiol Mol Plant Pathol 57: 47–54 [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y (1998) Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol 39: 500–507 [Google Scholar]
- Norman-Setterblad C, Vidal S, Palva ET (2000) Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol Plant Microbe Interact 13: 430–438 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, McGurl B, Ryan CA (1993) Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci USA 90: 8273–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parbery DG (1996) Trophism and the ecology of fungi associated with plants. Biol Rev 71: 473–527 [Google Scholar]
- Peña-Cortes H, Albrecht T, Prat S, Weiler EW, Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191: 123–128 [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, de Samblanx GW, Buchala A, Metraux J-P, Manners JM, Broekaert WF (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8: 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, van Wees SCM, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon JJA (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT (1999) Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta 209: 87–95 [DOI] [PubMed] [Google Scholar]
- Reuber TL, Plotnikova JM, Dewdney K, Rogers EE, Wood W, Ausubel FM (1998) Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J 16: 473–485 [DOI] [PubMed] [Google Scholar]
- Roetschi A, Si-Ammour A, Belbahri L, Mauch F, Mauch-Mani B (2001) Characterization of an Arabidopsis-Phytophthora pathosystem: Resistance requires a functional PAD2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J 28: 293–305 [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer P, Gees R, Mosinger E (1993) Effect of jasmonic acid on the interaction of barley (Hordeum vulgare L.) with the powdery mildew Erysiphe graminis f.sp. hordei. Plant Physiol 102: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart CD, Myers KL, Restrepo S, Martin GB, Fry WE (2003) Partial resistance of tomato to Phytophthora infestans is not dependent upon ethylene, jasmonic acid, or salicylic acid signaling pathways. Mol Plant Microbe Interact 16: 141–148 [DOI] [PubMed] [Google Scholar]
- Spoel S, Koornneef A, Claessens SMC, Korzelius JP, van Pelt JA, Mueller MJ, Buchala AJ, Metraux JP, Brown R, Kazan K, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15: 747–754 [DOI] [PubMed] [Google Scholar]
- Stout MJ, Duffey SS (1996) Characterization of induced resistance in tomato plants. Entomol Exp Appl 79: 273–283 [Google Scholar]
- Stout MJ, Fidantsef AL, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol 54: 115–130 [Google Scholar]
- Tally A, Oostendorp M, Lawton K, Staub T, Bassi B (1999) Commercial development of elicitors of induced resistance to pathogens. In AA Agrawal, S Tuzun, E Bent, eds, Inducible Plant Defenses against Pathogens and Herbivores. American Phytopathological Society Press, St. Paul, pp 357–369
- Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399: 686–688 [Google Scholar]
- Thaler JS, Bostock RM (2004) Interactions between abscisic acid mediated responses to osmotic stress and plant resistance to pathogens and insects. Ecology 85: 48–58 [Google Scholar]
- Thaler JS, Farag MA, Pare PW, Dicke M (2002. a) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5: 764–774 [Google Scholar]
- Thaler JS, Fidantsef AL, Duffey SS, Bostock RM (1999) Trade-offs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. J Chem Ecol 25: 1597–1609 [Google Scholar]
- Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM (2002. b) Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131: 227–235 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Stout MJ, Karban R, Duffey SS (1996) Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J Chem Ecol 22: 1767–1781 [DOI] [PubMed] [Google Scholar]
- Thomma B, Eggermont K, Broekaert WF, Cammue BPA (2000) Disease development of several fungi on Arabidopsis can be reduced by treatment with methyl jasmonate. Plant Physiol Biochem 38: 421–427 [Google Scholar]
- Thomma B, Penninckx I, Broekaert WF, Cammue BPA (2001. a) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- Thomma B, Tierens KFM, Penninckx I, Mauch-Mani B, Broekaert WF, Cammue BPA (2001. b) Different micro-organisms differentially induce Arabidopsis disease response pathways. Plant Physiol Biochem 39: 673–680 [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Shockey J, Levesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95: 7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19: 195–216 [DOI] [PubMed] [Google Scholar]
- Zhao YF, Thilmony R, Bender CL, Schaller A, He SY, Howe GA (2003) Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J 36: 485–499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.