Abstract
AIM: To assess the role of computed tomography (CT) and magnetic resonance imaging (MRI) and establish imaging criteria of lymph node metastasis in early colorectal cancer.
METHODS: One hundred and sixty patients with early colorectal cancer were evaluated for tumor location, clinical history of polypectomy, depth of tumor invasion, and lymph node metastasis. Two radiologists assessed preoperative CT and/or MRI for the primary tumor site detectability, the presence or absence of regional lymph node, and the size of the largest lymph node. Demographic, imaging, and pathologic findings were compared between the two groups of patients based on pathologic lymph node metastasis and optimal size criterion was obtained.
RESULTS: The locations of tumor were ascending, transverse, descending, sigmoid colon, and rectum. One hundred and sixty early colorectal cancers were classified into 3 groups based on the pathological depth of tumor invasion; mucosa, submucosa, and depth unavailable. A total of 20 (12.5%) cancers with submucosal invasion showed lymph node metastasis. Lymph nodes were detected on CT or MRI in 53 patients. The detection rate and size of lymph nodes were significantly higher (P = 0.000, P = 0.044, respectively) in patients with pathologic nodal metastasis than in patients without nodal metastasis. Receiver operating curve analysis showed that a cut-off value of 4.1 mm is optimal with a sensitivity of 78.6% and specificity of 75%.
CONCLUSION: The short diameter size criterion of ≥ 4.1 mm for metastatic lymph nodes was optimal for nodal staging in early colorectal cancer.
Keywords: Early colon cancer, Lymph node metastasis, Computed tomography, Magnetic resonance imaging, Lymph node size
Core tip: This study is the first study on the imaging criterion of lymph node metastasis in early colorectal cancer. The results suggest that the detection rate and the size of lymph nodes (LNs) were significantly higher in patients with pathologic nodal metastasis. The optimal size criterion for LN metastasis was ≥ 4.1 mm in early colorectal cancer.
INTRODUCTION
Early colorectal carcinoma is defined as invasive carcinoma that has not spread in the direction continuity beyond the submucosal layer[1]. Recent advances in endoscopic instruments and techniques have increased the detection of small colorectal lesions, early colon cancers and adenomas[2]. The accurate assessment of lymph node (LN) metastasis in early colorectal cancer (CRC) is crucial for deciding appropriate treatment strategies such as endoscopic resection or surgery as well as for a prognostic factor[3]. Mucosal colorectal carcinoma is believed to have no potential for lymph node metastasis; however, reported incidences of lymph node metastasis in patients with submucosal carcinoma vary from 3.5% to 38%[4]. Risk factors for the lymph node metastasis of early colorectal cancers are deep invasion of depth, invasion of polyp stalk, poorly differentiated adenocarcinoma and presence of lymphovascular invasion[5-8]. However, these risk factors can be assessed only after the endoscopic removal of tumors and preoperative or pre-procedural diagnosis of lymph node metastasis with computed tomography (CT) or magnetic resonance imaging (MRI) is difficult. There have been a number of different imaging criteria for lymph node metastasis in colorectal carcinomas[9-22]; however, to the best of our knowledge, there have only been limited studies on early colorectal carcinoma. This study evaluates the imaging risk factors for LN metastasis in early CRCs and develops adequate diagnostic size criteria for LN metastasis in patients with early CRC.
MATERIALS AND METHODS
Participants
This study received study-specific institutional review board approval and a waiver of informed consent was obtained. Patients with surgically proven early colorectal cancer who underwent CT and/or MRI before radical resection (surgical excision of tumor mass and regional lymph node dissection) were retrospectively analyzed. We enrolled 160 patients (age range: 20-85 years; mean age: 59.7 years, male: 90, female: 70) for this study. Out of 160 patients, 141 patients underwent CT, 61 patients underwent MR examination and 42 patients underwent CT and MR examination before surgery. Lymph node size on MR was used for subjects who underwent both exams.
CT
All CT scans were obtained with one of the following commercially available multidetector CT scanners (Sensation 64; Siemens Medical Solutions, Erlangen, Germany), LightSpeed VCT; (GE Medical Systems, Milwaukee, Wisconsin). Each patient received 120 mL of nonionic contrast agent (iopromide, Ultravist 300; Bayer Schering Healthcare, Berlin, Germany) at a rate of 3 mL/s. Single-phase contrast-enhanced scans were obtained with a scanning delay of 75 s after IV administration of the contrast agent with 5 mm section thickness. The scanning parameters using Sensation 64 and LightSpeed VCT were: detector configuration, 0.6 × 32 mm/0.625 × 64 mm; nominal section thickness, 0.75/0.625 mm; beam pitch, 1/1; gantry rotation time, 0.5/0.5 s; reconstruction interval, 0.75/0.625 mm; tube voltage, 120/120 kV(p). Automated tube current modulation was routinely used for all patients and performed with a 64-detector row CT scanner (CareDose 4D, Siemens Medical Solutions with 210 image quality reference milliampere-s/AutomA, GE Healthcare with a noise index of 14). The data were reformatted in the axial and coronal planes with a 5-mm section thickness and a 5-mm interval. All the CT images were reviewed with a picture archiving and communication system workstation (Marotech 5.4, Seoul, South Korea).
MRI
MRI was performed using a 3T MR scanner (Magnetom Verio; Siemens Medical Solutions, Erlangen, Germany) with a phased-array multi-coil. Before MR scanning, approximately 50-100 mL of sonography transmission gel was administered for an appropriate distension of the rectum. The MR images were performed with the following sequences: A sagittal image was obtained with a T2-weighted fast spin-echo sequence. The perpendicular plane to the long axis of the rectal cancer was selected for axial scanning: oblique axial T1-weighted fast spin-echo sequence [TR/TE of 750/10; flip angle of 150; field of view (FOV) of 200 × 200 mm; matrix size of 320 × 224; 2 NEX; slice thickness of 5 mm with no gap; and acquisition time of 4 min 31 s] and oblique axial T2-weighted fast spin echo sequence (TR/TE of 4000/118; flip angle of 140; FOV of 200 × 200 mm; matrix size of 320 × 224; 2 NEX; slice thickness of 5 mm with no gap; acquisition time of 3 min 27 s). Diffusion-weighted MR images were acquired in the sagittal and oblique axial plane using a single shot-echo planar imaging technique with b of 0, 500 and 1000 s/mm2; TR/TE of 6100/83; FOV of 200 mm; matrix size of 104 × 73; 2 NEX; slice thickness of 5 mm with no slice gap; and an acquisition time of 2 min 30 s. The contrast-enhanced T1-weighted image with fat suppression on the axial plane with TR/TE of 640/13; flip angle of 150; and slice thickness of 5 mm was obtained after an intravenous bolus injection of 0.1 mmol/kg Gadobutrol (Gadovist, Schering, Berlin, Germany) at a rate of 3 mL/s followed by a 25 mL saline flush.
Image interpretation
Two experienced board-certified radiologists (with 10- and 2-year experience in abdominal CT and MRI, respectively) were blinded for histological results and assessed preoperative CT and/or MR images for this study by consensus with access to the endoscopic findings of tumor location. Radiologists recorded the location and size of the mass, detectability of regional LNs 3 mm or larger and the size of regional LNs, when tumors were viewed on CT or MRI. Suspicious lymph nodes less than 3 mm were ignored because they cannot be differentiated from vascular structures or other non-specific soft tissue densities. The corresponding segment mentioned on endoscopy was evaluated for the evaluation of regional LNs, if the primary tumors were not visible; subsequently, radiologists evaluated the mesorectum for cases of rectal cancer and evaluated the sigmoid mesocolon for cases of sigmoid colon cancer. Two readers assessed primary tumor site detectability and the presence or absence of regional lymph nodes. They also measured the largest diameter of primary mass and short diameter of regional lymph nodes. A 3rd radiologist reviewed medical records for a clinical history of polypectomy (or endoscopic mucosal/submucosal resection) when patients were imaged and reviewed colonoscopic and histopathologic reports.
Pathology
A 3rd radiologist reviewed the pathologic reports for tumor depth of invasion, the presence or absence of lymph node metastasis and the number of metastatic lymph node. A 10-year experienced pathologist measured the size of 20 metastatic lymph nodes in short diameter.
Statistical analysis
Patients were divided into two groups based on pathologic lymph node metastasis. Differences in sex, age, tumor depth, tumor location, detectability of primary tumor site, detectability of regional lymph node, and lymph node size between those with lymph node metastasis and those without lymph node metastasis were tested. Univariate analysis was performed with Student’s t-test for numerical data or the χ2 test and Fisher’s exact test for categorical data. Differences were considered significant when the P-value was less than 0.05. Receiver operating characteristic (ROC) analysis was used to obtain optimal lymph node size criterion. The area under the ROC curve was evaluated for diagnostic performance.
RESULTS
A total of 52 patients underwent CT or MRI after preoperative polypectomy and 17 endoscopic tumor resection sites were detected on CT or MRI among 52 patients. Out of 160 primary colonic masses, 77 tumors (mean size 2.7 cm; range: 0.8-8 cm) were detected on CT or MRI. The tumor location was divided into 5 groups; ascending (n = 17), transverse (n = 15), descending (n = 8), sigmoid colon (n = 50), and rectum (n = 70). A total of 160 early colorectal cancers were divided into 3 groups based on the pathological depth of tumor invasion; mucosa (n = 17), submucosa (n = 133) and depth unavailable (n = 10). A total of 20 (12.5%) cancers with submucosal invasion showed lymph node metastasis; however, there was no lymph node metastasis in any patients with mucosal cancer or with early cancer with unavailable depth. Recognizable lymph nodes were detected in 53 patients on CT or MRI near the primary tumor (Figures 1 and 2) or corresponding colonic segment of endoscopic finding (mean short diameter of lymph node; 4.5 mm, range: 3-14 mm). The average short diameter of 20 pathologic metastatic lymph nodes was 4.8 mm (range: 1.9-8.5 mm).
Figure 1.
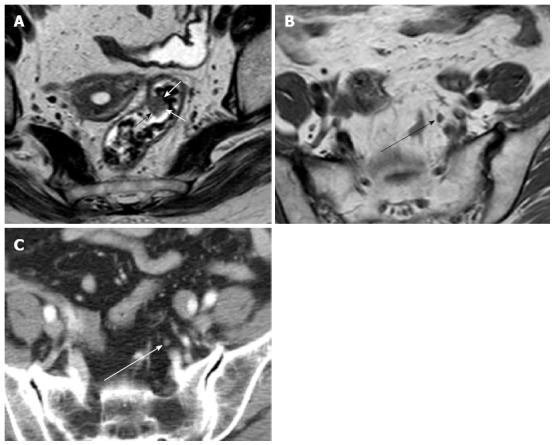
Early rectal carcinoma and lymph node metastasis on computed tomography and magnetic resonance imaging. A: Axial T2-weighted image shows polypoid rectal carcinoma (arrows); B: Axial T1-weighted image shows regional lymph node with 4.5 mm in short axis diameter (black arrow); C: Axial computed tomography scan shows the same regional lymph node (white arrow) as in B.
Figure 2.
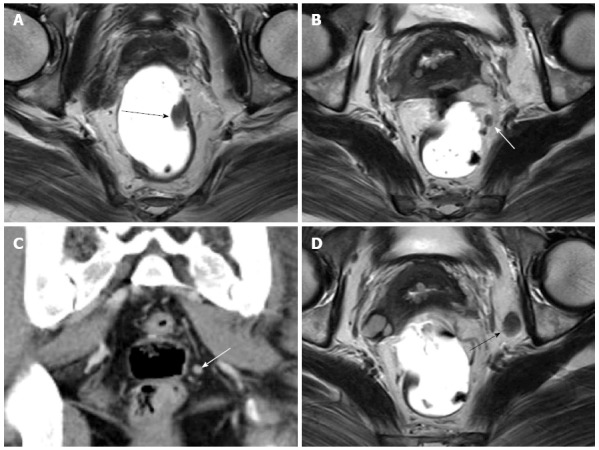
Early rectal carcinoma and lymph node metastasis on computed tomography and magnetic resonance imaging. A: Axial T2-weighted image shows polypoid rectal carcinoma (black arrow); B: Axial T2-weighted image shows perirectal lymph node (white arrow); C: Coronal computed tomography scan shows the same lymph node (white arrow); D: Axial T2-weighted image shows an enlarged left obturator lymph node (black arrow). These metastatic lymph nodes were one-to-one correlated pathologically.
Only the detectability of regional lymph nodes and lymph node size showed a significant difference between nodal metastatic and non-metastatic groups (Table 1). The detection rate of lymph nodes was significantly higher (P = 0.000) in the pathologic nodal metastatic group (15/20) than in the non-metastatic group (38/140). The mean short axis diameter of the largest regional lymph nodes was significantly higher (P = 0.044) in the nodal metastatic group (5.686 mm) than in the non-metastatic group (4.121 mm).
Table 1.
Demographic, imaging, and pathologic variables and lymph node metastasis
| Non-metastasis | Metastasis | P value | |
| Sex (n) | 0.547 | ||
| M | 80 | 10 | |
| F | 60 | 10 | |
| Age (yr) | 59.89 (20-85) | 58.75 (38-81) | 0.672 |
| Tumor depth (n) | 0.129 | ||
| Mucosa | 17 | 0 | |
| Submucosa | 113 | 20 | |
| Tumor location (n) | 0.756 | ||
| Ascending | 15 | 2 | |
| Transverse | 13 | 2 | |
| Descending | 6 | 2 | |
| Sigmoid | 45 | 5 | |
| Rectum | 61 | 9 | |
| Detectability of primary tumor site (n) | |||
| Yes | 82 | 12 | 1.000 |
| No | 58 | 8 | |
| Detectability of regional lymph node (n) | |||
| Yes | 38 | 15 | 0.0001 |
| No | 102 | 5 | |
| Lymph node size (mm) | 4.121 (3-6.5) | 5.686 (4-14) | 0.0442 |
The detection rate of lymph nodes was significantly higher (P = 0.000) in the pathologic nodal metastatic group (15/20) than in the non-metastatic group (38/140);
The mean short axis diameter of the largest regional lymph nodes was significantly higher (P = 0.044) in the nodal metastatic group (5.686 mm) than in the non-metastatic group (4.121 mm).
ROC analysis was performed on the lymph node size parameter to obtain optimal diagnostic criterion to diagnose lymph node metastasis. The area under the ROC curve was 0.809 and the ROC curve showed that a criterion of 4.1 mm was optimal to diagnose lymph node metastasis, with a sensitivity of 78.6% and specificity of 75% (Figure 3).
Figure 3.
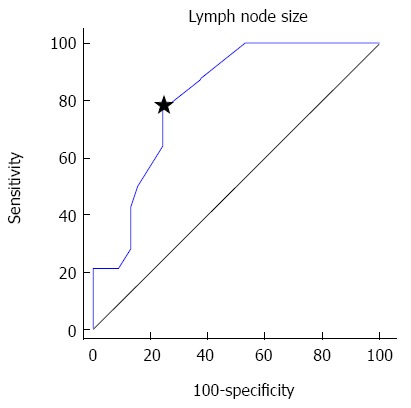
Receiver operating characteristic curve of short axis diameter. A criterion of 4.1 mm (star) showed optimal sensitivity (78.6%) and specificity (75%).
DISCUSSION
The incidence of lymph node metastasis in early colon cancer is 7%-15% and the risk of lymph node metastases rises with advancing mural invasion into submucosa, up to 23%-38.5% in cases with the tumor depth of invasion over two thirds of submucosa[23]. Our study showed a 12.5% lymph node metastasis in early CRC patients, which is compatible with previous studies.
Conventional radiologic lymph node metastasis evaluation is traditionally based on nodal size and shape. There have been significant efforts to assess lymph node metastasis by size or other criteria. In previous reports, one of the general size criteria for nodal metastasis was 1cm in the short diameter[24]. Continuous technological advancements in CT and MR equipment made it possible to detect LNs as small as 5 mm on CT and MRI due to improved image resolution. Additionally, a higher rate of nodal micro metastases smaller than 5 mm has been reported in pathologic reports. Recent studies for rectal cancer presented 5 mm as an optimal size criterion, which showed moderate sensitivity (68%) and specificity (78%)[14-16,21,22]. However, a 5 mm cut-off value for LN metastasis in our early CRC group resulted in a sensitivity of 50% and a specificity of 81.6%. This too low sensitivity was caused by small-sized metastatic LNs. According to the pathologic reports, 30%-50% of metastatic lymph nodes in rectal cancer including both advanced and early cancer were smaller than 5 mm in size[21,22]. In the review of pathologic reports of our early CRCs, the average short diameter of metastatic lymph nodes was 4.8 mm and 56% of metastatic LN was less than 5 mm. Consequently, we need a modified size criterion for early colorectal cancer. The ROC curve analysis of short axis diameter of LNs in our study showed that a criterion of 4.1 mm or larger was optimal to diagnose LN metastasis in early CRC.
One obstacle to a smaller cut-off value of LN diameter was the ability of imaging modalities to detect small lymph nodes. Previous studies indicated that the smallest lymph nodes that can be detected were 5 mm for CT, and 3 mm for MR with using a spiral CT and a 1.5-T MRI[16,22]. Our study differentiated lymph nodes as small as 3 mm from other structures (such as blood vessels) due to the improved resolution of a 64 channel multi-detector row CT.
Unlike our study, a prior study showed no significant difference in the MRI detectability of LNs between nodal metastatic and non-metastatic groups[9]. The difference in findings also can be explained by the different incidence of enlarged reactive LNs between advanced and early cancers. The reactive LNs are usually not visible on imaging studies, but some enlarged reactive LNs can be detected on CT/MRI. The prior study included mostly advanced stage rectal cancers (46/49) unlike our study which consisted of only early stage cancers. Therefore, we can presume that advanced cancers have a higher incidence of enlarged reactive LNs, which can be visualized on imaging, on the contrary to the lower incidence of enlarged reactive LNs in patients with early CRC. Consequently, we should pay more attention to lymph node detection for patients with early CRCs, because detectable LNs associated with early cancer are more likely metastatic rather than reactive LNs.
There are several limitations to our study. It was a retrospective study that may have various biases. Of particular note, most of metastatic LNs could not be correlated with the CT or MRI due to retrospective design. Nevertheless, our study showed significant difference in LN detectability of CT and MRI between metastatic and non-metastatic group. Therefore, our findings imply that LNs around early cancer on CT and MRI require attention in clinical practice. The number of metastatic lymph nodes were relatively small (n = 20); and may not reveal a real difference between the two groups. Selection bias is another inevitable component in a retrospective study. Although our study did not show significant difference among demographic data between nodal metastatic and non-metastatic groups, measurement error or inconsistent sensitivity of radiologists can be a confounding factor. To overcome this limitation, we need further prospective studies. In addition, we did not apply morphologic criteria, because morphologic evaluation was difficult in early CRC due to the small size of the lymph nodes. This study included patients without detectable primary tumors, and a limited evaluation of the corresponding mesocolon of primary tumor. However, our results indicated that the detectability of the primary tumor did not affect the pathologic LN metastasis. In clinical practice, radiologists should evaluate nodal status even in cases blinded to the exact location of the primary colon mass or when not visible due to small size, incomplete colon distention or post removal state. Our results indicated that a careful observation of the corresponding mesocolon segment is still important even in cases without detectable primary colon mass. The final limitation to our study was that the CT scanners used in our study were the most state-of-the-art equipment; 64 channel multi-detector CTs. Further studies are required for the reproducibility of small LN detection of 3 mm in lower-powered 8 or 16 channel CTs.
In conclusion, the advancement in imaging modalities will enable the detection of smaller lymph nodes and the establishment of more accurate size criteria for lymph node metastasis. Lymph node detectability and size of visible lymph nodes on CT/MR were significantly different between pathologic nodal metastatic and non-metastatic groups of patients with early colorectal carcinomas. A 4.1 mm short axis diameter criterion is believed optimal in the CT/MR evaluation of regional lymph node metastasis in patients with early colorectal carcinoma.
COMMENTS
Background
The accurate assessment of lymph node (LN) metastasis in early colorectal cancer (CRC) is crucial for deciding appropriate treatment strategies such as endoscopic resection or surgery as well as for use as a prognostic factor. Recent imaging studies considered 5 mm as an optimal size criterion for LN metastasis in colorectal cancer. However, previous studies have not focused on early colorectal carcinoma.
Research frontiers
Continuous development of computed tomography (CT) and magnetic resonance imaging (MRI) made it possible to detect small LNs by CT and MRI due to improved image resolution. This study evaluates the radiologic risk factors for LN metastasis in early CRCs and develops adequate diagnostic size criteria for LN metastasis in patients with early CRC.
Innovations and breakthroughs
Most of previous studies for evaluation of radiologic criteria included both advanced and early colorectal cancer, but the portion of included early cancer was very small. The size criteria (approximately 0.5-1 cm) for diagnosing metastatic LNs in previous study showed low to intermediate sensitivity, because the ranges of sizes of metastatic and non-metastatic LNs were overlapped. However, the sizes of LNs in the metastatic group and the non-metastatic group were as significantly different in this study. Authors presumed that this different result from previous study was because their study included only early CRCs. They concluded that the differentiated criteria for early CRC is needed and evaluated optimal size criteria for LN metastasis. The newly suggested size criterion for metastatic LNs in early CRC is 4.1 mm, slightly smaller compared to the existing criterion for both advance and early CRC.
Applications
For the CT or MRI evaluation of early colorectal cancer, an application of a differentiated size criterion from advanced cancer for LN metastasis could be helpful in management planning for early CRC.
Peer review
This is a good retrospective study in which the authors assessed the role of CT and MRI to establish the imaging criteria of LN metastasis in early colorectal cancer. The results are interesting and suggest that the detection rate and the size of LNs were significantly higher in patients with pathologic nodal metastasis. The optimal size criterion for LN metastasis was ≥ 4.1 mm in early colorectal cancer.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 19, 2014
First decision: June 10, 2014
Article in press: July 25, 2014
P- Reviewer: Moldovan R, Murayama Y, Padin-Iruegas ME, Zhu YL S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Kashida H, Kudo SE. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol. 2006;11:1–8. doi: 10.1007/s10147-005-0550-5. [DOI] [PubMed] [Google Scholar]
- 2.Kudo S, Kashida H, Nakajima T, Tamura S, Nakajo K. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997;21:694–701. doi: 10.1007/s002689900293. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura YJ, Sakuragi M, Togashi K, Okada M, Nagai H, Konishi F. Distribution of lymph node metastasis in T1 sigmoid colon carcinoma: should we ligate the inferior mesenteric artery? Scand J Gastroenterol. 2005;40:858–861. doi: 10.1080/00365520510015746. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka S, Haruma K, Teixeira CR, Tatsuta S, Ohtsu N, Hiraga Y, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol. 1995;30:710–717. doi: 10.1007/BF02349636. [DOI] [PubMed] [Google Scholar]
- 5.Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328–336. doi: 10.1016/0016-5085(85)90333-6. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HS, Deppisch LM, Gourley WK, Kahn EI, Lev R, Manley PN, Pascal RR, Qizilbash AH, Rickert RR, Silverman JF. Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology. 1995;108:1657–1665. doi: 10.1016/0016-5085(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 7.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286–1295. doi: 10.1007/BF02049154. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka H, Nakamura A, Sugiyama M, Hachiya J, Atomi Y, Masaki T. MRI diagnosis of mesorectal lymph node metastasis in patients with rectal carcinoma. what is the optimal criterion? Anticancer Res. 2004;24:4097–4101. [PubMed] [Google Scholar]
- 10.Butch RJ, Stark DD, Wittenberg J, Tepper JE, Saini S, Simeone JF, Mueller PR, Ferrucci JT. Staging rectal cancer by MR and CT. AJR Am J Roentgenol. 1986;146:1155–1160. doi: 10.2214/ajr.146.6.1155. [DOI] [PubMed] [Google Scholar]
- 11.de Lange EE, Fechner RE, Edge SB, Spaulding CA. Preoperative staging of rectal carcinoma with MR imaging: surgical and histopathologic correlation. Radiology. 1990;176:623–628. doi: 10.1148/radiology.176.3.2389016. [DOI] [PubMed] [Google Scholar]
- 12.Guinet C, Buy JN, Ghossain MA, Sézeur A, Mallet A, Bigot JM, Vadrot D, Ecoiffier J. Comparison of magnetic resonance imaging and computed tomography in the preoperative staging of rectal cancer. Arch Surg. 1990;125:385–388. doi: 10.1001/archsurg.1990.01410150107019. [DOI] [PubMed] [Google Scholar]
- 13.Okizuka H, Sugimura K, Ishida T. Preoperative local staging of rectal carcinoma with MR imaging and a rectal balloon. J Magn Reson Imaging. 1993;3:329–335. doi: 10.1002/jmri.1880030207. [DOI] [PubMed] [Google Scholar]
- 14.Hadfield MB, Nicholson AA, MacDonald AW, Farouk R, Lee PW, Duthie GS, Monson JR. Preoperative staging of rectal carcinoma by magnetic resonance imaging with a pelvic phased-array coil. Br J Surg. 1997;84:529–531. [PubMed] [Google Scholar]
- 15.Drew PJ, Farouk R, Turnbull LW, Ward SC, Hartley JE, Monson JR. Preoperative magnetic resonance staging of rectal cancer with an endorectal coil and dynamic gadolinium enhancement. Br J Surg. 1999;86:250–254. doi: 10.1046/j.1365-2168.1999.01019.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42:770–775. doi: 10.1007/BF02236933. [DOI] [PubMed] [Google Scholar]
- 17.Gualdi GF, Casciani E, Guadalaxara A, d’Orta C, Polettini E, Pappalardo G. Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum. 2000;43:338–345. doi: 10.1007/BF02258299. [DOI] [PubMed] [Google Scholar]
- 18.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 19.Kim NK, Kim MJ, Park JK, Park SI, Min JS. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000;7:732–737. doi: 10.1007/s10434-000-0732-3. [DOI] [PubMed] [Google Scholar]
- 20.Kulinna C, Eibel R, Matzek W, Bonel H, Aust D, Strauss T, Reiser M, Scheidler J. Staging of rectal cancer: diagnostic potential of multiplanar reconstructions with MDCT. AJR Am J Roentgenol. 2004;183:421–427. doi: 10.2214/ajr.183.2.1830421. [DOI] [PubMed] [Google Scholar]
- 21.Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, Chang GJ, Skibber JM, Ernst RD. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2012;32:389–409. doi: 10.1148/rg.322115122. [DOI] [PubMed] [Google Scholar]
- 22.Koh DM, Brown G, Husband JE. Nodal staging in rectal cancer. Abdom Imaging. 2006;31:652–659. doi: 10.1007/s00261-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 23.Cahill RA. Regional nodal staging for early stage colon cancer in the era of endoscopic resection and N.O.T.E.S. Surg Oncol. 2009;18:169–175. doi: 10.1016/j.suronc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Saunders TH, Mendes Ribeiro HK, Gleeson FV. New techniques for imaging colorectal cancer: the use of MRI, PET and radioimmunoscintigraphy for primary staging and follow-up. Br Med Bull. 2002;64:81–99. doi: 10.1093/bmb/64.1.81. [DOI] [PubMed] [Google Scholar]


