Abstract
INTRODUCTION
Fertility in women declines with increasing age. With the deferment of marriage and childbearing, couples are turning to assisted reproductive technology to counteract this decline. We aimed to evaluate the results of in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) in women of different age groups, and highlight the cost-effectiveness of IVF treatment in these groups while assessing its implications on the national healthcare provision model.
METHODS
Retrospective analysis of 3,412 stimulated IVF/ICSI cycles in a hospital-based IVF centre was performed from January 2008 to December 2010. Patients were stratified into seven age groups: < 30 years; 30–35 years; 36–37 years; 38 years; 39 years; 40–44 years; and ≥ 45 years.
RESULTS
Age had a significant effect on the number of cycles leading to embryo transfer (p < 0.001). The number of oocytes retrieved decreased across the various age groups (p < 0.001) and was the highest among women aged < 30 (mean 18.5 ± 10.3) years. With increasing age, there was a trend toward a lower fertilisation rate. Age also had a significant effect on the rates of clinical pregnancy, live birth and multiple pregnancies (p < 0.001).
CONCLUSION
Patients aged < 30 years had the best IVF outcomes, reflecting optimal reproductive capacity. Age-related decline in fertility starts after 30 years. Women opting for IVF should be counselled about age-specific success rates while taking into account individual risk factors.
Keywords: clinical pregnancy rate, female ageing, in vitro fertilisation outcome, live birth rate, miscarriage rate
INTRODUCTION
With increasing age, fertility in women declines.(1-3) Most women reach menopause by their early fifties, and biological infertility occurs about 10–12 years before menopause.(4,5) However, there is no universal definition of an advanced reproductive age for women, in part because the effects of increasing age occur as a continuum rather than as a threshold effect, and declining fertility is an individual event that differs in each woman.(6)
Nevertheless, it is well known that fecundability (i.e. the probability of achieving a pregnancy in one menstrual cycle) begins to decline significantly in the early thirties, with a more rapid decline a few years later (around 37 years of age).(7) In women aged 31–35 years, spontaneous cumulative pregnancy rates begin to decline, and by 35–39 years, one-third of women experience difficulty conceiving. By 40–44 years of age, half of all women have impaired reproductive capacity.(8)
The precise reason for the loss of fertility with female ageing is not fully understood, although it is thought to be due to genes encoded on the X chromosome and autosomes.(6) The mechanisms postulated to be responsible for such loss include decreasing ovarian reserve,(1) poorer oocyte quality,(9) lower embryo implantation rates,(10) altered hormonal environment resulting in ovulatory dysfunction(11,12) and uterine problems.(3,7) There is a higher propensity for acquired conditions such as endometriosis, fibroids and pelvic infections to occur in older women.(3) Lifestyle factors such as obesity and lower frequency of intercourse are also potential risk factors.(3)
With the deferment of marriage and childbearing, more couples nowadays are turning to assisted reproductive technology in an attempt to counteract age-related decline in female reproductive capacity. However, it is indisputable that the highest live birth rates in in vitro fertilisation (IVF) are still seen in younger women aged 25–30 years,(13) a finding which confirms that a woman’s age is an important determinant of IVF success.
The primary objective of the present study was to evaluate the results of IVF/intracytoplasmic sperm injection (ICSI) in women of different age groups. The secondary objective involved highlighting the cost-effectiveness of IVF treatment in these age groups while assessing the implications of IVF treatment on the national healthcare provision model in Singapore.
METHODS
The medical records of 2,900 women undergoing IVF at KK Women’s and Children’s Hospital IVF Centre, Singapore, from January 2008 to December 2010 were reviewed. A total of 3,412 fresh IVF cycles using autologous oocytes commenced during the study period. The causes of subfertility in couples seen at our centre included male factors, ovulatory disorders, tubal disease, endometriosis, failed intrauterine insemination, previous tubal ligation, premature ovulation failure and unexplained subfertility.
A retrospective review of all 3,412 cycles was performed. The ages of the women were recorded at the start of stimulation, and the patients were stratified into the following seven age groups: < 30 years; 30–35 years; 36–37 years; 38 years; 39 years; 40–44 years; and ≥ 45 years. The IVF outcomes of these age groups were reviewed. The indices reviewed included the average number of oocytes collected, mean duration of stimulation and fertilisation rates. The number of cycles reaching embryo transfer, clinical pregnancy rates, live birth rates, miscarriage rates and multiple pregnancy rates were also reported.
The average number of oocytes collected per cycle, average days of stimulation and fertilisation rate were reported as mean ± standard deviation. The fertilisation rate was reported in terms of percentage. Differences in these factors between the various age groups were tested using one-way analysis of variance (ANOVA). Where differences were found to be statistically significant, contrast analysis was performed to further analyse the difference for significance.
Frequency and percentages were reported for the variables – number of cycles with embryo transfer, number of clinical pregnancies, live births, miscarriages and multiple pregnancies. Differences in these factors between the age groups were analysed using the chi-square test. Odds ratio (OR) between the different age groups was also reported. A p-value of < 0.05 was considered to be statistically significant.
RESULTS
Of the 3,412 fresh cycles, the majority was noted in women aged 30–35 years (n = 1,583, 46.4%), followed by women aged 36–37 years (n = 569, 16.7%) and < 30 years (n = 386, 11.3%). Women aged 40–44 years, 39 years and 38 years accounted for 9.8% (n = 334), 7.9% (n = 271) and 7.7% (n = 263) of all cycles, respectively. Women aged ≥ 45 years made up the lowest group, accounting for merely 0.2% (n = 9) of all cycles. Table I summarises the IVF outcomes of women reviewed in the seven age groups.
Table I.
Outcomes of in vitro fertilisation in the various age groups.

The rate of miscarriage among women aged < 30 years was 15.1% (28/185). Miscarriage rate doubled to 30.0% (21/70) among women aged 38 years, and among those aged 39 years, this rate increased to 47.7% (21/44). Among women aged 40–44 years, the miscarriage rate was as high as 55.3% (21/38). For women aged ≥ 45 years, a total of 6 (0.2%) fresh cycles were started. However, only 3 (50.0%) women reached embryo transfer and none achieved a clinical pregnancy. Compared to women in the younger age groups, women aged ≥ 45 years had the lowest mean number of oocytes collected per cycle (mean 4.5 ± 2.3) and the lowest fertilisation rate (47.6% ± 42.1%; Table I).
The proportion of cycles reaching embryo transfer in the various age groups were as follows: < 30 years (95.9%, 370/386); 30–35 years (95.2%, 1,507/1,583); 36–37 years (95.3%, 542/569); 38 years (91.3%, 240/263); 39 years (92.3%, 250/271); 40–44 years (85.9%, 287/334); and ≥ 45 years (50.0%, 3/6; Table I & Fig. 1).
Fig. 1.

Percentage of fresh cycles reaching embryo transfer in the various age groups.
Age was found to have a significant effect on the number of cycles resulting in embryo transfer (χ2(6) = 69.2, p < 0.001). Contrast analysis of the ORs among the various age groups showed significant differences between women aged 39 years and those aged 40–44 years (OR 2.0, 95% CI 1.1–3.4), and between women aged 40–44 years and those aged ≥ 45 years (OR 6.1, 95% CI 1.2–31.2; Table II).
Table II.
Comparison of odds ratios among the various age groups.

The number of oocytes retrieved decreased across the various age groups, and the difference was statistically significant (F(6, 2,843) = 61.0, p < 0.001). The number of oocytes retrieved was highest among women aged < 30 years (mean 18.5 ± 10.3; Table I & Fig. 2). Contrast analysis among the various age groups showed statistically significant decreases when comparisons were made between the age groups: < 30 years vs. 30–35 years, 30–35 years vs. 36–37 years, 38 years vs. 39 years, 39 years vs. 40–44 years and 40–44 years vs. ≥ 45 years (p < 0.001).
Fig. 2.
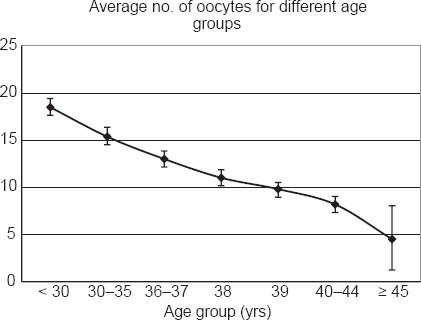
Comparison of the average number of oocytes collected per cycle among the various age groups.
There was a general downward trend in the embryo fertilisation rate among women with increasing age (Table I & Fig. 3). Significantly lower fertilisation rates were noted among women aged 40–44 years when compared to those aged 39 years (66.8% ± 30.8% vs. 69.8% ± 26.6%; p = 0.001). Embryo fertilisation rates among women aged ≥ 45 years were further reduced when compared to those aged 40–44 years (47.6% ± 42.1% vs. 66.8 ± 30.8; p = 0.047). Evaluation of the mean duration of stimulation among the different age groups showed that age did not have a significant effect on this interval (F(6, 3,395) = 4.4, p = 0.129; Fig. 4).
Fig. 3.

Comparison of the embryo fertilisation rates among the various age groups.
Fig. 4.

Comparison of the mean duration of stimulation among the various age groups.
Clinical pregnancy rates were highest among women aged < 30 years (50.0%; Table I & Fig. 5). Women who did not achieve embryo transfer were excluded from the analysis of clinical pregnancy rates. Age was found to have a significant detrimental effect on clinical pregnancy rates (χ2(6) = 198.3, p < 0.001). A statistically significant difference was found in the ORs for clinical pregnancy between women aged 30–35 years and those aged 36–37 years (OR 1.8, 95% CI 1.5–2.3; Table II). A higher OR for clinical pregnancy was also found for women aged 38 years, which was statistically significant, when compared to women aged 39 years (OR 1.9, 95% CI 1.3–3.0; Table II). No clinical pregnancies occurred in women aged ≥ 45 years.
Fig. 5.

Comparison of clinical outcomes among the various age groups.
Live birth rates were the highest in women aged < 30 years (40.0%), with a downward trend being observed with increasing age (Table I & Fig. 5). Age had a significant inverse relationship with live birth rates (χ2(6) = 221.6, p < 0.001). A statistically significant difference was evident in the ORs for achieving live birth per attempted embryo transfer cycle between women aged 30–35 years and those aged 36–37 years (OR 1.9, 95% CI 1.5–2.3; Table II). On comparing women aged 38 years with those aged 39 years, a statistically higher OR was found for achieving live birth among those in the younger age group (OR 2.5, 95% CI 1.5–4.3; Table II).
Women aged < 30 years had the highest multiple pregnancy rates (39.5%; Table I & Fig. 5), and age was found to have a significant effect on the incidence of multiple pregnancies (χ2(5) = 42.3, p < 0.001). Women aged < 30 years were 1.4 times more likely to have a multiple pregnancy than women aged 30–35 years, whereas women aged 30–35 years were 1.9 times more likely to have a multiple pregnancy than women aged 36–37 years (Table II). Age also had a significant effect on the incidence of miscarriages (χ2(5) = 88.0, p < 0.001).
DISCUSSION
As in many other developed countries, Singaporean women are delaying marriage and childbirth for a variety of reasons. These include the higher availability of contraceptive options, and prioritising of academic pursuits and work commitments over personal life. As data from the Singapore Department of Statistics shows, fewer women are getting married under the age of 30 now when compared to a decade ago (Fig. 6).(14) Furthermore, there was a reduction in the age-specific fertility rate of Singaporean women in 2010 when compared to 2000 (Fig. 7).(15) Data also revealed that the total fertility rate of Singaporean women had declined over the last 20 years, with the lowest total fertility rate per woman being reported in 2010 (Table III).(15)
Fig. 6.

Comparison of age-specific marriage rates in Singapore for 2000 and 2010. Source: Singapore Department of Statistics(14)
Fig. 7.

Comparison of age-specific fertility rates in Singapore for 2000 and 2010. Source: Singapore Department of Statistics(15)
Table III.
Total fertility rates in Singapore from 1980 to 2010.

Reflecting societal trends toward delayed marriages and deferment of childbearing, our study showed that the largest proportion of women undergoing IVF at our centre were over 30 years of age, specifically aged 30–35 years. This is certainly a concern, as age-related decline in fertility is known to commence as early as 30 years of age in women – this was reflected in the reduced number of oocytes retrieved per cycle and the lower pregnancy and live birth rates seen among women of older age groups in our study. Correspondingly, the best IVF outcomes were noted in women aged < 30 years. In a related finding, Menken et al reported that women aged over 30 years had significantly higher live birth rates when donor oocytes from younger women were used instead of their own oocytes.(8)
The decline in fertility with age corresponds to the natural biological attrition of the ovarian reserve. The maximum complement of oocytes (approximately 6–7 million) exists during fetal life (at 20 weeks of gestation), a number that undergoes attrition during the course of pregnancy and drops to about 1–2 million by birth.(1) At puberty, 300,000–500,000 oocytes remain, and by the age of 37 years (when the rate of attrition doubles, resulting in an increased rate of follicular loss), around 25,000 remain in women.(9) By the time of menopause, which is usually in the fifties (approximately age 49 years in Singaporean women),(16) only about 1,000 follicles remain.(10)
For women aged 40–44 years, clinical pregnancy rates fell to 13.2% in our study, indicating a nearly fourfold decrease when compared to women aged < 30 years.(17) Studies suggest that poorer IVF outcomes in women over the age of 40 years are due to the lower number of oocytes collected as well as poorer oocyte quality, giving rise to lower fertilisation and implantation rates, with fewer cycles reaching embryo transfer in this cohort.(11,18) These results reinforce the major role that age plays in IVF outcomes, and confirm that women aged over 40 years are likely to face more difficulties in achieving pregnancy.
Live birth rates are lower with increasing maternal age due to increased obstetric complications. A doubling in miscarriage rate, from 15.1% among women aged < 30 years to 30.0% among those aged 38 years, was found in our study. The incidence of miscarriages was further increased by fourfold to 55.3% among women aged 40–44 years. Compared to women aged < 30 years, live birth rates in those aged 40–44 years fell to 5.9%, indicating a nearly sevenfold decrease. Our findings are consistent with the live birth rates (range 2.0%–7.0%) reported in the literature for women aged 40 years and above.(15) Previous results from our centre have reported cumulative live birth rates of 16.0% in women over 40 years after three IVF cycles.(19) These outcomes suggest that women opting for IVF need to be informed about the increased obstetric risks, and lower pregnancy and live birth rates associated with increased age.
Given that female age plays a crucial role in IVF outcomes, we aimed to highlight the economic implications of IVF outcomes in the various age groups, as well as its ramifications for the national healthcare provision model in Singapore, as a secondary objective of this study. For older patients keen to embark on IVF treatment, counselling on success rates and financial costs is imperative. Patients need to realise the financial repercussions of incurred costs from cycle cancellations, higher drug dosages and the increased number of cycles required to achieve live birth.(19) From a national cost-effectiveness perspective, evidence suggests an increase of up to 3.6 times in the cost per delivery for older women,(20) as well as higher incremental costs per live birth with each respective IVF cycle.(21) These findings form the economic rationale behind Singapore’s co-funding scheme for couples – Enhanced Co-Funding For Assisted Reproduction Technology (ART) Treatment – which provides partial financial reimbursement to women under the age of 40 years undergoing a maximum of three fresh and three frozen ART cycles in public hospitals. The scheme is based on the relative cost-effectiveness of providing treatment to patient groups likely to have a better prognosis.
In our study, no clinical pregnancies were reported in women aged ≥ 45 years. This finding, which was consistent with the dismal IVF outcomes reported in the literature for women aged ≥ 44 years,(17) is one of the reasons why the Singapore Ministry of Health does not allow women aged ≥ 45 years into an ART programme, unless prior approval has been obtained.
In summary, there is evidence that fertility decreases with increasing maternal age, which, beyond a certain age, cannot be overcome by IVF treatment. Couples embarking on IVF in Singapore are provided financial counselling, which is an integral aspect of informed decision-making. It is especially important that older women are provided with centre-specific statistics to foster realistic expectations and awareness of increased treatment costs per live birth, given that government co-funding is no longer available to them. Clinicians could play an important role in reducing the financial burden incurred by patients due to ART by advising against the commencement of treatment in patients likely to have poor prognosis, as well as recommending against repeat cycles in repeat poor responders.
ACKNOWLEDGEMENT
We would like to acknowledge the generous contributions of Dr Sadhana Nadarajah, Senior Consultant, Director of KKIVF Centre and National Sperm Bank, Head of the Adolescent Gynaecology Unit, Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore, with respect to the publication of this study.
REFERENCES
- 1.Speroff L. The effect of aging on fertility. Curr Opin Obstet Gynecol. 1994;6:115–20. [PubMed] [Google Scholar]
- 2.Dicker D, Goldman JA, Ashkenazi J, et al. Age and pregnancy rates in in-vitro fertilization. J In Vitro Fert Embryo Transf. 1991;8:141–4. doi: 10.1007/BF01131703. [DOI] [PubMed] [Google Scholar]
- 3.Scwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N Engl J Med. 1982;306:404–6. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 4.London: Human Fertilization and Embryo Authority; 1996. Human Fertilization and Embryo Authority. The patients’ guide to DI and IVF clinic. [Google Scholar]
- 5.Rutherford AJ, Subak-Sharpe RJ, Dawson KJ, et al. Improvement of in-vitro fertilization after treatment with buserelin, an agonist of luteinizing hormone releasing hormone. Br Med J (Clin Res Ed) 1988;296:1765–8. doi: 10.1136/bmj.296.6639.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lass A, Croucher C, Duffy S, et al. One thousand initiated cycles of in-vitro fertilization in women >or =40 years of age. Fertil Steril. 1998;70:1030–4. doi: 10.1016/s0015-0282(98)00353-7. [DOI] [PubMed] [Google Scholar]
- 7.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 8.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–94. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 9.Simpson JL, Lobo RA, Kelsey J, Marcus R. San Diego: Academic Press; 2000. Genetic programming in ovarian development and oogenesis. Menopause: biology and pathobiology; pp. 77–94. [Google Scholar]
- 10.Navot D, Bergh PA, Williams MA, et al. Poor oocytes quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–7. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- 11.Hull MGR, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: A quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril. 1996;65:783–90. doi: 10.1016/s0015-0282(16)58214-4. [DOI] [PubMed] [Google Scholar]
- 12.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42:629–36. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 13.Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilization treatment. Lancet. 1996;348:1402–6. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 14.Department of Statistics Singapore. Age Specifi c Marriage Rates. Copyright Government of Singapore [Google Scholar]
- 15.Department of Statistics Singapore. Age Specifi c Fertility Rates Copyright Government of Singapore [Google Scholar]
- 16.Loh FH, Khin LW, Saw SM, Lee JJ, Gu K. The age of menopause and the menopause transition in a multiracial population: a nation-wide Singapore study. Maturitas. 2005;52:169–80. doi: 10.1016/j.maturitas.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Ron-El R, Raziel A, Strassburger D, et al. Outcome of assisted reproductive technology in women over the age of 41. Fertil Steril. 2000;74:471–5. doi: 10.1016/s0015-0282(00)00697-x. [DOI] [PubMed] [Google Scholar]
- 18.Viardot-Foucault V, Tai BC, Oo SY, et al. Reproductive Endocrinology and ART. Canada: A celebratory congress; 2011. Cumulative success rates after IVF treatment: longitudinal study from a single centre. [Google Scholar]
- 19.Warbuton D, Kline J, Stein Z, et al. Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet. 1987;41:465–83. [PMC free article] [PubMed] [Google Scholar]
- 20.Suchartwatnachai C, Wongkularb A, Srisombut C, et al. Cost effectiveness of IVF in women 38 years and older. Int J Gynaecol Obstet. 2000;69:143–8. doi: 10.1016/s0020-7292(99)00215-5. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths A, Dyer SM, Lord SJ, et al. A Cost effectiveness analysis of in-vitro fertilization by maternal age and number of treatment attempts. Hum Reprod. 2010;25:924–31. doi: 10.1093/humrep/dep418. [DOI] [PubMed] [Google Scholar]


