Abstract
Clinical drug development remains a mostly empirical, costly enterprise, in which decision-making is often based on qualitative assessment of risk, without properly leveraging all the relevant data collected throughout the development programme. Model-based drug development (MBDD) has been proposed by regulatory agencies, academia and pharmaceutical companies as a paradigm to modernize drug research through the quantification of risk and combination of information from different sources across time. We present here a historical account of the use of MBDD in clinical drug development, the current challenges and further opportunities for its application in the pharmaceutical industry.
Keywords: clinical trial simulation, model-based drug development, modelling, PK/PD, simulation
Motivation for model-based drug development (MBDD)
Increasing late phase attrition rates, decreasing approvals of new compounds, expiring patents of blockbuster drugs and increasing price control pressure from payers have all led to the so-called pipeline problem in the pharmaceutical industry. Stakeholders have sponsored various initiatives to evaluate the root causes of the problem and propose ways to address them [1]. A recurrent reason identified through these initiatives is the archaic nature of the drug development process itself, which is based on a model that has evolved little since the 1960s, although improvement in some regions has been observed over the last decade. Another plausible reason for the current problem is the conservative nature of the pharmaceutical industry compounded by the fear that regulatory agencies may be reluctant to accept novel approaches as bases for regulatory approval. Therefore, it is imperative that modern approaches to drug development worldwide are established quickly, before the cost : benefit ratio for developing new drugs becomes unsustainable.
Modelling has long been used in drug development as an efficient tool to summarize and combine information from a variety of data sources [2]. In addition to modelling exercises conducted in the field of statistics, pharmacokinetic (PK) and pharmacodynamic (PD) models have a long and successful history of describing what the body does to drugs and how drugs affect the body, respectively. They have been used in all critical phases of clinical development, providing supportive information for study design and drug labelling. Usually, the sophistication and impact of modelling, which requires assumptions, decrease if a study is regarded as confirmatory from a regulatory submission aspect, i.e. primary analyses in pivotal studies rarely involve more than a simple statistical model, such as an analysis of covariance (ancova) or a Cox proportional hazards model. This is partly due to the fear of reliance on assumptions and lack of familiarity with non-standard methods. However, considerable efficiency gains can be obtained by utilizing modern modelling approaches, even in critical analyses in registration studies [3].
Clinical trial simulation should always go hand-in-hand with modelling. It functions as a foundation for modern protocol development by simulating trials under a range of designs, scenarios and assumptions, thereby providing operating characteristics (e.g. statistical power, probability of success) that help understand the effects of changes in study design on trial results. Traditional study design typically focuses on a power calculation for a single primary endpoint under a fixed set of assumptions that are often derived from the results of a previous study with a smaller population. Clinical trial simulations are often shunned because of the comfort level of clinical team members with traditional hypothesis-testing paradigms and the relative ease of sample size calculation with conventional software. However, the actual scientific question(s) to be addressed by a study may not quite fit into the hypothesis-testing framework of the traditional approach (e.g. dose selection), and there may be a high degree of uncertainty about the validity of the underlying assumptions for a power calculation (e.g. response rate based on a small sample population).
Taken together, modelling and simulation (M&S) provide a powerful paradigm for modernizing clinical study design and analysis. M&S allows proper quantification of risk at each decision-making point in drug development (e.g. go/no-go decision after a proof-of-concept [POC] study by calculating the probability of success of the next study); provides an efficient framework for combining information from different studies and other sources (e.g. Bayesian models); and facilitates an objective assessment of the sensitivity to assumptions that often rely on flimsy information. MBDD takes M&S to a higher level: it aims to represent critical components of clinical study results via a network of inter-related models that allow simulation of clinical outcomes from specific aspects of a single study to entire development programmes. MBDD is expected to facilitate quantitative decision-making at a portfolio level, greatly improving upon the current, qualitative and less-scientific decision process.
While various tools for and aspects of MBDD deserve discussion, it is difficult to review all of these in one paper. Several review articles in the literature offer focused discussion on specific MBDD topics [4–7], some of which include specific application cases. This review intends to provide an encompassing, high level evaluation of the past, present and future of MBDD. It also touches upon representative papers describing M&S tools for MBDD, which can be referred to for detailed information.
MBDD: The early days
M&S principles have long been used to forecast outcomes in various fields, including meteorology (e.g. the path of hurricane Sandy), economics (e.g. return of unemployment to the pre-Great Recession levels) and manufacturing (e.g. safety of new batteries in the Boeing Dreamliner). In the field of clinical drug development, the Center for Drug Development Sciences at Georgetown University, led by Carl Peck, played an important role in the late 1990s by championing applications of M&S in practice. The Center organized conferences, demonstrated the value of M&S to pharmaceutical companies when it was not well appreciated [8], encouraged the development of user-friendly commercial software, trained scientists in the appropriate use of M&S tools and influenced the Food and Drug Administration (FDA) Modernization Act of 1997. This early MBDD movement was also stirred by Lewis Sheiner's seminal learn-confirm paradigm manifesto to expedite the drug development process through greater utilization of model-based approaches [9].
From the early 2000s, the practical value of M&S in improving the efficiency of drug development has been increasingly appreciated across the pharmaceutical industry. As a result, M&S became a hot topic at major pharmaceutical conferences such as the European Medicines Agency-European Federation of Pharmaceutical Industries and Associations (EMA-EFPIA) Workshop [10], the American Association of Pharmaceutical Scientists (AAPS) and the American Society for Clinical Pharmacology & Therapeutics (ASCPT), as well as other smaller workshops. Papers and books were published on the benefits and challenges of MBDD, including case studies [4,6,8,11–13]. As of August 2013, a PubMed search found 22 papers that specifically included MBDD as a keyword, although many more papers related to MBDD have been published under various keywords discussed in this commentary. Hence it is difficult to list so many successful applications of MBDD related works. Regulatory agencies have also shared their experiences and expectations on the use of M&S as a tool to guide regulatory decisions [14–17]. The FDA Critical Path Initiative Report is the most notable document reflecting the clear regulatory support for M&S in clinical development [18]. It systematically examined various roadblocks to improving the efficiency of then current drug development, and made impactful suggestions to promote change and drive innovation. The centrepiece of the proposed strategy was the adoption of quantitative clinical trial M&S that would help improve trial designs and predict outcomes.
The application of M&S in decision-making often involves thorough analyses to justify the choice of a particular study design after considering an optimal balance between cost and the quality of knowledge to be obtained. Miller et al. [3] shared their experiences of M&S application for more appropriate compound selection in early drug development to differentiate candidate compounds and to achieve more informed decision-making during phase 2 to phase 3 and beyond. A survey of 10 large and mid-sized pharmaceutical companies showed the positive impact of M&S in their decision-making process [19]. These success stories caught the attention of senior management in pharmaceutical companies and led to an increased interest in the potential of M&S to improve the efficiency of drug development. Combined with further evidence of the acceptance of M&S approaches by regulatory agencies [14–17], the appreciation of the potential benefits of M&S eventually led to the establishment of groups, or entire departments, dedicated to its application in pharmaceutical companies. Consulting companies for MBDD were also formed to cater to specialized needs and/or to provide routine pharmacometric services.
Current applications of M&S across all clinical development phases
An enormous amount of information is produced during the drug development process, and using it efficiently to guide decision-making across the various phases is challenging. Models are particularly useful in summarizing essential information in a succinct and efficient manner, allowing the integration of knowledge from different studies and external sources. Coupled with simulations and by using appropriate assumptions, such models can explore the potential outcomes of yet-to-be-conducted studies, enabling optimization of the study design to increase the probability of success and de-risk investment.
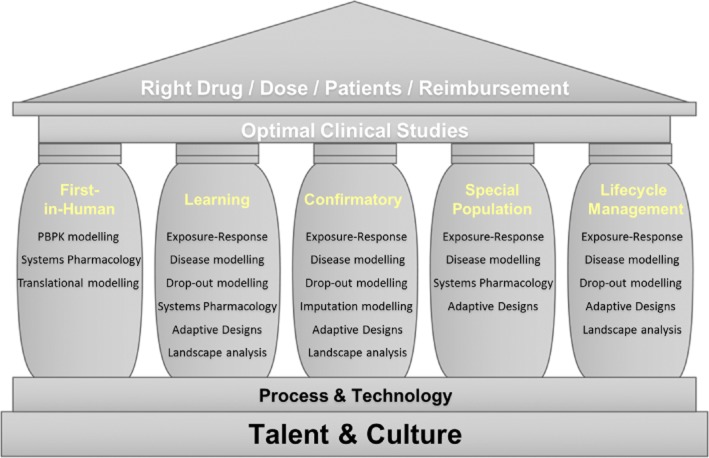
Quantitative decision-making can be facilitated by using various modelling approaches in each phase of drug development (Figure 1), but there is no definite consensus on the best-suited approach for different kinds of studies. In order to design clinical studies that can identify the optimal match of drug, dose and patient population, pharmaceutical companies should educate talented employees to apply M&S in an MBDD-supportive company culture. This would also lead to the right reimbursement for the companies. To conduct M&S efficiently, a practical application of the MBDD paradigm with the help of rapidly improving technology is critical. Further in this review, we discuss various M&S examples of the current use of MBDD in each pillar (study) of clinical drug development that may be also used under other pillar categories.
Figure 1.
Structure of model-based drug development. The pillars list various models to be considered for designing optimal clinical studies
Dose selection for first-in-human studies
Traditionally, the dose for a first-in-human study is selected through allometry combined with information on the safety margin for the compound, which is obtained from dose toxicology studies in the most sensitive species. Regarding the selection of a starting dose based on both efficacy and safety data from pre-clinical studies, there is increasing interest in methods that improve predictions of the full time course of PK in humans via mechanistic understanding of the underlying processes affecting the PK. A physiologically based pharmacokinetic (PBPK) modelling approach [20] can be utilized with the understanding of (i) drug specific properties, which include tissue affinity, plasma protein binding affinity, membrane permeability, enzymatic stability and transporter activities, (ii) system specific properties, which include organ mass or volume and blood flow between anatomical structures of tissues or organs and (iii) anatomical structure, which is common for all mammalian species and independent of the drug.
Compared with this allometric or empirical compartment modelling, PBPK modelling has the potential to more reliably predict the PK properties (absorption, distribution, metabolism and elimination) of a new drug in humans. Setting up a PBPK model in early drug development also helps improve the market formulation, if necessary, by refining it with drug specific properties derived from early phase 1 data. Such PBPK approaches have been intensively discussed as a tool to improve the drug development process, especially in early development [20]. Several software programmes (e.g. Simcyp®, Gastroplus® and PK-Sim/MoBi®) have incorporated relevant PBPK equations, making the application of such models easier and widespread. Recent publications on the experiences of regulatory agencies with PBPK methods have further encouraged the use of such methods across the pharmaceutical industry [21]. The systems pharmacology approach can be also used in this drug development phase, and it is discussed further in ‘Further opportunities for MBDD’ [22].
Designing a study in the learning phase
A POC (phase 2a) study is designed with an aim to demonstrate a sufficiently positive efficacy signal and to detect a clear toxicity signal, such that the results warrant further investment after understanding the mechanism of action of the investigational drug. A phase 2b study is intended to collect sufficient information to derive an exposure–response relationship that guides the dosage regimen decision, with an appropriate benefit : risk balance for the next large, costly Phase 3 study. In these learning phase studies, M&S is an essential tool for knowledge building and decision support for optimizing trial performance.
PK knowledge gained from the first-in-human study, together with pre-clinical biomarker information, provides the basis for M&S application in learning phase studies. Further, the estimated between- and within-individual PK variability, obtained from the fit of phase 1 study data, is a basis for the PK simulation model that can be used to simulate exposure–response relationships, providing useful information for the design of POC studies. In addition to pre-clinical PD information, the PD of competitor drugs (either from internal or external sources) with similar mechanisms of action can also be utilized to derive dose– or exposure–response relationships that can simulate the responses in a POC study before it is conducted [23]. To avoid potential biases due to the specific characteristics of a competitor drug, a meta-analysis that includes several competing drugs may be performed to extract the overall exposure vs. efficacy or toxicity relationship [24].
To ensure that simulated results resemble reality as much as possible, dropouts should be also included during simulation as part of a longitudinal disease progression/drug–effect model [25]. During the phase 2 study design process, when no patient dropout data are available, a dropout model possibly developed using competitor drugs with a similar indication and/or mechanism of action can be used. In general, when certain information required for simulations is unavailable, sensitivity of the assumed information should be tested, as this would help not only in effectively relating the uncertainty in the simulated output with that of the input, but also in identifying influential model parameters that change sensitively to the assumptions made during simulation. This indicates the need for better understanding of the parameters to predict clinical outcomes with greater certainty [26].
To maximize the knowledge creating opportunity in a learning phase study, the application of Bayesian adaptive design methods has recently been advocated [27,28]. Such models allow for reductions in the number of subjects allocated to ineffective doses, while providing adequate statistical power to detect the treatment effect of an effective dose. However, conduct of an adaptive design study requires careful logistical, operational and analytical planning due to its greater complexity, and its benefits and drawbacks need be evaluated beforehand via various assumptions and simulations.
Innovation in the design and interpretation of confirmatory studies
Once a model representing drug specific data from an early study is available, realistic confirmatory study results can be simulated using hypothetical study designs by integrating indication-specific, but drug independent, information. Wang et al. [29] used four registration studies to develop a drug independent model that linked biomarkers (e.g. tumour size) to clinical outcome (overall survival) in oncology. They used baseline prognostic factors and early change in tumour size as predictors. With this type of general indication specific model, one can predict clinical outcomes based on the understanding of the drug specific relationship between a candidate drug's exposure and its biomarker level [30], facilitating the selection of an appropriate design for an expensive, large scale study. Such an understanding of dose (or exposure)–response relationships for the desired (effectiveness) and undesired (toxicity) effects obtained after a phase 2a and a phase 2b study can be discussed with the FDA at an end of phase 2a [31] and end of phase 2b meeting, respectively, and dose selection can thus be optimized for subsequent studies.
Predicting the effect size is as important for the reference arm (i.e. active comparator or placebo) as for the drug under investigation, because confirmation of efficacy is based on comparisons between the reference and the drug. If an active competitor drug is used as a comparator, a model describing the time course of the active comparator response could be set up using published data. On the other hand, if a placebo is used, placebo data (or natural disease progression data without placebo given, if available) from studies with similar patient populations could be used to develop a disease progression model [32], with which the time course of the placebo response could be simulated for virtual statistical comparison to evaluate candidate clinical study designs of interest.
During the analysis of a confirmatory study, handling of missing data plays an important role in influencing conclusions regarding the study outcome. M&S approaches can be used to impute values for missing data in a variety of scenarios, including missing at random. Sensitivity analyses can then be used to assess the robustness of the results [33]. Models can also be used under a missing-at-random assumption to analyze the observed data only (including intermediate visit data), through likelihood or Bayesian estimation. Sponsors should discuss the results of such sensitivity analyses with regulatory agencies to agree on a suitable method for handling missing data to facilitate objective interpretation of study results.
Special population studies
The most important information in a drug label is the dosage regimen derived by balancing efficacy and safety data for all types of patients. However, considering the heterogeneity of the patient population in clinical trials, studying all kinds of factors such as use of co-medications, specific organ impairment, ethnicity and co-morbid conditions (e.g. obesity) may not be feasible. If factors that differentiate one patient population from another can be foreseen, dedicated studies to evaluate the impact of these factors may need to be conducted based on the knowledge of the drug molecule and its mechanism of action. However, with the use of PBPK modelling [21,34], systems pharmacology [22] or covariate analysis of population modelling [35], certain special population studies may be exempted following scientific discussions with the appropriate regulatory agency.
M&S approaches find a special role in the design and analysis of paediatric studies, wherein blood sample volume is limited owing to the smaller body size. The population modelling approach allows prediction of individual PK/PD profiles from sparse samples by borrowing information from other patients [36].
Lifecycle management
For post-marketing lifecycle management, it is important to assess the competitive landscape by comparing clinical effect sizes, time to achieve a target response, and safety signals of competitive drugs using meta-analyses, and the information thus obtained can help differentiate a drug under development from others in the market [24]. Additionally, when a sponsor develops a lifecycle management strategy after a drug is approved for a certain indication with a given formulation, predicting the clinical outcome profile of a new strategic drug product and comparing it with competitors can not only facilitate the management's go/no-go decisions on the strategy but also help demonstrate the superiority, if any, of the approved drug over other drugs [37].
Challenges to MBDD
Despite the increased interest and use of MBDD in clinical development, there remain considerable hurdles to its broader adoption by pharmaceutical companies and regulatory agencies. The first challenge is the natural resistance to change in a highly conservative industry such as biopharmaceuticals. A common reason for sticking with traditional approaches is the notion that regulators may not approve of deviations from previously used methods. Since regulatory agencies have themselves played a key role in advocating the modernization of drug development, this argument does not hold, although the regulatory position towards MBDD is still highly dependent on the division and disease under consideration [38]. Open and frequent dialogue between industry and regulatory agencies, presentations and publications of case studies using MBDD approaches with buy-in from regulators and release of regulatory guidelines focusing on M&S approaches would all contribute towards increasing the industry's comfort level with MBDD. Communication skills also play an important role in the acceptance and understanding of MBDD approaches and need to be encouraged among both sponsors and regulators. Part of the reluctance to embrace the change presented by the MBDD paradigm may be related to a loss of decision making power, perceived or real, that some stakeholders might fear. For example, M&S results could highlight the futility of a study before or during its conduct, which can form the basis for terminating the study instead of continuing to collect data in the hope of a good result. Therefore, educating clinical team personnel on the basic principles and benefits of MBDD, as well as clear upper management support to changes, would go a long way to addressing this hurdle.
Another challenge is a self-inflicted one, resulting from overpromises on the benefits of MBDD that often failed to be fully substantiated, leading to frustration and backlash. MBDD certainly has the potential to improve greatly the efficiency of drug development, but it should not be construed as a panacea. It is incumbent upon those leading the MBDD implementation efforts to provide a realistic picture of the advantages of M&S in order to avoid potential hypes that may eventually fail to meet expectations. Indeed, this problem is not unique to MBDD [39]. It has been observed in many other paradigm-shifting disciplines such as artificial intelligence and data mining. As the area matures and gains greater acceptance, the temptation to oversell benefits for short term gains will disappear to achieve a plateau of productivity, making this a transient challenge to MBDD, hence realizing its full potential.
An internal challenge to the success of MBDD application in clinical drug development is related to ownership: which traditional function within clinical drug development, if any, should house MBDD activities and groups? As a discipline, MBDD lies at the intersection of several traditional drug development functions, most notably biostatistics and clinical pharmacology. Historically, clinical pharmacology has played a more active role in the development of the methodological underpinnings of MBDD, but many of those methods also fall within the realm of biostatistics. The issue is not due to infrastructural division, but rather due to competition for influence in development programmes and on clinical teams. Different quantitative disciplines often regard themselves as the owners of the M&S space, leading to confusion and inefficiencies caused by duplication of effort. Alignment among different M&S stakeholders is critical for the future of the discipline. Perhaps, because of this contest for the ownership of MBDD, the new discipline of pharmacometrics has been created to embody the principles of MBDD, with pharmacometricians specialized in the methodological development and application of MBDD. Consistent with this trend, some pharmaceutical companies and regulatory agencies have opted to create separate functional departments to house MBDD activities. This process is still unfolding and the prevailing paradigm is yet to be ascertained.
Further opportunities for MBDD
An enormous amount of effort and resources are required to translate the pre-clinical success of new molecular entities into demonstrated efficacy and safety in the clinical setting, with the ultimate goal of regulatory approval and subsequent benefit to patients. The conventional paradigm of utilizing assumption poor models and analysis methods to guide decision-making in clinical development has led to costly programmes, both in terms of the size of clinical studies and the limited knowledge gained from them. In this section, we briefly consider MBDD tools that have recently drawn attention and that are likely to experience increasing use in future.
Systems pharmacology
Drug research has mainly focused on identifying molecular targets without fully understanding the consequent physiological interactions. Although a successful early POC study is possible even with this limited understanding, clinical outcomes that reflect systemic changes may turn out to be negative at a later stage of drug development. Systems pharmacology is a relatively new discipline that lies at the interface between systems biology and PK/PD [22,40]. It is a response to the growing concern that pharmaceutical companies need to reduce the high late attrition rate for drugs in their pipeline caused by insufficient efficacy or missed toxicity signals in POC or phase 2 studies. This discipline is intended to provide a framework for integrating the information gained from understanding pathophysiological pathways (perturbed system due to disease vs. normal body function system), which can be used as pharmacological targets [41], in order to help select compounds that are more likely to translate into clinical efficacy and safety through iterative learning from modelling and experimentation. However, adoption of this approach in certain disease areas may take a while because quantitative prediction of pharmacological modulation of biological targets and their cascades requires more thorough research on pathophysiological systems than currently available.
Optimal sampling design
Clinical studies based on optimal sampling techniques provide more accurate and precise estimates of model parameters, resulting in better predictions of clinical outcomes [42]. Although the theory of optimal sampling has been available for a long time [43], logistic and operational difficulties often make recommended optimal sampling times infeasible in practice. However, more effort should be made to balance operational concerns with the value of information collected in a study (e.g. increase in precision of the parameters of interest).
Decision analysis
The application of MBDD in clinical development has frequently focused on either efficacy or safety endpoints in isolation within a single study. The use of a clinical utility index to combine efficacy and safety information, even from several clinical trials, into a single metric has considerable promise within the MBDD paradigm [44]. If acceptable clinical utility index criteria can be agreed upon by a clinical team, M&S can be used to determine optimal treatments (e.g. dosage regimen), which can subsequently lead to effective study designs aimed at identifying or confirming such optimal treatments.
Many studies are conducted before the confirmatory study for learning purposes. In these exploratory studies, the probability of reaching a correct decision or achieving a target value should be the main concern rather than a statistical metric (e.g. P value) for treatment vs. control. Such decision analysis tools, coupled with M&S, can be used to quantify the risk : benefit ratio of alternative portfolio strategies, providing useful information to guide decision making in governance bodies. Bayesian methods, which combine previous and current information, are particularly useful in this context [45]. Another area in which MBDD can and should be more effectively applied is portfolio level decision making support. Novel methods and tools (e.g. the DDMoRe project) are being developed to support this effort [46,47].
Adherence monitoring: pharmionics
Pharmionics is the study of whether patients actually take prescribed medicines. A meta-analysis of 95 clinical studies showed that almost 40% of treated subjects stopped taking medications by the end of 1 year [48]. Partial adherence to the dosage regimen specified in a study protocol may complicate the interpretation of study results. It can lead to inappropriate conclusions such as lack or underestimation of efficacy and serious toxicity. To achieve increased adherence for drugs already on the market, software applications that remind patients to take their medication and record drug administration (e.g. http://www.care4today.com) have been introduced in clinical practice. Such tools, in addition to devices that record the opening of a pill bottle or a pill package, will enhance the understanding of drug effectiveness.
Final remarks
In its early days during the mid-1990s, M&S was mainly regarded as a supporting tool used as part of troubleshooting efforts to explain unexpected and undesirable findings during a regulatory review or post-marketing surveillance. Recently, many pharmaceutical companies have realized the benefits of model-based drug development in the early phases, rather than model-supported drug development. As a result, M&S groups have sprouted across the pharmaceutical industry and in regulatory agencies. One of the factors limiting the growth of MBDD is the lack of M&S experts. Academic departments in leading universities have only recently established programmes focused on training new pharmacometricians [49]. This limitation may remain a hurdle for the broader cross-industry implementation of MBDD in the coming years. Training clinical pharmacologists, biostatisticians and other quantitative scientists in the use of pharmacometrics tools could address this problem in the short term.
With more pharmacometricians trained in each country, more regulatory reviewers who can appreciate the usefulness of M&S have become available. However, since regulatory policies vary widely around the world, pharmaceutical companies often have to contend with the ‘lowest common denominator’. In other words, if one regulatory agency is not willing to accept novel MBDD approaches, pharmaceutical companies end up using conventional, and sometimes inefficient, approaches, which negates the value of M&S. It would be beneficial to harmonize the policy for MBDD to some extent, similar to the International Conference on Harmonization guidelines for clinical trials.
The drug development process is in dire need of improvement in efficiency, pace, success rate and costs, to deliver the promise of exciting new treatments offered by the explosive growth in scientific discoveries (e.g. mapping of the human genome) over the past decade. Modelling provides an essential tool for representing and combining information collected in development programmes, which can be used to improve greatly the efficiency of drug development. MBDD alone will not transform drug development into a highly efficient, cost effective engine to produce new treatments, but it will certainly play a critical role in the effort to embed scientific thinking into the drug development process itself.
Acknowledgments
The authors appreciate comments from the manuscript reviewers and discussion within the Department of Model Based Drug Development at Janssen R&D, LLC.
Competing Interests
Both authors are employees of Janssen Research & Development, LLC.
Both authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: HK and JP had support from Janssen Research & Development, LLC, for the submitted work. There are no other financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin Pharmacol Ther. 2011;89:183–188. doi: 10.1038/clpt.2010.286. [DOI] [PubMed] [Google Scholar]
- 2.Peck CC, Barr WH, Benet LZ, Collins J, Desjardins RE, Furst DE, Harter JG, Levy G, Ludden T, Rodman JH. Opportunities for integration of pharmacokinetics, pharmacodynamics, and toxicokinetics in rational drug development. J Clin Pharmacol. 1994;34:111–119. doi: 10.1002/j.1552-4604.1994.tb03974.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller R, Ewy W, Corrigan BW, Ouellet D, Hermann D, Kowalski KG, Lockwood P, Koup JR, Donevan S, El-Kattan A, Li CSW, Werth JL, Feltner DE, Lalonde RL. How modeling and simulation have enhanced decision-making in new drug development. J Pharmacokinet Pharmacodyn. 2005;32:185–197. doi: 10.1007/s10928-005-0074-7. [DOI] [PubMed] [Google Scholar]
- 4.Milligan PA, Brown MJ, Marchant B, Martin SW, van der Graaf PH, Benson N, Nucci G, Nichols DJ, Boyd RA, Mandema JW, Krishnaswami S, Zwillich S, Gruben D, Anziano RJ, Stock TC, Lalonde RL. Model-based drug development: a rational approach to efficiently accelerate drug development. Clin Pharmacol Ther. 2013;93:502–514. doi: 10.1038/clpt.2013.54. [DOI] [PubMed] [Google Scholar]
- 5.Holford NH, Kimko HC, Monteleone JP, Peck CC. Simulation of clinical trials. Annu Rev Pharmacol Toxicol. 2000;40:209–234. doi: 10.1146/annurev.pharmtox.40.1.209. [DOI] [PubMed] [Google Scholar]
- 6.Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA, Corrigan BW, Lockwood PA, Marshall SA, Benincosa LJ, Tensfeldt TG, Parivar K, Amantea M, Glue P, Koide H, Miller R. Model-based drug development. Clin Pharmacol Ther. 2007;82:21–32. doi: 10.1038/sj.clpt.6100235. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Pfister M, Meibohm B. Concepts and challenges in quantitative pharmacology and model-based drug development. AAPS J. 2008;10:552–559. doi: 10.1208/s12248-008-9062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimko HC, Reele SSB, Holford NHG, Peck CC. Prediction of the outcome of a phase 3 clinical trial of an antischizophrenic agent (quetiapine fumarate) by simulation with a population pharmacokinetic and pharmacodynamic model. Clin Pharmacol Ther. 2000;68:568–577. doi: 10.1067/mcp.2000.110975. [DOI] [PubMed] [Google Scholar]
- 9.Sheiner LB. Learning and confirming in clinical drug development. Clin Pharmacol Ther. 1997;61:275–291. doi: 10.1016/S0009-9236(97)90160-0. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. 2012. EFPIA-EMA modeling and simulation workshop report. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/05/WC500127118.pdf (last accessed 14 March 2014)
- 11.Kimko H, Duffull SB, editors. Simulation for Designing Clinical Trials: A Pharmacokinetic-Pharmacodynamic Modeling Perspective. New York: Marcel Dekker; 2003. [Google Scholar]
- 12.Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. New York: Springer Science; 2011. [Google Scholar]
- 13.Ette E, Williams P, editors. Pharmacometrics: The Science of Quantitative Pharmacology. Hoboken, NJ: John Wiley & Sons; 2007. [Google Scholar]
- 14.Bhattaram VA, Bonapace C, Chilukuri DM, Duan JZ, Garnett C, Gobburu JV, Jang SH, Kenna L, Lesko LJ, Madabushi R, Men Y, Powell JR, Qiu W, Ramchandani RP, Tornoe CW, Wang Y, Zheng JJ. Impact of pharmacometric reviews on new drug approval and labeling decisions – a survey of 31 new drug applications submitted between 2005 and 2006. Clin Pharmacol Ther. 2007;81:213–221. doi: 10.1038/sj.clpt.6100051. [DOI] [PubMed] [Google Scholar]
- 15.Bhattaram VA, Booth BP, Ramchandani RP, Beasley BN, Wang Y, Tandon V, Duan JZ, Baweja RK, Marroum PJ, Uppoor RS, Rahman NA, Sahajwalla CG, Powell JR, Mehta MU, Gobburu JV. Impact of pharmacometrics on drug approval and labeling decisions: a survey of 42 new drug applications. AAPS J. 2005;7:E503–E512. doi: 10.1208/aapsj070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jönsson S, Henningsson A, Edholm M, Salmonson T. Contribution of modeling and simulation studies in the regulatory review: a European regulatory perspective. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 15–36. [Google Scholar]
- 17.Garnett CE, Lee JY, Gobburu JVS. Contribution of modeling and simulation in the regulatory review and decision-making: U.S. FDA perspective. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 37–57. [Google Scholar]
- 18.U.S. Food and Drug Administration. 2004. FDA critical path initiative. Available at http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ucm076689.htm (last accessed 14 March 2014)
- 19.Stone JA, Banfield C, Pfister M, Tannenbaum S, Allerheiligen S, Wetherington JD, Krishna R, Grasela DM. Model-based drug development survey finds pharmacometrics impacting decision making in the pharmaceutical industry. J Clin Pharmacol. 2010;50(Suppl):20S–30S. doi: 10.1177/0091270010377628. [DOI] [PubMed] [Google Scholar]
- 20.Rowland M, Peck C, Tucker G. Physiologically based pharmacokinetics in drug development and regulatory science. Annu Rev Toxicol Pharmacol. 2011;51:45–73. doi: 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
- 21.Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu TC, Booth BP, Rahman NA, Reynolds KS, Gil Berglund E, Lesko LJ, Huang S-M. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89:259–267. doi: 10.1038/clpt.2010.298. [DOI] [PubMed] [Google Scholar]
- 22.Sorger PK, Allerheiligen SRB, Abernethy DR, Altman RB, Brouwer KLR, Califano A, D'Argenio DZ, Iyengar R, Jusko WJ, Lalonde R, Lauffenburger DA, Shoichet B, Stevens JL, Subramaniam S, Van der Graaf P, Vicini P, Ward R. 2011. Quantitative and systems pharmacology in the post-genomic era: new approaches to discovering drugs and understanding therapeutic mechanisms. An NIH white paper by the QSP Workshop Group. October. Available at http://www.nigms.nih.gov/Training/Documents/SystemsPharmaWPSorger2011.pdf (last accessed 14 March 2014)
- 23.Kowalski KG, Olson S, Remmers AE, Hutmacher MM. Modeling and simulation to support dose selection and clinical development of SC-75416, a selective COX-2 inhibitor for the treatment of acute and chronic pain. Clin Pharmacol Ther. 2008;83:857–866. doi: 10.1038/sj.clpt.6100374. [DOI] [PubMed] [Google Scholar]
- 24.Mandema JW, Boyd RA, DiCarlo LA. Therapeutic index of anticoagulants for prevention of venous thromboembolism following orthopedic surgery: a dose–response meta-analysis. Clin Pharmacol Ther. 2011;90:820–827. doi: 10.1038/clpt.2011.232. [DOI] [PubMed] [Google Scholar]
- 25.Hu C, Sale ME. A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn. 2003;30:83–103. doi: 10.1023/a:1023249510224. [DOI] [PubMed] [Google Scholar]
- 26.Abraham AK, Krzyzanski S, Mager DE. Partial derivative – based sensitivity analysis of models describing target-mediated drug disposition. AAPS J. 2007;9:E181–190. doi: 10.1208/aapsj0902020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie F, Ji Y, Tremmel L. A Bayesian adaptive design for multi-dose, randomized, placebo-controlled phase I/II trials. Contemp Clin Trials. 2012;33:739–748. doi: 10.1016/j.cct.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro J, Bretz F, Hsu CH. Adaptive trial designs. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 109–130. [Google Scholar]
- 29.Wang Y, Sung C, Dartois C, Ramchandani R, Booth BP, Rock E, Gobburu J. Elucidation of relationship between tumor size and survival in non-small cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167–174. doi: 10.1038/clpt.2009.64. [DOI] [PubMed] [Google Scholar]
- 30.Claret L, Lu JF, Bruno R, Hsu CP, Hei YJ, Sun YN. Simulations using a drug-disease modeling framework and phase II data predict phase III survival outcome in first-line non-small-cell lung cancer. Clin Pharmacol Ther. 2012;92:631–634. doi: 10.1038/clpt.2012.78. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration. 2009. Guidance for industry: end-of-phase 2A meetings. Available at http://www.fda.gov/downloads/Drugs/…/Guidances/ucm079690.pdf (last accessed 14 March 2014)
- 32.Schmidt S, Post TM, Boroujerdi M, Kesteren C, Ploeger BA, Della Pasqua OE, Danhof M. Disease progression analysis: towards mechanism-based models. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 437–459. [Google Scholar]
- 33.Shaffer ML, Chinchilli VM. Including multiple imputation in a sensitivity analysis for clinical trials with treatment failures. Contemp Clin Trials. 2007;28:130–137. doi: 10.1016/j.cct.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhao P, Vieira M, Grillo J, Song P, Wu T, Zheng J, Arya V, Berglund E, Atkinson A, Sugiyama Y, Pang K, Reynolds K. Evaluation of exposure change of nonrenally eliminated drugs in patients with chronic kidney disease using physiologically based pharmacokinetic modeling and simulation. J Clin Pharmacol. 2012;52:91S–108S. doi: 10.1177/0091270011415528. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. 1999. Guidance for industry: population pharmacokinetics. Available at http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM133184.pdf (last accessed 14 March 2014)
- 36.Barrett J. Modeling and simulation in pediatric research and development. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 401–433. [Google Scholar]
- 37.Samtani MN, Sheehan JJ, Fu D-J, Remmerie B, Sliwa JK, Alphs L. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol Adv Appl. 2012;4:25–40. doi: 10.2147/CPAA.S32735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehmann F, Papaluca Amati M, Salmonson T, Posch M, Vamvakas S, Hemmings R, Eichler HG, Schneider CK. Gatekeepers and enablers: how drug regulators respond to a challenging and changing environment by moving toward a proactive attitude. Clin Pharmacol Ther. 2013;93:425–432. doi: 10.1038/clpt.2013.14. [DOI] [PubMed] [Google Scholar]
- 39.Perez C. Technological Revolutions and Financial Capital: The Dynamics of Bubbles and Golden Ages. London: Elgar; 2002. [Google Scholar]
- 40.van der Graaf PH, Benson N. Systems pharmacology: bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development. Pharm Res. 2011;28:1460–1464. doi: 10.1007/s11095-011-0467-9. [DOI] [PubMed] [Google Scholar]
- 41.Wajima T, Isbister GK, Duffull SB. A comprehensive model for the humoral coagulation network in humans. Clin Pharmacol Ther. 2009;86:290–298. doi: 10.1038/clpt.2009.87. [DOI] [PubMed] [Google Scholar]
- 42.Ogungbenro K, Dokoumetzidis A, Aarons L. Application of optimal design methodologies in clinical pharmacology experiments. Pharm Stat. 2009;8:239–252. doi: 10.1002/pst.354. [DOI] [PubMed] [Google Scholar]
- 43.D'Argenio DZ. Optimal sampling times for pharmacokinetic experiments. J Pharmacokinet Biopharm. 1981;9:739–756. doi: 10.1007/BF01070904. [DOI] [PubMed] [Google Scholar]
- 44.Carrothers TJ, Hodge FL, Korsan R, Poland W, Dykstra K. Decision-Making in Drug development: application of a clinical utility index. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 85–107. [Google Scholar]
- 45.Smith M, French J, Kowalski K, Hutmacher M, Ewy W. Decision-Making in Drug development: application of a model based framework for assessing trial performance. In: Kimko H, Peck C, editors. Clinical Trial Simulations: Applications and Trends. 1st edn. New York: Springer Science; 2011. pp. 61–83. [Google Scholar]
- 46.Patel NR, Ankolekar S, Antonijevic Z, Rajicic N. A mathematical model for maximizing the value of phase 3 drug development portfolios incorporating budget constraints and risk. Stat Med. 2013;32:1763–1777. doi: 10.1002/sim.5731. [DOI] [PubMed] [Google Scholar]
- 47.Harnisch L, Matthews I, Chard J, Karlsson MO. Drug and disease model resources: a consortium to create standards and tools to enhance model-based drug development. CPT Pharmacometrics Syst Pharmacol. 2013;2:e34. doi: 10.1038/psp.2013.10. doi: 10.1038/psp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blaschke TF, Lars Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 49.Holford N, Karlsson MO. Time for quantitative clinical pharmacology: a proposal for a pharmacometrics curriculum. Clin Pharmacol Ther. 2007;82:103–105. doi: 10.1038/sj.clpt.6100231. [DOI] [PubMed] [Google Scholar]