Abstract
Background and Purpose
Lipoxins can function as endogenous ‘breaking signals’ in inflammation and play important roles in the progression of endometriosis. In this study, we further investigated the molecular mechanism by which lipoxin A4 (LXA4) suppresses the development of endometriosis.
Experimental Approach
Primary endometriotic stromal cells (ESCs) were treated with IL-1β, or pre-incubated with LXA4 before incubation with IL-1β. The LXA4 receptor (ALX receptor) antagonist Boc-2 and gene-silencing approaches were used to study the involvement of the ALX receptor in anti-inflammatory signalling responses in ESCs. An animal model of endometriosis was induced in BALB/c mice by i.p. injection of an endometrium-rich fragment.
Key Results
Decreased levels of LXA4 and 15-LOX-2 expression but increased expression of AXL receptors were observed in endometriotic tissues. LXA4 inhibited the release of inflammatory factors and phosphorylation of p38 MAPK in IL-1β-induced ESCs, an effect mediated by ALX receptors. LXA4 inhibited the proliferation of ESCs, as indicated by reduced DNA replication, caused G0/G1 phase cell cycle arrest and down-regulated the expression of proliferating cell nuclear antigen in ESCs. LXA4 also attenuated the invasive activity of ESCs mainly by suppressing the expression and activity of MMP-9. In vivo, we further confirmed that LXA4 could inhibit the progression of endometriosis by acting as an anti-inflammatory.
Conclusions and Implications
LXA4 exerted anti-inflammatory, anti-proliferative and anti-invasive effects on endometriosis through a mechanism that involved down-regulating the activities of p38 MAPK, which was mediated by ALX receptors.
Table of Links
| TARGETS | LIGANDS |
|---|---|
| ALX receptor | LXA4 |
| 15-LOX-2 | IL-1β |
| 15-LOX-1 | IL-6 |
| p38 MAPK | MCP-1 |
| MMP-9 | TNF-α |
| VEGF | |
| SB203580 |
This Table lists key protein targets and ligands in this document, which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., b2013a).
Introduction
Endometriosis (EM), characterized by the presence of uterine endometrial tissue outside of the normal location, is a common gynaecological disorder with a complex, multifactorial aetiology that caused chronic pelvic pain, dysmenorrhoea, and even infertility (Giudice and Kao, 2004). The underlying pathophysiological mechanism responsible for EM remains unknown. Growing evidence suggests that aberrant immune responses and inflammatory reactions play an important role in the pathogenesis of EM. Many of pro-inflammatory cytokines derived from endometriotic cells, activated macrophages and other immune cells in the abdominal cavities of women with EM have been thought to play a crucial role in the development of the disorder (Podgaec et al., 2007).
The resolution of inflammation is now understood to be an active process that involves the action of anti-inflammatory and pro-resolution modulators, the lipoxins, resolvins and protectins, which activate specific biochemical pathways necessary for the resolution of acute inflammatory responses (Serhan et al., 2008b). Lipoxins (LXs), a class of arachidonic acid (AA) metabolites, are typically formed by transcellular metabolism through distinct biosynthetic pathways depending on the cellular context, include the naturally occurring lipoxin A4 (LXA4) and B4 (LXB4) and the aspirin-triggered lipoxins (LXA4) 15-epimeric lipoxin A4 (15-epi-LXA4) and 15-epimeric lipoxin B4 (15-epi-LXB4; Maderna and Godson, 2009). Three human lipoxygenases (LOXs), included 5-LOX, 12-LOX and 15S-lipoxygenase-2 (15-LOX-2), have been confirmed to involve the formation of LXs. The expression of these three LOXs was aberrant in many diseases including adenomatous polyps, human epidermoid carcinoma and EM (Melstrom et al., 2008; Agarwal et al., 2009; Borghese et al., 2009). LXs mediate a number of physiologic and pathologic processes, including regression of proinflammatory cytokine production, inhibition of cell proliferation, modulation of cytokine-stimulated metalloproteinase activity and stimulation of macrophage clearance of apoptotic polymorphonuclear neutrophils (McMahon et al., 2001; Bonnans et al., 2006). These diverse actions are mainly mediated by various membrane receptors including the LXA4 receptor (ALX receptor), cysteinyl leukotriene receptor (CysLT1), GPCR 32 (GPR32), oestrogen receptor α (ERα, also known as NR3A1) and unidentified high-affinity surface-binding receptors (Maderna and Godson, 2009; Krishnamoorthy et al., 2010; Russell et al., 2011). However, because the A-type LX has been shown to bind with high affinity to the ALX receptor, more and more studies have focused on how LXA4 exerts actions via it. The ALX receptor was known as formyl peptide receptor-like 1 (FPRL1) and formyl peptide receptor 2 (FPR2), a distinct GPCR of the formyl peptide receptor superfamily. LXA4 is a dual acting mediator and activates specific cellular pathways via ALX receptor to elicit both anti-inflammatory and pro-resolution effects (Serhan et al., 2008b). Studies also revealed that LXA4 could attenuate NF-κB activation and inhibit phosphorylation of p38 MAPK, ERK1/2, and Akt (Cezar-de-Mello et al., 2008; Baker et al., 2009), which suggested that LXA4 might play its role in regulating the activation of these signalling pathways.
It is noteworthy that many of the factors that are attenuated by the LX–ALX receptor interaction are involved in the development of EM. Endometrium in experimental models of EM in rats and in women with EM showed higher expression of LXA4 receptor compared with the normal tissues (Motohashi et al., 2005), which suggested a possible role of LXA4 and the receptor under pathophysiological condition of EM. We have previously reported that LXA4 suppressed the growth of endometriotic lesion in an experimental mouse model of EM possible by inhibiting the expression and activities of MMP (Chen et al., 2010a). Xu also found that LXA4 could inhibit the progression of EM in mice by playing its roles of anti-inflammation and anti-angiogenesis (Xu et al., 2012).
Recently, increasing evidence indicated that p38 MAPK, an intracellular signal-transducing molecule, might be involved in the pathogenesis of EM (Seval et al., 2006; Yoshino et al., 2006). Our previous studies found that SB203580, a p38 MAPK inhibitor, could suppress the development of EM in a murine model by inhibiting the expression of proinflammatory cytokines and proteolytic factors (Zhou et al., 2010). Collectively, we suppose that LXA4 may play its anti-inflammatory and anti-invasive roles in EM via p38 MAPK signalling pathway.
In this study, we first investigated the levels of LXA4, expressions of 15-LOX-2 and ALX receptor in human endometriotic tissues. Then, the effects of LXA4 on proinflammatory cytokine production in vivo or in vitro were investigated. We also analysed the impacts of LXA4 on cell proliferative and invasive activity in endometriotic stromal cells (ESCs). Furthermore, the effects of LXA4 and its receptor ALX receptor on p38 MAPK signalling pathway were studied, which may explain the mechanism of LXA4 inhibiting the progression of EM.
Methods
Patients and samples
Endometrial tissue samples (Supporting Information Table S1) were obtained from 53 patients who underwent surgery from September 2011 through to August 2012 for diagnosis or treatment of EM or for other benign gynaecological disorders (controls). To prevent the effect of tissue quality on PCR results, six cases were excluded from the study due to incompetent tissue quality as determined by the gynaecological pathologist. Finally, 47 patients were enrolled in this study. Among the patients, 20 patients with laparoscopically confirmed absence of EM, and 27 patients with both laparoscopically and histologically confirmed EM. All the EM patients had ovarian endometriotic cysts. The women's menstrual cycle phase were established according to their menstrual history and confirmed by endometrial histology using the criteria as described previously (Noyes et al., 1975). Therefore, the division of the menstrual cycle was as follows: proliferative phase, days 1–14; secretory phase, days 15–29. The use of these tissues was approved by Ethics Committee of the First Affiliated Hospital of Xiamen University, and informed consent was obtained from each patient. These patients had not received hormonal treatment or any anti-inflammatory treatment for at least 6 months before surgery.
Isolation and culture of human ESCs
Primary ESC culture was all from ovarian endometriotic cyst tissues, based on previously published procedure with minor modifications (Lin et al., 2012). Briefly, after endometriotic tissues were minced into small pieces and incubated in type IV collagenase, endometrial epithelial cells and stromal cells were separated from the cell suspensions by nylon cell strainers. Isolated ESCs were cultured in complete medium at 37°C in 5% CO2. When the cells became 90% confluent, they were used for experiments. The purity of the endometrial stromal cells were assessed by staining with vimentin and CD 10, and the purity of the endometrial epithelial cells were determined by immunocytochemical staining with cytokeratin 19. As shown in Supporting Information Fig. S1, for ESCs in monolayer culture after the second passage, the percentages of vimentin- and CD 10-positive cells were approximately 96 and 89%, respectively, and remained consistent with passage. ESCs were used between passages 2 and 6.
Quantitative real-time PCR analysis
Total RNA was extracted from ESCs by using RNAiso Plus (Takara, Kyoto, Japan) following the manufacturer's protocol. Intact total RNA was confirmed by determining appropriate sharp 28 S and 18 S rRNA bands by agarose gel electrophoresis; 1 μg of total RNA was reverse transcribed in a system of 20 μL volume using PrimeScript RT reagent Kit (Takara). Real-time quantitative PCR assays were carried out by employing SYBR PrimeScript RT-PCR kit (Takara) using Rotor-Gene 6000 (Corbett Research, Sydney, Australia) as previously described (Zhou et al., 2010). The primers used for RT-PCR and qRT-PCR were provided in Supporting Information Table S2. The 2-ΔΔCT method was used to calculate the relative mRNA level of each gene (Schmittgen and Livak, 2008).
Western blotting analysis
Western blot analysis was performed as described previously (Zhou et al., 2010). Briefly, protein was isolated from ESCs or endometriotic tissues using RIPA lysis buffer (Applygen Technologies Inc., Beijing, China) containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS and protease inhibitor cocktail (Roche, Basel, Switzerland). Then protein concentration was determined by using a BCA assay (Applygen Technologies Inc.). Protein lysates (cell for 30 μg per lane, tissue for 45 μg per lane) were separated on 10% SDS-PAGE under denatured conditions. The separated samples were transferred to PVDF membranes and exposed to primary antibodies included anti-15-LOX-2 (1:100), anti-ALX receptor (1:200), anti-p38 MAPK (1:1000), anti-phospho-p38MAPK (1:1000), anti-β-actin (1:1000) and β-tubulin (1:1000). The membranes were subsequently incubated with a HRP-conjugated secondary antibody (1:10000; Pierce, Rockford, IL, USA) for 1 h at room temperature and visualized using enhanced chemiluminescence (Pierce) and X-ray film.
Matrigel invasion assay
Effect of LXA4 on the invasion ability of ESCs was determined using Matrigel invasion chambers (pore size: 8 mm, 24-well; BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer's protocol. The cells were trypsinized after serum-starvation for 24 h, and 1 × 105 cells plated on transwell chambers pre-coated with 20 μg Matrigel. 500 μL medium-containing 10% FBS in the lower chamber served as chemoattractant. After 24 h of co-incubation with LXA4 (100 nM) or ethanol as a vehicle, cells that have invaded through the Matrigel and the 8.0 μm pore size membrane were fixed with 95% ethanol, stained by using crystal violet and counted.
ALX receptor and MMP-9 gene silencing in ESCs
The cells were transiently transfected with small interfering RNA (siRNA) against human ALX receptor or MMP-9 using TurboFect™ siRNA Transfection Reagent (Fermentas, Glen Burnie, MD, USA) according to the manufacturer's instructions. ALX receptor siRNA were 19-nucleotide long duplexes designed and synthesized by Shanghai GenePharma (Shanghai, China). The ALX receptor siRNA sequence is 5′-GAGGGAUUAUCCGGUUUGU-3′ (forward) and 5′-ACAAACCGGAUAAUCCCUC-3′ (reverse). The MMP-9 siRNA sequence is 5′-UCACCUUCACUCGCGUGUA-3′ (forward) and 5′-UACA CGCGAGUGAAGGUGA-3′ (reverse). As a negative control, a non-specific siRNA duplex with a random nucleotide sequence (Shanghai GenePharma) was used.
Statistical analysis
Statistical analysis was performed using SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA). Results are reported as the mean ± SEM. After testing the data for normal distribution and equal variance, differences between two groups were analysed by Student's unpaired t-test, and differences between multiple groups were analysed by one-way anova. Each assay was repeated in triplicate. P < 0.05 was considered statistically significant.
Results
Decreased LXA4 levels and 15-LOX-2 expression in human endometriotic tissues
The levels of LXA4 in normal endometrial (control) and endometriotic tissues (EM) were detected by elisa. As shown in Figure 1A, both in proliferative phase and secretory phase, the levels of LXA4 in EM group (n = 16) were significantly decreased compared with control group (n = 10; P < 0.01), but no difference was found in serum sample (data not shown). In proliferative phase, 15-LOX-2 mRNA expression was shown to be significantly reduced in EM group (n = 9) compared with control group (n = 10; P < 0.01; Figure 1B), but no difference was found in secretory phase. We also analysed the expression and distribution of 15-LOX-2 by Western blot and immunohistochemistry. As shown in Figure 1C, 15-LOX-2 expression was shown to be significantly decreased in EM group (n = 8) compared with control group (n = 8; P < 0.01), and 15-LOX-2 was found in nucleus of glandular and stromal cells (Figure 1D). These data suggest that the low levels of LXA4 may relate to abnormal expression of 15-LOX-2 in endometriotic tissues.
Figure 1.
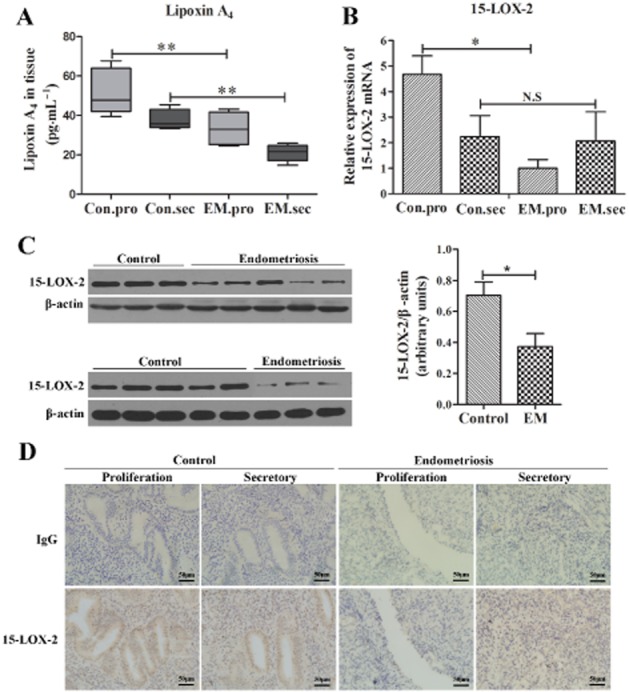
The expression of LXA4 and 15-LOX-2 is down-regulated during the menstrual cycle in human endometriotic tissues. (A) LXA4 levels in normal endometrial (control, n = 10) and endometriotic tissues (EM, n = 16) were evaluated by elisa. (B) Gene expression of 15-LOX-2 was detected by real-time RT-PCR and normalized to GAPDH expression. 15-LOX-2 mRNA expression was significant lower in the EM group (n = 8) versus control group (n = 9) in the proliferative phase, but no difference was found in the secretory phase. (C) Western blot analysis of 15-LOX-2 protein expression between normal endometrial (control, n = 8) and endometriotic tissues (EM, n = 8). Ratios of 15-LOX-2 to β-actin were determined following densitometry measurements of the specific protein bands. (D) Immunohistochemical staining for 15-LOX-2 in the human endometriotic tissues (EM, n = 12) and normal endometrium (control, n = 10) both in the proliferative phase and secretory phase (×400), scale bar = 50 μm. Sections were immunostained with 15-LOX-2 or IgG. Data are presented as means ± SEM; *P < 0.05, **P < 0.01.
Up-regulation of ALX receptors in human ectopic endometrium
As shown in Figure 2A, the mRNA expression of ALX receptors in endometrium with EM was significantly higher than in control endometrium by RT-PCR (P < 0.05; Figure 2A). Real-time RT-PCR results further showed that, in proliferative phase, there was a 2.4-fold increase in mRNA expression of ALX receptor in EM group (n = 9) compared with that expressed in control group (n = 8; P < 0.05; Figure 2B). Immunolocalization of ALX receptors showed positive cytoplasmic staining in glandular epithelial and stromal cells in endometrium (Figure 2C). The results demonstrated a significant increase of ALX receptor protein expression in ectopic endometrial tissues compared with in the control endometrium. The expression of ALX receptor in ESCs was also examined. RT-PCR analysis, immunocytochemical staining and immunofluorescence assay demonstrated that the ALX receptor was expressed in ESCs (Figure 2D–F). Western blot analysis showed that ALX receptor protein expression in ESCs was significantly higher than in control endometrial stromal cells (NESCs; P < 0.05; Figure 2G). These results suggest that higher expression of ALX receptors in the ESCs derived from women with EM may be associated with their unique biological characteristics.
Figure 2.
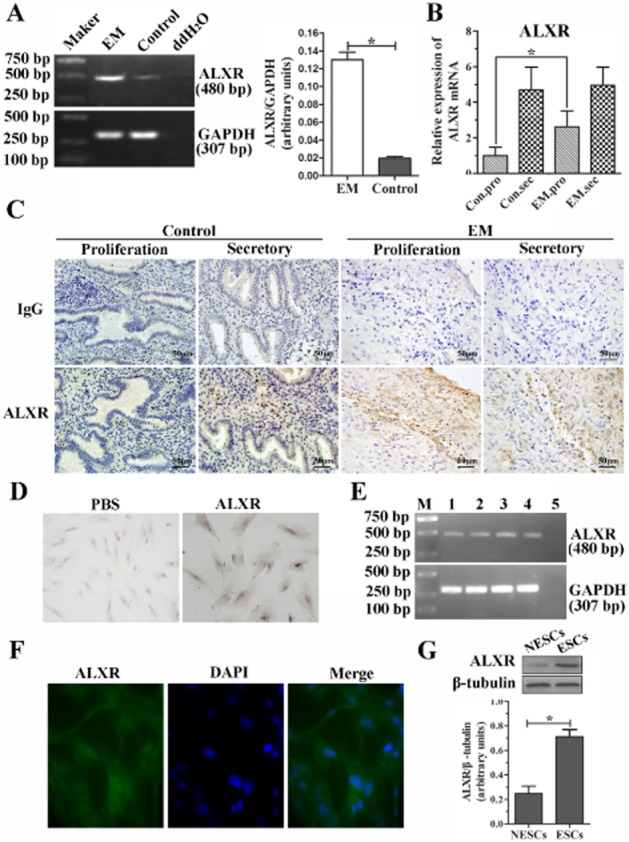
ALX receptor mRNA and protein are increased in endometriotic tissues and ESCs. (A) RT-PCR amplification of a 480 bp fragment from ectopic endometrial tissues and normal endometrium. Densitometric analysis of results are shown in column graph. (B) The ALX receptor mRNA expression was verified further in both control (n = 20) and ectopic endometrial tissues (n = 27) by real-time RT-PCR. The mRNA expression of ALX receptors was significantly greater in the EM group (n = 8) versus control group (n = 9) in the proliferative phase, but no difference was found in the secretory phase, *P < 0.05. (C) Immunohistochemical staining for ALX receptor in the human endometriotic tissues (EM, n = 12) and normal endometrium (control, n = 10). Sections were immunostained with anti-human ALX receptor antibody (ALXR) and IgG, scale bar = 50 μm. (D and E) Expression of ALX receptors in ESCs detected by immunocytochemical staining (D, ×400), RT-PCR (E), and immunofluorescence assay (F, ×400). (G) Western blot analysis of ALX receptor protein expression between normal (NESCs) and ectopic ESCs. A ratio of ALX receptor to β-tubulin was determined following densitometric measurements of the specific protein bands. Values are the mean ± SEM of the combined data from three independent experiments; *P < 0.05.
LXA4 inhibits IL-1β-induced cytokines release of ESCs via ALX receptors
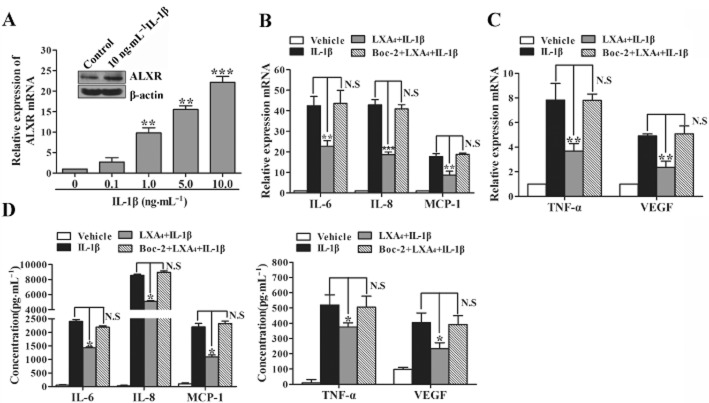
In previous studies, we have demonstrated that the expression of ALX receptors in ESCs was higher than in NESCs. Here, we found that IL-1β could up-regulate ALX receptor mRNA and protein expressions in ESCs (Figure 3A). To investigate whether the ALX receptor is involved in LXA4 effect on cytokines release induced by IL-1β in ESCs, the Boc-2 peptide, an effective antagonist of the ALX receptor, was used to block ALX receptor function. Pre-exposure of ESCs with Boc-2 (100 μmol·L−1, 15 min) abolished LXA4 effect on IL-1β-induced gene and protein expressions of IL-6, IL-8, MCP-1, TNF-α and VEGF (Figure 3B–D), showing the involvement of ALX receptors in LXA4 anti-inflammatory actions on human ESCs.
Figure 3.
LXA4 inhibition of IL-1β-induced cytokine release in ESCs is mediated by the ALX receptor. (A) ESCs were stimulated with IL-1β (0.1 to 10 ng·mL−1) or vehicle (medium) for 24 h, and mRNA expression of the ALX receptor was determined by real-time PCR. Inset is a representative Western blot result showing that expression of ALX receptor protein is induced by IL-1β (10 ng·mL−1). (B–D) ESCs were incubated with Boc-2 (100 μmol·L−1, 15 min) or vehicle, then exposed to LXA4 (10 nM, 30 min) and stimulated with IL-1β (1 ng·mL−1, 24 h). Total RNA extracts were generated and converted into cDNA. The mRNA expressions of IL-6, IL-8, MCP-1, TNF-α and VEGF were detected by real-time PCR (B and C). Concentrations of IL-6, IL-8, MCP-1, TNF-α and VEGF were measured in supernatants by elisa (D). Results were expressed as mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. N.S., no significant difference.
LXA4 suppresses the proliferative activity and cell cycle progression of ESCs
To investigate the role of LXA4 in proliferative activity of ESCs, the EdU incorporation assay, cell cycle analysis and proliferating cell nuclear antigen (PCNA) staining were conducted after ESCs were treated with LXA4 (100 nmol·L−1; Figure 4). Following pretreatment with LXA4, the percentage of EdU-positive cells was reduced in ESCs compared with the controls (Figure 4A and B). Moreover, the proportion of cells corresponding to the proportion of cells in the S and G2/M phase was lower in LXA4-treated ESCs (40.19 vs. 5.51%) than that in control cells (48.61 vs. 13.31%; Figure 4C). The G0/G1 phase was increased in LXA4-treated ESCs (54.30%) compared with control cells (38.09%). It seems that treatment with LXA4 resulted in cells that were blocked in G0/G1 phase and could not enter into S phase for DNA synthesis. Further analysis revealed that LXA4 significantly decreased the proliferation index of ECSs (P < 0.05) (Figure 4D). PCNA is retained one of the most important index to estimate cell proliferation. The positive nuclear PCNA staining in LXA4-treated ESCs was fewer than in control cells (Figure 4E). Counts of PCNA-positive cells were significantly decreased in LXA4-treated ESCs compared with control cells (P < 0.05; Figure 4F).
Figure 4.
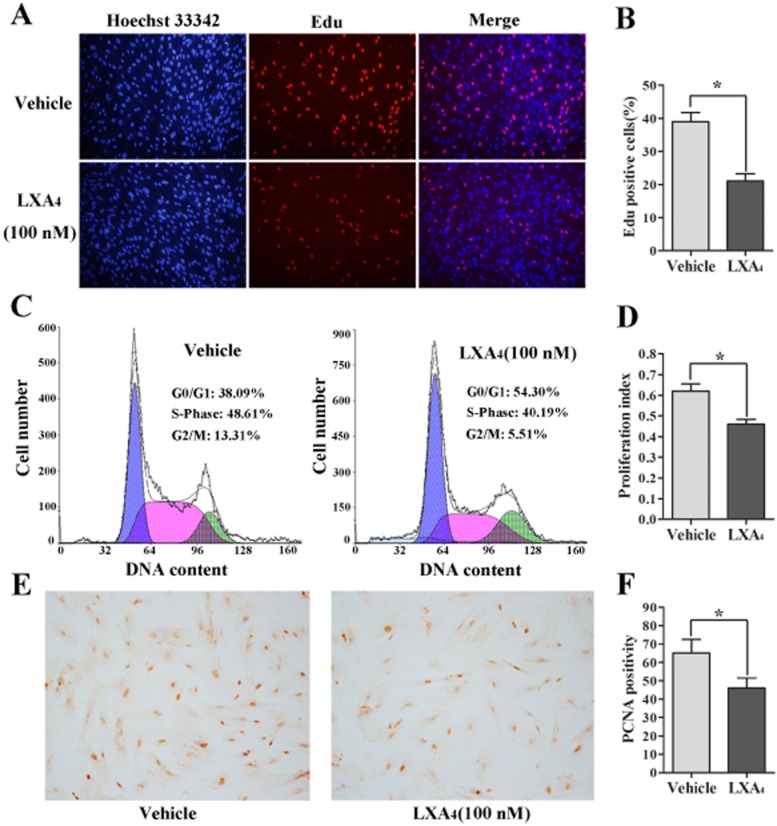
LXA4 inhibits the proliferative activity of ESCs. (A) LXA4 (100 nmol·L−1) inhibited DNA replication in ESCs compared with controls as determined by the EdU incorporation assay, Original magnifications: ×200. EdU-positive cell counts were analysed by using software of the light microscope (B), *P < 0.05. (C) Cell cycle analysis indicated the increased population of cells in the G0/G1 phase and decreased S and G2/M phase cells in ESCs treated with LXA4 (100 nmol·L−1). (D) Proliferation index = (S phase + G2/M phase)/(G0/G1 phase + S phase + G2/M phase). (E) LXA4 decreased PCNA protein expression in ESCs detected by imunocytochemical staining. Original magnifications: ×100. PCNA-positive cell counts were analysed by using software of the light microscope (F). These experiments were done three times using different batches of cells and data are means ± SEM; *P < 0.05.
LXA4 attenuates the migration and invasion of ESCs
We first assessed the effect of LXA4 on the migration in ESCs by wound healing assay. As shown in Supporting Information Fig. S2A and B, pretreatment with LXA4 (100 nM) for 48 h resulted in a remarkable decrease in cell migration ability relative to control group (P < 0.05). We further investigated the effect of LXA4 on the invasion of ESCs. As shown in Figure 5A and B, pretreatment with LXA4 (10 and 100 nM) significantly reduced IL-1β-stimulated (1 ng·mL−1) the mRNA and protein expressions of MMP-2 and MMP-9 in ESCs. To examine the effect of LXA4 on IL-1β-induced activities of MMP-2 and MMP-9 of ESCs, gelatin zymography assay was used. Only MMP-9 activity was detected in cell culture supernatants, which could be induced by IL-1β in a dose-dependent manner. Treatment with LXA4 (10 nM) significantly inhibited MMP-9 activity in ESCs stimulated by IL-1β (1 ng·mL−1; P < 0.05) (Figure 5C). Cell Matrigel invasion assays were performed to evaluate the change in invasive ability of ESCs after LXA4 treatment (100 nM), and the results showed that LXA4 significantly decreased the invasive activity of ESCs (P < 0.05; Figure 5D). These results imply that LXA4 could attenuate the invasive activity of ESCs by inhibiting the activity of MMP-9.
Figure 5.
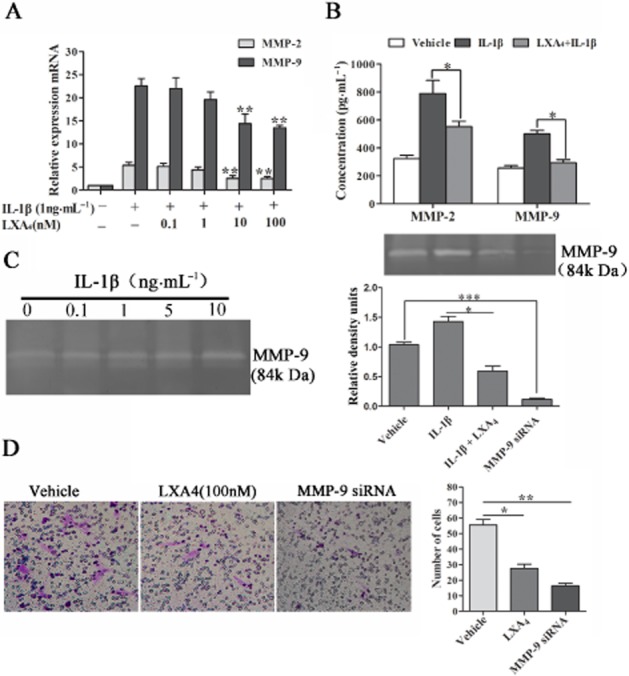
LXA4 attenuates the invasion activity of ESCs by reducing the expression of MMP-9. (A and B) ESCs were stimulated with IL-1β (1 ng·mL−1) for 15 min or pre-incubated with LXA4 (0.1 to 100 nM) for 30 min before IL-1β treatment. The mRNA and protein expression of MMP-2 and MMP-9 were determined by real-time PCR and elisa. (C) ESCs were stimulated with IL-1β (0.1 to 10 ng·mL−1) or vehicle (medium) for 24 h, and protein activities of MMP-9 was assessed by the Gelatin Zymography assay (left). The IL-1β-increased MMP-9 was significantly inhibited by LXA4 (right), (compared with IL-1β alone). (D) Cell Matrigel invasion assays indicated the decreased number of ESCs, which invaded from upper chamber to lower chamber treated with LXA4 (100 nM). The band intensities were analysed using Quantity One software. Data are presented as means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
LXA4 inhibits IL-1β-induced phosphorylation of p38 MAPK of ESCs in an ALX receptor-dependent manner
We first examined the phosphorylated p38 MAPK immunoreactivity in both normal and ectopic endometrial tissues (Figure 6A). As was shown, phospho-p38 MAPK expressions were mainly detected in ESCs of ectopic endometrial tissues, which were barely detected in normal endometrial tissues. Phosphorylated/total p38 MAPK ratio was significantly higher in peritoneal cells of EM mice than in the control mice (P < 0.01; Figure 6B). Similarly, we also found that the phosphorylation level of p38 MAPK in ESCs was significantly higher than in NESCs (P < 0.05; data not shown).
Figure 6.
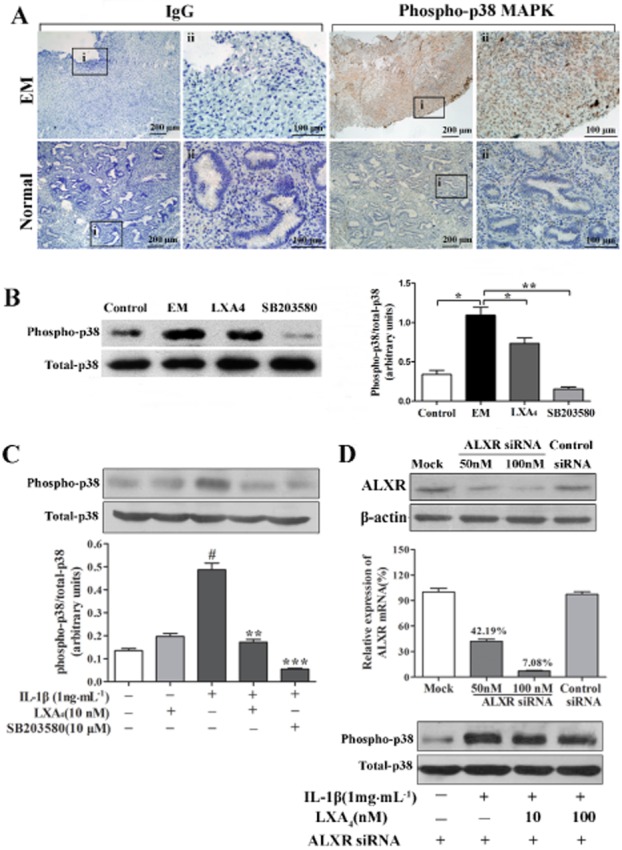
LXA4 inhibition of IL-1β-induced phosphorylation of p38 MAPK in ESCs is ALX receptor-dependent. (A) Immunohistochemical staining for phosphorylated p38 MAPK in ectopic endometrial tissues (EM, n = 12) and normal endometrial tissues (control, n = 10). Sections were immunostained with phosphorylated p38 MAPK (phospho-p38 MAPK) antibody and IgG. Original magnification: (i) ×100 and (ii) ×400. (B) A representative Western blot result demonstrated that expression of phospho-p38 MAPK was inhibited by LXA4 in peritoneal cells of EM mice. *P < 0.05, **P < 0.01. (C) IL-1β-induced p38 MAPK phosphorylation was inhibited by LXA4. ESCs were pre-incubated with LXA4 for 30 min before IL-1β treatment for an additional 15 min. The band intensities were analysed using Quantity One software. #P < 0.001 (compared with vehicle); **P < 0.01, ***P < 0.001 (compared with IL-1β alone). (D) ALX receptor knockdown with siRNA blocked the inhibitory effect of LXA4 on IL-1β-induced p38 MAPK phosphorylation in ESCs. Cells were transiently transfected with siRNA duplexes for the ALX receptor or scrambled siRNA (control) for 48 h. ALX receptor mRNA and protein levels significantly decreased in the presence of ALX receptor siRNA (D) Mock: the cells were transfected with transfection reagent alone. A representative Western blot result showed that LXA4 had no effect on IL-1β-induced p38 MAPK phosphorylation in ESCs transfected with ALX receptor siRNA. Experiments were repeated in ESCs from at least three different subjects.
In vitro, IL-1β (1 ng·mL−1) significantly stimulated the phosphorylation of p38 MAPK in ESCs (P < 0.01; Figure 6C). To further clarify the effect of LXA4 on the phosphorylation of p38 MAPK, ESCs were pretreated with LXA4 or vehicle (0.01% ethanol) for 30 min before a further stimulation with IL-1β for 15 min. Pretreatment with LXA4 (10 nM) significantly reduced IL-1β-stimulated phosphorylation of p38 MAPK (P < 0.01; Figure 6C). SB203580, a p38 MAPK specific inhibitor, significantly inhibited the phosphorylation of p38 MAPK induced by IL-1β (P < 0.001). Interestingly, the inhibitory effect of SB203580 was also found in the peritoneal cells of EM mice (Figure 6B). To determine whether ALX receptor mediates LXA4 attenuation of p38 MAPK phosphorylation in ESCs induced by IL-1β, we used ALX receptor-specific siRNA to silence the expression of ALX receptor by RNA interference. Transfection of ESCs with ALX receptor siRNA for 48 h resulted in strong reduction of ALX receptor mRNA and protein levels relative to mock-transfected cells (mock) or cells transfected with scrambled siRNA (control siRNA; Figure 6D). LXA4 had no effect on IL-1β-induced phosphorylation of p38 MAPK in ESCs transfected with ALX receptor siRNA (Figure 6D).
LXA4 suppresses the growth of endometriotic lesion and the expressions of inflammatory factors in EM mouse model
To investigate the effect of LXA4 on the progression of EM, we measured the weight and size changes of endometriotic lesions in EM mouse model after being treated by LXA4 for 21 days. The results showed that LXA4 inhibited the growth of endometriotic lesions in EM mice (Figure 7A–C). The weight of endometriotic lesions in the LXA4 group (22.62 ± 12.66 mg) was significantly less than in EM group (67.62 ± 13.44 mg; P < 0.05; Figure 7B). LXA4 also decreased the size of endometriotic lesions from 8.76 ± 1.88 mm3 to 3.38 ± 1.23 mm3 (P < 0.05) (Figure 7C).
Figure 7.
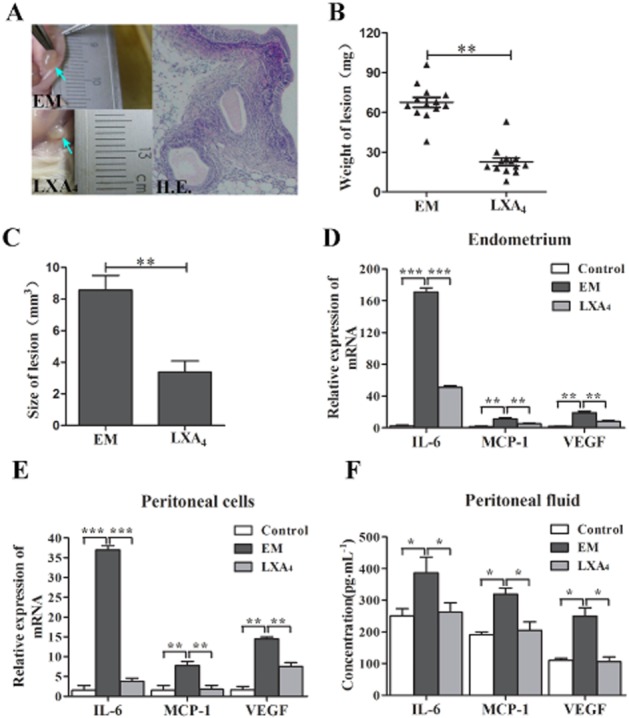
LXA4 inhibits the proliferative activity of endometriotic lesions and the production of IL-6, MCP-1 and VEGF in EM mice. (A) LXA4 suppressed the growth of the endometriotic lesions in EM mice. Three days before the EM induction, the LXA4 group was i.p. injected LXA4 at a dose of 5 μg·kg−1 on a daily basis for 24 days. Arrows indicate endometriotic lesions. The weight (B) and size (C) of endometriotic lesions were significantly reduced by LXA4 treatment. Horizontal bars represent the mean weight of endometriotic lesions. *P < 0.05. (D and E) The mRNA expressions of IL-6, MCP-1 and VEGF in both endometrial tissues and peritoneal cavity cells were detected by real-time PCR. (F) Concentrations of IL-6, MCP-1 and VEGF in peritoneal fluids were measured by elisa. Results are expressed as mean ± SEM of at least three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001).
Furthermore, we investigated the effects of LXA4 on synthesis of IL-6, MCP-1 and VEGF in EM mice. The results showed that high levels of IL-6, MCP-1 and VEGF were observed in EM mice compared with control mice. LXA4 significantly decreased the mRNA levels of IL-6, MCP-1 and VEGF in both endometriotic lesions and peritoneal cells of EM mice (Figure 7D and E). The concentrations of IL-6, MCP-1 and VEGF in the peritoneal fluid were significantly lower in LXA4 group than in EM group (P < 0.05). LXA4 decreased the concentrations of IL-6 from 386.82 ± 49.33 pg·mL−1 to 262.25 ± 29.12 pg·mL−1, MCP-1 from 319.10 ± 19.26 pg·mL−1 to 205.43 ± 26.36 pg·mL−1 and VEGF from 250.09 ± 25.92 pg·mL−1 to 105.51 ± 15.60 pg·mL−1 (Figure 7F).
Discussion
15-LOX is an intracellular enzyme which has two distinct isoforms: 15-LOX-1 and 15-LOX-2. In terms of enzymatic characteristics, 15-LOX-1 preferentially metabolizes linoleic acid to 13-(S)-HODE, but also metabolizes AA to 15-hydroxyeicosatetraenoic acid (15-HETE). 15-LOX-2, on the other hand, converts AA exclusively to 15-HETE and plays a key role in the synthesis of LXA4. The majority of earlier research on the role of 15-LOX focused on 15-LOX-1. For example, 13-(S)-HODE, the 15-LOX-1 metabolite, played an important role in activating the MAPK signalling pathway and subsequently decreasing PPAR expression in human colorectal carcinoma cells (Hsi et al., 2002). However, growing evidence showed that 15-LOX-2 and its metabolite (15-HETE) exerted an important role in some diseases. It was reported that the expression of 15-LOX-2 mRNA and protein declined in the prostate cancer tissue compared with the normal tissue (Tang et al., 2009), suggesting 15-LOX-2 may play an important role in the development of prostate cancer, and 15-HETE could inhibit the MAPK signalling pathway and up-regulate a downstream target of MAPK signalling like PPAR (Hsi et al., 2002). Interestingly, oestrogen negatively regulated the intrinsic LXA4 formation, especially ERβ-specific regulation of epithelial 15-LOX in the cornea, could provide novel mechanism that 15-LOX was down-regulated in some inflammatory disease (Wang et al., 2012). The present study found that 15-LOX-2 existed in the endometriotic tissues, which was previously shown (Russell et al., 2011), but the expression of 15-LOX-2 decreased comparing with control endometrial tissues in both proliferative and secretory phase. Collectively, the decreased levels of LXA4 in endometriotic tissues suggest that the endometrium is a source of LXA4 production and decreased expression of 15-LOX-2 may lead to the reduction of LXA4 in endometriotic tissues.
It has been demonstrated that ectopic endometrial cells need to undergo a three-step procedure (attachment-aggression-angiogenesis) to develop into EM (Robboy and Bean, 2010). Inflammatory reactions during this process might be associated with the development of EM. IL-1β was well known to play a critical role in the pathogenesis of this disorder by inducing the growth, adhesion, invasiveness and angiogenesis of endometrial fragments outside the uterus. IL-6 was a multifunctional cytokine participating in immune response and had angiogenic effects, some of which were mediated via the induction of VEGF expression (Wu and Ho, 2003). Many studies have reported increased expression of IL-6 in EM (Umezawa et al., 2008). IL-8, a CXC chemokine, was elevated in women with EM and the levels correlate with the severity of the disease (Ulukus et al., 2005). MCP-1 was a chemotactic cytokine that could influence both innate immunity through its effects on monocytes and adaptive immunity via T helper cell polarization (Gu et al., 2000). Findings indicated that MCP-1 might result in the development of immunotolerance by increasing apoptosis of leukocytes and thereby supporting the survival of ectopic endometrial cells (Selam et al., 2006). VEGF was an important vasoactive growth factor being involved in the development of peritoneal EM (Dziunycz et al., 2009). Our previous studies have found an increase production of MMP2 and MMP-9 in EM mice, which might associate with the development of EM (Chen et al., 2010b). In the present study, we also observed that IL-1β stimulated the release of IL-6, IL-8, MCP-1, TNF-α and VEGF in ESCs (Supporting Information Fig. S3). Together, inflammation plays an important role in the pathophysiological processes of EM.
Recently, LXA4 was described a anti-inflammatory and pro-resolution lipid mediator, which elicits its actions via the GPCR ALX receptor (Serhan and Chiang, 2008a). LXA4 and its receptor ALX receptor involved regulating inflammatory events in the human endometrium and decidua of early pregnancy (Macdonald et al., 2011). The ectopic endometrial tissues from women with EM showed an elevated expression of LXA4 receptor compared with the normal tissues (Motohashi et al., 2005; Canny and Lessey, 2013). The present finding also demonstrated a significant increase of ALX receptor gene and protein expression in ectopic endometrial tissues compared with the control endometrium. ALX receptor protein expression in ESCs was significantly higher than in normal endometrial stromal cells (NESCs). Therefore, we speculated that the high expression of ALX receptor in EM patients was responsible for responding to the inflammatory reactions, although the LXA4 levels in ectopic endometrial tissues were significantly decreased. These findings suggest a possible role of LXA4 and its receptor under pathological condition as EM. In another study, we found that LXA4 could regulate ER in ESCs. Treatment with LXA4 (100 nM) for 48 h, LXA4 could attenuate ERα and augment ERβ expression in ESCs, and inhibit oestrogen response element activity (data not shown). The results suggest that LXA4 may exect its biological function in EM via ALX receptor or ERβ.
LXA4 has been previously demonstrated to elicit anti-inflammatory, anti-proliferative, and anti-angiogenic actions in a variety of cell types, including epithelial cells, endothelial cells, fibroblasts, by reducing the expression of inflammatory factors and growth factors (Baker et al., 2009; Janakiram et al., 2011). In the present study, the elevated expressions of proinflammatory cytokines in IL-1β-induced ESCs were significantly inhibited by pre-treatment with LXA4. It has been shown that LXA4 exerted its biological actions through its specific receptor named FPRL1 or ALX receptor. In this study, we found that Boc-2, an antagonist of ALX receptor, could abolish inhibition of LXA4 on IL-1β-induced expressions of IL-6, IL-8, MCP-1, TNF-α and VEGF in ESCs. These data indicate the involvement of ALX receptor in LXA4 anti-inflammatory actions on EM.
In in vivo experiment, LXA4 could suppress the weight and size of endometriotic lesions in EM mouse model. In in vitro experiment, we further found that LXA4 could inhibit the DNA replication and cause G0/G1 phase cell cycle arrest in ESCs, suggesting an anti-proliferative potential of LXA4 with endometriotic lesion. LXA4 could also down-regulate synthesis of PCNA protein in ESCs. PCNA is a cofactor of DNA polymerases that coordinates cell proliferation by recruiting crucial players to the DNA replication fork (Moldovan et al., 2007). It was reported that the positive nuclear PCNA staining of human endometrium in women with EM showed a significant persistence compared with in fertile healthy women (Hapangama et al., 2009). Therefore, PCNA may play an important role in the development of EM, and LXA4 may decrease the proliferative activity of endomeitriotic lesion by inhibiting the synthesis of PCNA. Moreover, some evidence indicated that LXA4 could play a critical role in regulating cell invasion and migration (Zhou et al., 2009; Hao et al., 2011), and few was reported about its role in EM. In the present study, we also observed that LXA4 attenuated the invasive activity of ESCs possibly by inhibiting the activity of MMP-9, which was consistent with the previous reported in vivo. Wound healing assay also demonstrated that LXA4 attenuated the ESCs migration ability. Therefore, these results imply that LXA4 may be involved in suppressing the implantation of endometriotic cells in ectopic sites.
P38 MAPK, an intracellular signal-transducing molecule, has been demonstrated to involve in the regulation of many cellular processes including inflammatory reaction, cell differentiation, cell proliferation and cell death. Activation of p38 MAPK by extracellular stimuli, such as bacterial pathogens and cytokines, mediates signal transduction into the nucleus to turn on the responsive genes (Ono and Han, 2000). In the present study, we found that IL-1β significantly stimulated the phosphorylation of p38 MAPK in endometriotic cells. High levels of phosphorylated p38 MAPK were also observed in human ectopic endometrial tissues. These findings suggest that p38 MAPK may serve as an essential signal transducer and thereby play a key role in the pathogenesis of EM. P38 MAPK signalling pathway was reported to involve inhibition of pro-inflammatory cytokine production in endothelial cells by LXA4 (Wu et al., 2008). In the present investigation, the attenuation of IL-1β-induced p38 MAPK phosphorylation by LXA4 in ESCs was observed. In addition, the RNA interference experiment demonstrated that LXA4 had no effect on IL-1β-induced phosphorylation of p38 MAPK in ESCs transfected with ALX receptor siRNA. These data suggest that LXA4 may reduce the expression of pro-inflammatory cytokines in EM by inhibiting p38 MAPK activation, which was AXL receptor-dependent.
In summary, our findings have uncovered a novel role of LXA4 in regulating the pathological processes of EM. These results demonstrated that LXA4 markedly suppressed the development of EM by inhibiting activation of p38 MAPK through the ALX receptor, which led to the down-regulation of pro-inflammatory cytokines and proteolytic factors. Crosstalk between these factors presents a mechanism for the inhibitory effects of LXA4 in EM. In addition, these studies point to a novel therapeutic strategy for endogenous anti-inflammatory lipid mediators in EM.
Acknowledgments
The present investigation was supported by the Natural Science Foundation of Fujian Province (2013 D001), by the Science and Technology Planning Project of Xiamen City (3502Z20134002), by the China Postdoctoral Science Foundation Grant (2013 M541860) and by the National Science Foundation for Fostering Talents in Basic Research of the National Natural Science Foundation of China (Grant No. J1310027/J0106).
Glossary
- 15-LOX-2
15S-lipoxygenase-2
- ALX receptor
lipoxin A4 receptor
- EM
endometriosis
- ESCs
endometriotic stromal cells
- LXA4
lipoxin A4
Author contributions
R. W. and W. Z. contributed equally to this work. They mainly achieved all the experimentation. .S. C., Y. S., L. S. and M. Z. participated in clinical material collection, cell culture, elisa test, immunohistochemistry. Q. C. and Q. C. contributed towards designing of this study, supervision of experiments, interpretation of data and composed the article.
Conflict of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12816
The immunocytochemistry identified of human endometrial glandular epithelial and stromal cells (40×), scale bar = 50 μm. (A) Endometrial glandular epithelial cells (left, negative control IgG; right, CK19); (B) Endometrial stromal cells (left, negative control IgG; right, vimentin 9); (C) Endometrial stromal cells (left, negative control IgG; right, CD10);
LXA4 inhibited the migration of ESCs. (A) A wound healing assay was performed on ESCs (5 × 105, 6 cm plate) incubated with either ethanol (control) or LXA4 (100 nM). The ‘wounded’ areas were photographed by Nikon Eclipse 50i fluorescent microscope at various time points (0 h, 24 h or 48 h). One representative experiment was shown, scale bar = 50 μm. (B) Quantitative analysis of wound-induced migration assay from (A). The results were presented as mean ± SEM of three experiments done in duplicate and differences with P < 0.05 on the Student's t-test were considered statistically significant (*).
LXA4 inhibits IL-1β-induced expressions of IL-6 (A), IL-8 (B), MCP-1 (C), TNF-α (D), and VEGF (E) mRNA in a dose-dependent manner. ESCs were pretreated for 30 min with vehicle (0.01% ethanol) or with the indicated concentrations of LXA4 before IL-1β (1 ng·mL−1) treatment for an additional 24 h. Total RNA extracts were generated and converted into cDNA. Changes in the expressions of IL-6, IL-8, MCP-1, TNF-α and VEGF mRNA were detected by real-time PCR. To determine the dose effects of IL-1β on cytokines synthesis of ESCs, cells were stimulated with IL-1β (0.1 to 10 ng·mL−1) or vehicle (medium) for 24 h, and mRNA expressions of IL-6 (A), IL-8 (B), MCP-1 (C), TNF-α (D) and VEGF (E) were also determined by real-time PCR. The results were presented mean ± SEM of three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001).
According to the manufacturer's instructions and previous reports, appropriate positive control was used with each antibody during the staining run. (A) Immunohistochemical staining for 15-LOX-2 in the human normal renal tissues (n = 3). Sections were immunostained with anti-human 15-LOX-2 antibody (15-LOX-2) and negative control (IgG). Scale bar = 50 μm; (B) Immunohistochemical staining for ALX receptor in the human gastric cancer tissues (n = 3). Sections were immunostained with anti-human ALX receptor antibody (ALXR) and negative control (IgG). Scale bar = 50 μm; (C) Immunohistochemical staining for Phospho-p38 MAPK in the human gastric cancer tissues (n = 3). Sections were immunostained with anti-human Phospho-p38 MAPK antibody and negative control (IgG). Scale bar = 50 μm.
Clinical characteristics of patients and controls.
Primer sequences for RT-PCR and quantitative real-time PCR.
Supplemental materials and methods.
References
- Agarwal S, Achari C, Praveen D, Roy KR, Reddy GV, Reddanna P. Inhibition of 12-LOX and COX-2 reduces the proliferation of human epidermoid carcinoma cells (A431) by modulating the ERK and PI3K-Akt signalling pathways. Exp Dermatol. 2009;18:939–946. doi: 10.1111/j.1600-0625.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1862. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, O'Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–3826. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese B, Gayet V, Chiche JD, Vernerey D, de Ziegler D, Bonaiti-Pellie C, et al. Absence of association between a functional polymorphism of ALOX15 gene and infertility in endometriosis. Fertil Steril. 2009;91(4 Suppl):1414–1416. doi: 10.1016/j.fertnstert.2008.05.039. [DOI] [PubMed] [Google Scholar]
- Canny GO, Lessey BA. The role of lipoxin A4 in endometrial biology and endometriosis. Mucosal Immunol. 2013;6:439–450. doi: 10.1038/mi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezar-de-Mello PF, Vieira AM, Nascimento-Silva V, Villela CG, Barja-Fidalgo C, Fierro IM. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br J Pharmacol. 2008;153:956–965. doi: 10.1038/sj.bjp.0707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Zhou WD, Pu DM, Huang QS, Li T, Chen QX. 15-Epi-lipoxin A4 inhibits the progression of endometriosis in a murine model. Fertil Steril. 2010a;93:1440–1447. doi: 10.1016/j.fertnstert.2009.01.107. [DOI] [PubMed] [Google Scholar]
- Chen QH, Zhou WD, Su ZY, Huang QS, Jiang JN, Chen QX. Change of proinflammatory cytokines follows certain patterns after induction of endometriosis in a mouse model. Fertil Steril. 2010b;93:1448–1454. doi: 10.1016/j.fertnstert.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Dziunycz P, Milewski L, Radomski D, Barcz E, Kaminski P, Roszkowski PI, et al. Elevated ghrelin levels in the peritoneal fluid of patients with endometriosis: associations with vascular endothelial growth factor (VEGF) and inflammatory cytokines. Fertil Steril. 2009;92:1844–1849. doi: 10.1016/j.fertnstert.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Hao H, Liu M, Wu P, Cai L, Tang K, Yi P, et al. Lipoxin A4 and its analog suppress hepatocellular carcinoma via remodeling tumor microenvironment. Cancer Lett. 2011;309:85–94. doi: 10.1016/j.canlet.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner MA, Drury JA, Quenby S, Hart A, Maddick M, et al. Sustained replication in endometrium of women with endometriosis occurs without evoking a DNA damage response. Hum Reprod. 2009;24:687–696. doi: 10.1093/humrep/den416. [DOI] [PubMed] [Google Scholar]
- Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate. Alteration in peroxisome proliferator-activated receptor gamma. J Biol Chem. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- Janakiram NB, Mohammed A, Rao CV. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer Metastasis Rev. 2011;30:507–523. doi: 10.1007/s10555-011-9311-2. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Wang CC, Wu MH, Yang SH, Li YH, Tsai SJ. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J Clin Endocrinol Metab. 2012;97:1515–1523. doi: 10.1210/jc.2012-1450. [DOI] [PubMed] [Google Scholar]
- Macdonald LJ, Boddy SC, Denison FC, Sales KJ, Jabbour HN. A role for lipoxin A4 as an anti-inflammatory mediator in the human endometrium. Reproduction. 2011;142:345–352. doi: 10.1530/REP-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon B, Mitchell S, Brady HR, Godson C. Lipoxins: revelations on resolution. Trends Pharmacol Sci. 2001;22:391–395. doi: 10.1016/s0165-6147(00)01771-5. [DOI] [PubMed] [Google Scholar]
- Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Motohashi E, Kawauchi H, Endo H, Kondo H, Kitasato H, Kuramoto H, et al. Regulatory expression of lipoxin A4 receptor in physiologically estrus cycle and pathologically endometriosis. Biomed Pharmacother. 2005;59:330–338. doi: 10.1016/j.biopha.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, Spedding M, Yu W, Harmar AJ NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgaec S, Abrao MS, Dias JA, Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373–1379. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Bean SM. Pathogenesis of endometriosis. Reprod Biomed Online. 2010;21:4–5. doi: 10.1016/j.rbmo.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Russell R, Gori I, Pellegrini C, Kumar R, Achtari C, Canny GO. Lipoxin A4 is a novel estrogen receptor modulator. FASEB J. 2011;25:4326–4337. doi: 10.1096/fj.11-187658. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Selam B, Kayisli UA, Akbas GE, Basar M, Arici A. Regulation of FAS ligand expression by chemokine ligand 2 in human endometrial cells. Biol Reprod. 2006;75:203–209. doi: 10.1095/biolreprod.105.045716. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008a;153(Suppl. 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008b;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seval Y, Cakmak H, Kayisli UA, Arici A. Estrogen-mediated regulation of p38 mitogen-activated protein kinase in human endometrium. J Clin Endocrinol Metab. 2006;91:2349–2357. doi: 10.1210/jc.2005-2132. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang MT, Chen Y, Yang D, Che M, Honn KV, et al. Downregulation of vascular endothelial growth factor and induction of tumor dormancy by 15-lipoxygenase-2 in prostate cancer. Int J Cancer. 2009;124:1545–1551. doi: 10.1002/ijc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulukus M, Ulukus EC, Seval Y, Zheng W, Arici A. Expression of interleukin-8 receptors in endometriosis. Hum Reprod. 2005;20:794–801. doi: 10.1093/humrep/deh675. [DOI] [PubMed] [Google Scholar]
- Umezawa M, Sakata C, Tanaka N, Kudo S, Tabata M, Takeda K, et al. Cytokine and chemokine expression in a rat endometriosis is similar to that in human endometriosis. Cytokine. 2008;43:105–109. doi: 10.1016/j.cyto.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012;26:1506–1516. doi: 10.1096/fj.11-198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–296. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- Wu SH, Liao PY, Dong L, Chen ZQ. Signal pathway involved in inhibition by lipoxin A(4) of production of interleukins induced in endothelial cells by lipopolysaccharide. Inflamm Res. 2008;57:430–437. doi: 10.1007/s00011-008-7147-1. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhao F, Lin F, Chen J, Huang Y. Lipoxin A4 inhibits the development of endometriosis in mice: the role of anti-inflammation and anti-angiogenesis. Am J Reprod Immunol. 2012;67:491–497. doi: 10.1111/j.1600-0897.2011.01101.x. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Ruimeng X, et al. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J Reprod Immunol. 2006;72:85–93. doi: 10.1016/j.jri.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Zhou WD, Yang HM, Wang Q, Su DY, Liu FA, Zhao M, et al. SB203580, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis by down-regulating proinflammatory cytokines and proteolytic factors in a mouse model. Hum Reprod. 2010;25:3110–3116. doi: 10.1093/humrep/deq287. [DOI] [PubMed] [Google Scholar]
- Zhou XY, Li YS, Wu P, Wang HM, Cai ZY, Xu FY, et al. Lipoxin A(4) inhibited hepatocyte growth factor-induced invasion of human hepatoma cells. Hepatol Res. 2009;39:921–930. doi: 10.1111/j.1872-034X.2009.00520.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The immunocytochemistry identified of human endometrial glandular epithelial and stromal cells (40×), scale bar = 50 μm. (A) Endometrial glandular epithelial cells (left, negative control IgG; right, CK19); (B) Endometrial stromal cells (left, negative control IgG; right, vimentin 9); (C) Endometrial stromal cells (left, negative control IgG; right, CD10);
LXA4 inhibited the migration of ESCs. (A) A wound healing assay was performed on ESCs (5 × 105, 6 cm plate) incubated with either ethanol (control) or LXA4 (100 nM). The ‘wounded’ areas were photographed by Nikon Eclipse 50i fluorescent microscope at various time points (0 h, 24 h or 48 h). One representative experiment was shown, scale bar = 50 μm. (B) Quantitative analysis of wound-induced migration assay from (A). The results were presented as mean ± SEM of three experiments done in duplicate and differences with P < 0.05 on the Student's t-test were considered statistically significant (*).
LXA4 inhibits IL-1β-induced expressions of IL-6 (A), IL-8 (B), MCP-1 (C), TNF-α (D), and VEGF (E) mRNA in a dose-dependent manner. ESCs were pretreated for 30 min with vehicle (0.01% ethanol) or with the indicated concentrations of LXA4 before IL-1β (1 ng·mL−1) treatment for an additional 24 h. Total RNA extracts were generated and converted into cDNA. Changes in the expressions of IL-6, IL-8, MCP-1, TNF-α and VEGF mRNA were detected by real-time PCR. To determine the dose effects of IL-1β on cytokines synthesis of ESCs, cells were stimulated with IL-1β (0.1 to 10 ng·mL−1) or vehicle (medium) for 24 h, and mRNA expressions of IL-6 (A), IL-8 (B), MCP-1 (C), TNF-α (D) and VEGF (E) were also determined by real-time PCR. The results were presented mean ± SEM of three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001).
According to the manufacturer's instructions and previous reports, appropriate positive control was used with each antibody during the staining run. (A) Immunohistochemical staining for 15-LOX-2 in the human normal renal tissues (n = 3). Sections were immunostained with anti-human 15-LOX-2 antibody (15-LOX-2) and negative control (IgG). Scale bar = 50 μm; (B) Immunohistochemical staining for ALX receptor in the human gastric cancer tissues (n = 3). Sections were immunostained with anti-human ALX receptor antibody (ALXR) and negative control (IgG). Scale bar = 50 μm; (C) Immunohistochemical staining for Phospho-p38 MAPK in the human gastric cancer tissues (n = 3). Sections were immunostained with anti-human Phospho-p38 MAPK antibody and negative control (IgG). Scale bar = 50 μm.
Clinical characteristics of patients and controls.
Primer sequences for RT-PCR and quantitative real-time PCR.
Supplemental materials and methods.