Abstract
Background
The data on the prognostic values of high sensitivity C-reactive protein (hsCRP) levels in patients with advanced symptomatic heart failure (HF) receiving cardiac resynchronization therapy (CRT) are scarce. The aim of present study was to investigate the association of serum hsCRP levels with left ventricle reverse remodeling after six months of CRT as well as long-term outcome.
Methods
A total of 232 CRT patients were included. The assessment of hsCRP values, clinical status and echocardiographic data were performed at baseline and after six months of CRT. Long-term follow-up included all-cause mortality and hospitalizations for HF.
Results
During the mean follow-up periods of 31.3 ± 31.5 months, elevated hsCRP (> 3 mg/L) prior to CRT was associated with a significant 2.39-fold increase (P = 0.006) in the risk of death or HF hospitalizations. At 6-month follow-up, patients who responded to CRT showed significant reductions or maintained low in hsCRP levels (–0.5 ± 4.1 mg/L reduction) compared with non-responders (1.7 ± 6.1 mg/L increase, P = 0.018). Compared with patients in whom 6-month hsCRP levels were reduced or remained low, patients in whom 6-month hsCRP levels were increased or maintained high experienced a significantly higher risk of subsequent death or HF hospitalizations (Log-rank P < 0.001). The echocardiographic improvement was also better among patients in whom 6-month hsCRP levels were reduced or remained low compared to those in whom 6-month hsCRP levels were raised or maintained high.
Conclusions
Our findings demonstrated that measurement of baseline and follow-up hsCRP levels may be useful as prognostic markers for timely potential risk stratification and subsequent appropriate treatment strategies in patients with advanced HF undergoing CRT.
Keywords: Cardiac resynchronization therapy, Clinical outcome, Heart failure, High sensitivity C-reactive protein
1. Introduction
It has been firmly shown that cardiac resynchronization therapy (CRT) is an established treatment option for a subgroup of appropriately selected patients with diverse heart failure (HF) severity to improve left ventricular (LV) reverse remodeling, reduce clinical symptoms and decrease cardiovascular mortality and morbidity.[1] Despite the indubitable advantages of CRT, the response to CRT appears to vary substantially and up to approximately 30% patients fail to benefit from CRT, leading to a desire to identify of so-called non-responders prior to CRT implantation.[2] High sensitivity C-reactive protein (hsCRP) is synthesized and secreted by hepatocytes in response to proinflammatory cytokines, namely IL-6, and it seems to be the most reliable biomarkers to assess inflammatory processes in clinical practice.[3] Plasma hsCRP levels are commonly elevated in patients with depressed LV function and have been regarded as an independent and powerful predictor of adverse cardiac events in advanced HF patients.[4]–[6] However, data regarding the association of hsCRP levels with LV reverse remodeling as well as long-term outcome in the subset of patients with HF receiving CRT are limited, and the value of hsCRP levels in predicting mortality or morbidity in CRT recipients currently remains unclear.
Therefore, aims of the present study were to: (1) investigate the relation between baseline hsCRP levels and long-term outcome after CRT; (2) evaluate the impact of CRT on the concentrations of hsCRP during 6-month follow-up; and (3) explore the prognostic value of follow-up hsCRP assessment following CRT.
2. Methods
2.1. Study population
A total of 232 consecutive chronic HF patients who underwent successful implantation of a CRT system between January 1999 and December 2013 at Fuwai hospital were included. All subjects received CRT therapy according to the accepted criteria of New York Heart Association (NYHA) class III or IV despite optimal medical therapy, LV ejection fraction (LVEF) ≤ 35%, QRS width ≥ 120 ms and left ventricular end-diastolic diameter (LVEDD) ≥ 55 mm.[1],[7] Data including demographics, echocardiographic parameters, laboratory values, and medications at the initial evaluation were retrospectively obtained from the electronic medical record. Long-term follow-up evaluations after device implantation were performed through the chart review, device interrogation or a telephone interview. All of the patients signed informed consent forms, and the study complied with the Declaration of Helsinki and was approved by the Research Ethics Board of Fuwai Hospital.
2.2. Definitions
Venous blood samples were obtained from the entire patient cohort before CRT implantation for measurement of hsCRP. We used the recommended conventional cut-off value for hsCRP of 3.0 mg/L provided by consensus conference of the Centers of Disease Control (CDC) and the American Heart Association (AHA) on the use of hsCRP in clinical practice,[8] and patients at baseline were dichotomized into high (> 3.0 mg/L) and low hsCRP groups (≤ 3.0 mg/L) according to enrollment hsCRP levels. During 6-month follow-up, there was a subset of 198 (85.3%) patients with routinely acquired blood samples for hsCRP assessment. Follow-up changes in hsCRP levels from baseline to 6-month were categorized as high/high (baseline/6-month), high/low, low/high, and low/low. Patients were classified as responders to CRT in terms of improvement in NYHA class by ≥ 1 combined with an absolute increase ≥ 5% in LVEF during 6-month follow-up.[9] Patients who died, underwent heart transplantation or hospitalized for HF within 6-month were regarded as non-responders. Primary endpoint events were defined as all-cause mortality (due to HF, sudden cardiac death, or non-cardiac cause), cardiac transplantation and hospitalizations for decompensated HF.
2.3. Echocardiographic evaluation
Echocardiograms were obtained at baseline for all patients and at 6-month were available for 215 (92.7%) patients, respectively. Echocardiographic parameters including the left atrium diameters (LAD) and LVEDD were measured using a commercially available system (iE33; Philips Medical Systems) equipped with a 3.5-MHz transducer according with the recommendations of the American Society of Echocardiography protocols.[10] LVEFs were calculated using the modified biplane Simpson's rule from apical imaging planes.[11] The severity of mitral regurgitation (MR) was assessed as the average area of the regurgitant jet area to left atrium using the color-flow Doppler images at the parasternal long-axis.[12]
2.4. CRT implantation procedure
The LV lead was inserted transvenously via the subclavian route. A coronary sinus venogram was obtained using a balloon catheter, and the LV pacing lead was inserted through the coronary sinus with the help of an 8-F or 9-F guiding catheter and positioned as far as possible in the venous system, preferably in the lateral or posterolateral vein. The atrial and right ventricular leads were placed routinely in the right atrial appendage and the right ventricular apex. All leads were connected to a dual-chamber biventricular implantable cardiac device. The decision to use a CRT device with defibrillator function (CRT-D) was based on primary or secondary prevention for episodes of sustained ventricular tachycardia or inducible ventricular arrhythmia. The atrioventricular interval was optimized by Ritter's method with transthoracic echocardiography.
2.5. Statistics
Continuous data are presented as mean ± SD, and dichotomous data are expressed as numbers and percentages. Comparison of data between patient groups was performed using the independent-samples t test for continuous data. The Fisher's exact tests or χ2 tests were used to compare dichotomous data. Survival of patients was evaluated with the Kaplan-Meier method and log rank test was utilized to compare the survival curves. Variables significant in univariate analysis were entered in a multivariate Cox proportional hazards model to determine the independent predictors of event-free survival using a backward stepwise selection. At each step, the least significant variable was discarded from the model until all variables in the model reached a P value below 0.25. The analyses were conducted using the SPSS 16.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
Table 1 shows the baseline demographics and clinical characteristics of 232 patients according to the initial hsCRP levels. Patients were subdivided into high (> 3 mg/L) and low (≤ 3 mg/L) hsCRP groups, with 105 patients in the high hsCRP group (7.8 ± 4.3 mg/L) and 127 subjects in the low hsCRP group (1.3 ± 1.6 mg/L). The mean age of study population was 59.7 ± 10.4 years, and 154 (66.4%) patients were male. CRT recipients showed depressed LV systolic function (mean LVEF, 27.7% ± 6.7%) with QRS prolongation (158.1 ± 20.0 ms). Compared to those with low hsCRP, patients with high hsCRP tended to be much older and more symptomatic, and presented less left bundle branch block (LBBB) and larger LAD during their initial evaluation. In addition, the concentrations of biochemical markers including serum creatinine, blood urea nitrogen (BUN) and N-terminal pro-brain natriuretic peptide (NT-proBNP) were significantly higher in patients with elevated hsCRP than in those with low hsCRP. No differences regarding the comorbidities and treatment strategies between elevated hsCRP group and low hsCRP group existed at baseline.
Table 1. Clinical baseline characteristics of the study population according to the enrolled hsCRP levels.
| hsCRP ≤ 3 mg/L (n = 127) | hsCRP > 3 mg/L (n = 105) | P-value | |
| Age, yrs | 57.6 ± 10.2 | 62.2 ± 10.0 | 0.001 |
| Male | 83 (65.4%) | 71 (67.6%) | 0.781 |
| LBBB | 108 (85.0%) | 78 (74.3%) | 0.041 |
| NYHA class | 3.1 ± 0.3 | 3.3 ± 0.4 | 0.013 |
| Intrinsic QRS duration, ms | 157.5 ± 18.4 | 158.9 ± 21.8 | 0.594 |
| Serum creatinine, mg/dL | 1.1 ± 0.4 | 1.3 ± 0.5 | 0.001 |
| BUN, mmol/L | 8.1 ± 2.8 | 9.5 ± 4.7 | 0.014 |
| hsCRP, mg/L | 1.3 ± 1.6 | 7.8 ± 4.3 | < 0.001 |
| NT-proBNP, pmol/L | 1879.3 ± 1286.7 | 2487.9 ± 2040.3 | 0.011 |
| Comorbidities | |||
| Ischaemic cardiomyopathy | 25 (19.7%) | 29 (27.6%) | 0.155 |
| Hypertension | 43 (33.9%) | 39 (37.1%) | 0.602 |
| Hypercholesterolemia | 45 (35.4%) | 29 (27.6%) | 0.204 |
| Diabetes mellitus | 27 (25.7%) | 26 (20.5%) | 0.344 |
| Atrial fibrillation | 7 (5.5%) | 11 (10.5%) | 0.159 |
| PAH | 25 (19.7%) | 32 (30.5%) | 0.057 |
| Echocardiography | |||
| LVEF, % | 27.6 ± 6.8 | 27.8 ± 6.4 | 0.785 |
| LAD, mm | 43.4 ± 7.1 | 45.5 ± 7.7 | 0.033 |
| LVEDD, mm | 71.2 ± 9.0 | 71.0 ± 10.2 | 0.866 |
| RVEDD, mm | 21.7 ± 4.9 | 22.7 ± 5.5 | 0.130 |
| MR grade | 2.5 ± 0.9 | 2.5 ± 1.0 | 0.826 |
| Treatments | |||
| ACEI or ARB | 103 (81.1%) | 83 (79.0%) | 0.696 |
| β-blockers | 118 (92.9%) | 100 (95.2%) | 0.459 |
| Digoxin | 93 (73.2%) | 76 (72.4%) | 0.855 |
| Amiodarone | 27 (21.3%) | 26 (24.8%) | 0.527 |
| CRT-D | 59 (46.5%) | 62 (59.0%) | 0.056 |
The data are presented as the n (%) or the means ± SD. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BUN: blood urea nitrogen; CRT-D: cardiac resynchronization therapy-defibrillator; hsCRP: high-sensitivity C-reactive protein; LAD: left atrial diameter; LBBB: left bundle branch block; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; MR: mitral regurgitation; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA class: New York Heart Association functional class; PAH: pulmonary arterial hypertension; RVEDD: right ventricular end-diastolic diameter.
3.2. Baseline hsCRP and clinical outcome
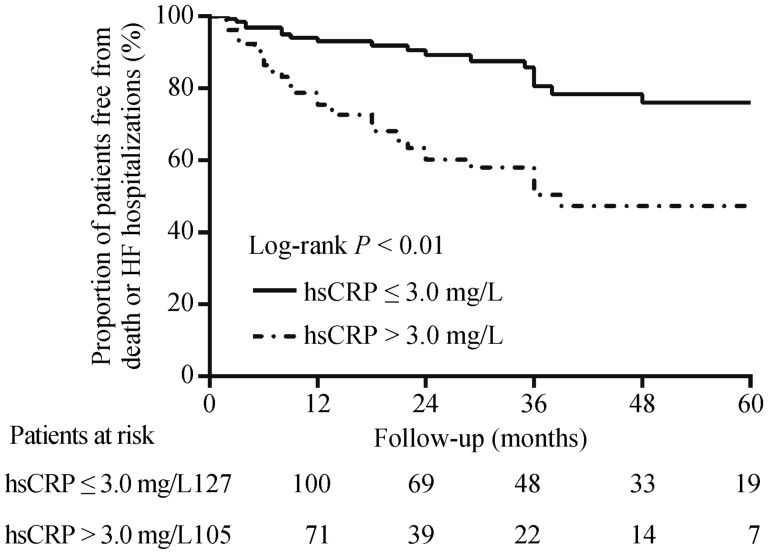
In our study, the mean follow-up duration was 31.3 ± 31.5 months. Thirty-two patients (13.8%) experienced all-cause mortality including 24 patients with HF-related death, five patients with sudden death and three patients with non-cardiac death. Four patients (1.7%) underwent heart transplantation and 56 patients (24.1%) were hospitalized for worsening HF. A total of 59 patients (25.4%) experienced the combined endpoint of death or HF hospitalizations. Over the long-term follow-up, patients with hsCRP > 3 mg/L had a significantly lower probability of surviving without suffering the combined endpoint of death or HF hospitalizations as compared to those with hsCRP ≤ 3 mg/L (log-rank P < 0.01, Figure 1). After controlling for confounding factors, elevated hsCRP levels (> 3 mg/L) at baseline were shown to independently predict adverse clinical response (HR: 2.39, 95%CI: 1.28–4.47, P = 0.006, Table 2). When assessed as a continuous variable, each 1 mg/L increase in hsCRP was associated with a corresponding 8.3% increase (P = 0.005) in the risk of the combined endpoint of death or HF hospitalizations.
Figure 1. Kaplan-Meier estimates of the cumulative probability of the combined endpoint of death or HF hospitalizations in CRT patients with high (> 3 mg/L) and low (≤ 3 mg/L) baseline hsCRP.
CRT: cardiac resynchronization therapy; HF: heart failure; hsCRP: high sensitivity C-reactive protein.
Table 2. Uni- and multivariate Cox proportional hazards models for death or heart failure hospitalizations.
| Univariable |
Multivariable |
||||
| HR (95%CI) | P-value | HR | P-value (95%CI) | ||
| Age, yrs | 1.02 (0.99–1.05) | 0.133 | |||
| Male | 1.51 (0.85–2.68) | 0.160 | |||
| Ischaemic cardiomyopathy | 1.15 (0.62–2.13) | 0.666 | |||
| QRS duration, ms | 0.99 (0.98–1.01) | 0.406 | |||
| LBBB | 0.54 (0.30–0.96) | 0.034 | |||
| NYHA class | 2.59 (1.52–4.41) | < 0.001 | |||
| LVEDD, mm | 1.01 (0.98–1.04) | 0.575 | |||
| LVEF,% | 1.01 (0.97–1.05) | 0.663 | |||
| MR grade | 1.57 (1.14–2.12) | 0.005 | |||
| Serum creatinine, per 1 mg/dL increase | 4.36 (2.66–7.17) | < 0.001 | 2.88 (1.71–4.85) | < 0.001 | |
| BUN, per 1 mmol/L increase | 1.13 (1.06–1.20) | < 0.001 | |||
| HsCRP > 3mg/L | 3.56 (2.04–6.22) | < 0.001 | 2.39 (1.28–4.47) | 0.006 | |
| NT-proBNP, per 100 pmol/L increase | 1.04 (1.02–1.05) | < 0.001 | 1.03 (1.01–1.04) | 0.003 | |
| ACE-I or ARB | 0.78 (0.40–1.50) | 0.451 | |||
| β-blockers | 0.04 (0.01–2.66) | 0.134 | |||
| CRT-D | 0.57 (0.34–0.97) | 0.038 | |||
ARB: angiotensin receptor blocker; BUN: blood urea nitrogen; CRT-D: cardiac resynchronization therapy-defibrillator; hsCRP: high-sensitivity C-reactive protein; LBBB: left bundle branch block; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; MR: mitral regurgitation; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA class: New York Heart Association functional class.
3.3. Effect of CRT on hsCRP levels
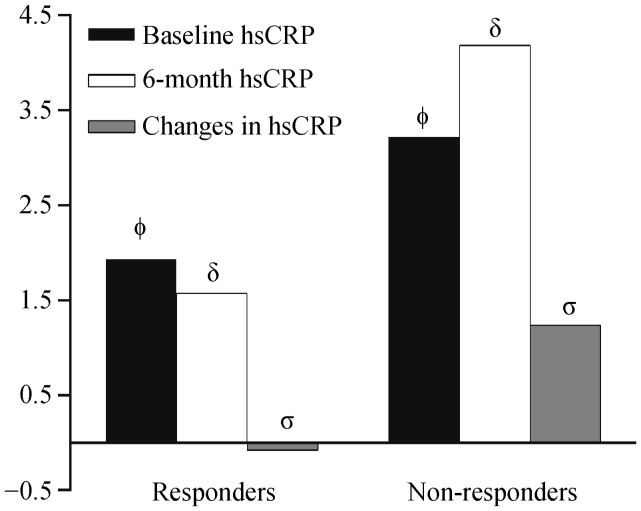
During 6-month follow-up, a total of 170 of 232 (73.3%) patients were classified as responders. Baseline hsCRP values as well as 6-month hsCRP levels were significantly lower in patients who responded to CRT than in those who did not (P = 0.001 and P < 0.001, respectively), with a mean 0.5 ± 4.1 mg/L reduction from baseline for patients who were responders and 1.7 ± 6.1 mg/L increase from baseline for those who were non-responders (P = 0.018). Both baseline and 6-month hsCRP values for responders and non-responders to CRT are displayed in Figure 2.
Figure 2. Mean baseline, 6-month, and absolute changes in hsCRP in patients with and without response to CRT at 6-month follow-up.
Absolute changes in hsCRP were calculated as the difference between 6 month and baseline hsCRP levels, among the 198 patients with available paired baseline and 6-month hsCRP assessment. ΦP = 0.001, δP < 0.001, σP = 0.018. CRT: cardiac resynchronization therapy; hsCRP: high sensitivity C-reactive protein.
3.4. HsCRP change and subsequent outcome
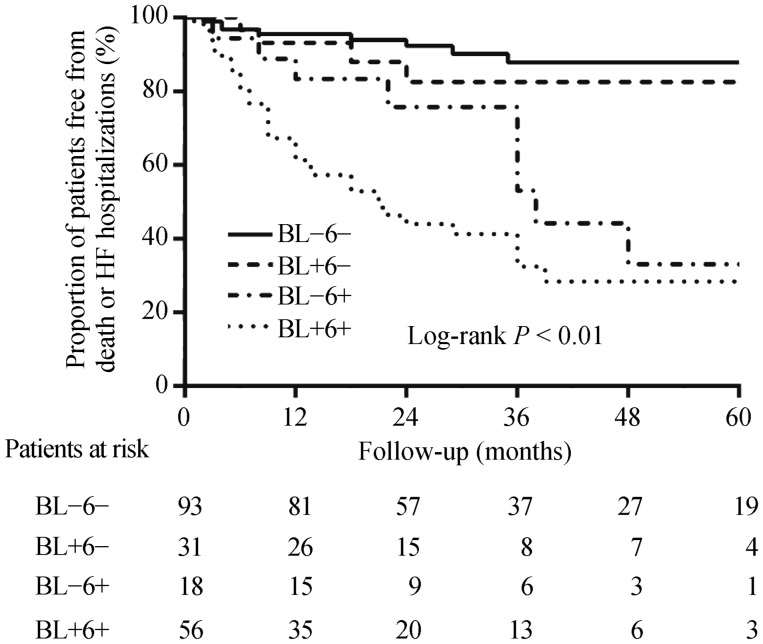
The survival curve for all-cause mortality or HF hospitalizations among patients with for each of the baseline to 6-month hsCRP change group is shown in Figure 3. During long-term follow-up, compared with those with low/high baseline and low 6-month hsCRP values, patients with low/high baseline and high 6-month hsCRP values displayed higher rate of the combined endpoint of death or HF hospitalizations (log-rank P < 0.01). After multivariate adjustment, compared with those with 6-month hsCRP values which stayed low, patients with 6-month hsCRP levels that remained high experienced the highest rate of death or HF hospitalizations (HR: 7.6, P < 0.001), and patients whose levels increased from low baseline to high 6-month hsCRP values suffered secondary risk (HR: 2.9, P = 0.079), and patients whose levels declined from a high baseline to low 6-month hsCRP values underwent similar risk (HR: 1.4, P = 0.576, Table 3). When assessed as a continuous variable, each 1 mg/L increase in 6-month hsCRP levels was related to a corresponding 9.6% increase (P = 0.001) in the risk of death or HF hospitalizations.
Figure 3. Kaplan-Meier estimates of the cumulative incidence of the combined endpoint of death or HF hospitalizations according to the pattern of hsCRP change after six months of CRT.
BL: baseline; 6: 6 months; HF: heart failure; hsCRP: high sensitivity C-reactive protein; (+): high hsCRP group (> 3 mg/L); (−): low hsCRP group (≤ 3 mg/L).
Table 3. Risk of death or heart failure hospitalizations in CRT patients by the change pattern of hsCRP.
| hsCRP change group | HR | 95%CI | P-value |
| High BL hsCRP and high 6-mo hsCRP, n = 56 | 7.6 | 3.1−18.5 | < 0.001 |
| Low BL hsCRP and high 6-mo hsCRP, n = 18 | 2.9 | 0.9−9.3 | 0.079 |
| High BL hsCRP and low 6-mo hsCRP, n = 31 | 1.4 | 0.4−5.0 | 0.576 |
| Low BL hsCRP and low 6-mo hsCRP, n = 93 | 1.00 | ||
The multivariable cox regression analysis controls for age, gender, ischaemic cardiomyopathy, LBBB, NYHA class, LVEDD, LVEF, BUN, serum creatinine, NT-proBNP, β-blocker, and ACEI/ARB. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BL: baseline; CRT: cardiac resynchronization therapy; hsCRP: high sensitivity C-reactive protein; LBBB: left bundle branch block; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA class: New York Heart Association functional class; 6-mo: 6-month.
3.5. HsCRP change and LV reverse remodeling
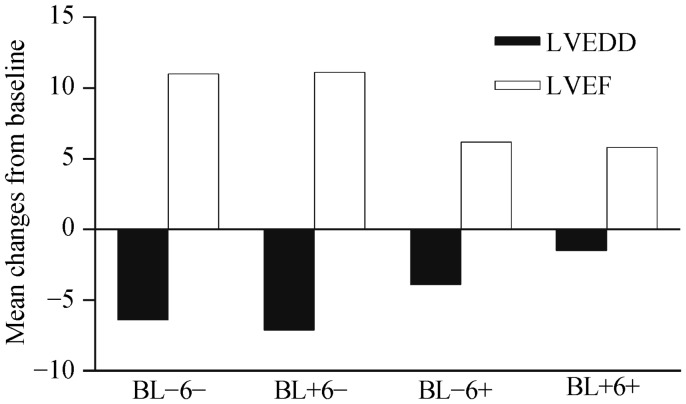
The relationship between the pattern of hsCRP change and LV reverse at 6-month among the 198 patients with paired echocardiograms and hsCRP values is shown in Figure 4. There were no significant differences in baseline LVEDD and LVEF between the low and high baseline hsCRP groups. Similar to the association of clinical outcome with hsCRP change, patients with low 6-month hsCRP values displayed the largest concurrent mean reductions in LVEDD and increases in LVEF (−6.4 ± 7.7 mm reduction in LVEDD and 11.0% ± 8.6 % increase in LVEF among those with low baseline hsCRP, and −7.1 ± 8.9 mm reduction in LVEDD and 11.1% ± 8.5 % increase in LVEF among those with high baseline hsCRP). In contrast, patients with high 6-month hsCRP values showed significantly lower reductions in LVEDD and increases in LVEF (−3.9 ± 5.9 mm reduction in LVEDD and 6.2% ± 8.3% increase in LVEF among those with low baseline hsCRP and −1.5 ± 9.8 mm reduction in LVEDD and 5.8% ± 9.4% increase in LVEF among those with high baseline hsCRP; P < 0.01 for the overall difference). No significant differences in LVEDD reduction or LVEF increases were noted between the two groups with low (low/low and high/low) and high (low/high and high/high) 6-month hsCRP values.
Figure 4. Comparison of absolute 6-month changes (mean) in LVEDD and LVEF according to the pattern of hsCRP change after 6 months of CRT.
Absolute changes in LVEF and LVEDD were calculated as the difference between 6-month and baseline values. For comparison across the four groups, there were significant differences in LVEDD and LVEF changes (P = 0.003 and P = 0.002, respectively). BL: baseline; hsCRP: high sensitivity C-reactive protein; LVEDD: left ventricular end diastolic dimension; LVEF: left ventricular ejection fraction; 6: 6 months; (+): high hsCRP group (> 3 mg/L); (−): low hsCRP group (≤ 3 mg/L).
4. Discussion
The current analysis explored several significant implications on the prognostic value of hsCRP levels for severe HF patients receiving CRT. The main findings can be summarized as follows. First, elevated hsCRP prior to CRT device implantation was identified as an independent determinant of adverse survival and more HF hospitalizations. Secondly, during 6-month follow-up, plasma levels of hsCRP were reduced or remained low in patients who were responders to CRT, yet a similar improvement was not observed among patients who were non-responders. Finally, the pattern of hsCRP changes after six months of CRT was significantly associated with the LV reverse parameters and subsequent clinical outcome.
To date, there have been relatively limited available studies regarding the relationship between baseline hsCRP levels and long-term outcome after CRT. Kamioka, et al.[13] found, in a small cohort of 65 patients, that hsCRP levels before CRT implantation were identified as the strongest predictive factor for cardiac death irrespective of other prognostic factors and that being in the high hsCRP group (> 3 mg/L) at baseline was also associated with worse survival. This finding is similar to our results that hsCRP levels pre-CRT implant independently predict all-cause mortality or hospitalizations for HF following CRT after adjusting other established risk factors. What the exact potential mechanisms are for the observed the heightened risk of mortality and morbidity in patients with high hsCRP levels before CRT implantation currently remain unresolved. Several prior reports have demonstrated that elevated levels of hsCRP, as a biomarker of inflammation, was a powerful independent predictor of prognosis in chronic HF,[4]–[6] and a recent study has shown that increased hsCRP can directly predict progressive regional myocardial functional deterioration independent of confounding cardiovascular events.[14] The similar effect of hsCRP levels on the long-term outcome may also occur in CRT recipients, this hypothesis, however, cannot be directly confirmed by the current or prior results.
Moreover, a few previous studies have evaluated the association of changes in hsCRP levels after CRT and the subsequent clinical and echocardiographic response. In 140 patients undergoing CRT, Michelucci, et al.[15] reported that CRT reduced hsCRP status only in patients who showed LV reverse and reduction of hsCRP levels were associated with the absence of adverse cardiac events. On the contrary, Glick, et al.[16] reported that, although hsCRP decreased significantly within two weeks after CRT implantation, levels of hsCRP or their drop after two weeks of CRT did not correlate with clinical outcome. This discrepancy may possibly be due to the small sample size (32 patients) as well as a relatively short follow-up period. Another small observational study of 27 patients with advanced HF showed similar results to ours, in that hsCRP levels at 6-month follow up decreased in responders while such improvements were not observed in non-responders.[17] However, the authors did not investigate whether changes in hsCRP levels were associated with clinical outcome after CRT. The findings of these small retrospective studies were mostly congruent with our results that patients who showed significant LV reverse remodeling experienced significant reductions or maintained low 6-month hsCRP values, whereas patients who do not exhibit obvious cardiac reverse remodeling experienced a slight increase or remained high with regard to their 6-month hsCRP levels after CRT. Additionally, when patients were further stratified into four subgroups according to hsCRP change, we found that the pattern of hsCRP change from baseline to 6-month was associated with a lack of favorable LV reverse remodeling parameters with respect to LV dimensions and LVEF, and independently predicted subsequent all-cause mortality or HF hospitalizations. The pathophysiological mechanisms responsible for improvement of LV systolic function resulting in reduction or maintenance in hsCRP levels is likely complicated. Restoration of synchronization induced by CRT may lead to attenuated sympathetic and neurohormonal over-activation, reduced LV wall stress as well endothelial oxidative stress, which consequently exerts beneficial effects on the observed reduction in concentration of proinflammatory cytokines such as hsCRP.[18]–[20] In turn, the alleviation of inflammatory status may also result in improved clinical prognosis after CRT. Further studies are needed to elucidate the mechanisms underlying the changes in hsCRP levels and LV reverse remodeling as well as prognosis in patients undergoing CRT.
4.1. Study limitations
Firstly, this study is a nonrandomized retrospective analysis, and it involved a relatively small sample size, which may lead to statistical limitations and subsequently affect our findings. Moreover, the present study was only designed to examine the hsCRP levels but not to other inflammatory biomarkers such as IL-6, TNF-α and TGF-1β which have also been identified as stronger predictors of clinical events in patients with advanced HF treated with CRT.[15],[21],[22] Furthermore, although debate exists regarding the clinical utility of different hsCRP cut-off values for predicting a worse outcome, we used a conventional cut point of 3.0 mg/L for the definition of high versus low hsCRP in current study. Additionally, the measurement of 6-month follow-up hsCRP values and echocardiographic parameters was not available for the whole patients which may potentially influence our conclusions. Finally, further studies are needed to elucidate the underlying pathophysiologic mechanisms of the interaction between hsCRP levels and LV reverse remodeling as well as clinical prognosis in patients undergoing CRT.
4.2. Conclusions
We have shown that hsCRP levels at baseline could predict the risk of death or HF hospitalizations in CRT recipients. In addition, a reduction measured at 6-months or maintenance of low hsCRP levels was observed in responders to CRT, and patients who had persistently high or exhibited an increase in hsCRP levels showed inferior LV reverse remodeling and subsequently had an increase in adverse clinical outcomes. Taken together, our results suggest that the important prognostic information can be obtained via an early assessment of hsCRP levels before and after CRT implantation and that this information may be useful for risk stratification and could influence clinical management strategies in CRT recipients.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 2.Bax JJ, Gorcsan JR. Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–1943. doi: 10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 4.Yin WH, Chen JW, Jen HL, et al. Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. Am Heart J. 2004;147:931–938. doi: 10.1016/j.ahj.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Windram JD, Loh PH, Rigby AS, et al. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007;153:1048–1055. doi: 10.1016/j.ahj.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Lamblin N, Mouquet F, Hennache B, et al. High-sensitivity C-reactive protein: potential adjunct for risk stratification in patients with stable congestive heart failure. Eur Heart J. 2005;26:2245–2250. doi: 10.1093/eurheartj/ehi501. [DOI] [PubMed] [Google Scholar]
- 7.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 8.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 9.Fornwalt BK, Sprague WW, BeDell P, et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–1991. doi: 10.1161/CIRCULATIONAHA.109.910778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 11.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) J Am Coll Cardiol. 2003;42:954–970. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 12.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–e142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Kamioka M, Suzuki H, Yamada S, et al. High sensitivity C-reactive protein predicts nonresponders and cardiac deaths in severe heart failure patients after CRT implantation. Int Heart J. 2012;53:306–312. doi: 10.1536/ihj.53.306. [DOI] [PubMed] [Google Scholar]
- 14.Choi EY, Yan RT, Fernandes VR, et al. High-sensitivity C-reactive protein as an independent predictor of progressive myocardial functional deterioration: the multiethnic study of atherosclerosis. Am Heart J. 2012;164:251–258. doi: 10.1016/j.ahj.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelucci A, Ricciardi G, Sofi F, et al. Relation of inflammatory status to major adverse cardiac events and reverse remodeling in patients undergoing cardiac resynchronization therapy. J Card Fail. 2007;13:207–210. doi: 10.1016/j.cardfail.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Glick A, Michowitz Y, Keren G, et al. Neurohormonal and inflammatory markers as predictors of short-term outcome in patients with heart failure and cardiac resynchronization therapy. Isr Med Assoc J. 2006;8:391–395. [PubMed] [Google Scholar]
- 17.Shinohara T, Takahashi N, Saito S, et al. Effect of cardiac resynchronization therapy on cardiac sympathetic nervous dysfunction and serum C-reactive protein level. Pacing Clin Electrophysiol. 2011;34:1225–1230. doi: 10.1111/j.1540-8159.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- 18.Seifert M, Schlegl M, Hoersch W, et al. Functional capacity and changes in the neurohormonal and cytokine status after long-term CRT in heart failure patients. Int J Cardiol. 2007;121:68–73. doi: 10.1016/j.ijcard.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 19.Hasper D, Hummel M, Kleber FX, et al. Systemic inflammation in patients with heart failure. Eur Heart J. 1998;19:761–765. doi: 10.1053/euhj.1997.0858. [DOI] [PubMed] [Google Scholar]
- 20.Livanis EG, Flevari P, Theodorakis GN, et al. Effect of biventricular pacing on heart rate variability in patients with chronic heart failure. Eur J Heart Fail. 2003;5:175–178. doi: 10.1016/s1388-9842(02)00257-x. [DOI] [PubMed] [Google Scholar]
- 21.Osmancik P, Herman D, Stros P, et al. Changes and prognostic impact of apoptotic and inflammatory cytokines in patients treated with cardiac resynchronization therapy. Cardiology. 2013;124:190–198. doi: 10.1159/000346621. [DOI] [PubMed] [Google Scholar]
- 22.Tarquini R, Guerra CT, Porciani MC, et al. Effects of cardiac resynchronization therapy on systemic inflammation and neurohormonal pathways in heart failure. Cardiol J. 2009;16:545–552. [PubMed] [Google Scholar]