Abstract
Angiotensin II (Ang II) stimulates water and saline intakes when injected into the brain of rats. This arises from activation of the AT1 Ang II receptor subtype. Acute repeated injections, however, decrease the water intake response to Ang II without affecting saline intake. Previous studies provide evidence that Ang II-induced water intake is mediated via the classical G protein coupling pathway, whereas the saline intake caused by Ang II is mediated by an ERK 1/2 MAP kinase signaling pathway. Accordingly, the different behavioral response to repeated injections of Ang II may reflect a selective effect on G protein coupling. To test this hypothesis, we examined the binding of a radiolabeled agonist (125I-sarcosine1 Ang II) and a radiolabeled antagonist (125I-sarcosine1, isoleucine8 Ang II) in brain homogenates and tissue sections prepared from rats given repeated injections of Ang II or vehicle. Although no treatment-related differences were found in hypothalamic homogenates, a focus on specific brain structures using receptor autoradiography, found that the desensitization treatment reduced binding of both radioligands in the paraventricular nucleus of the hypothalamus (PVN) and median preoptic nucleus (MnPO), but not in the subfornical organ (SFO). Because G protein coupling is reported to have a selective effect on agonist binding without affecting antagonist binding, these findings do not support a G protein uncoupling treatment effect. This suggests that receptor number is more critical to the water intake response than the saline intake response, or that pathways downstream from the G protein mediate desensitization of the water intake response.
Keywords: Angiotensin, Brain, Hypertension, desensitization, radioligand, water intake, saline intake, AT1 angiotensin receptors, thirst
1. Introduction
Angiotensin II (Ang II) potently stimulates drinking behavior. Repeated injections of Ang II have different effects, dependent upon the timing of the injections. Specifically, daily injections of Ang II or continuous infusion over days sensitizes its dipsogenic and natriorexigenic potencies (Bryant et al., 1980; Moellenhoff et al., 2001; Pereira et al., 2010), but acute repeated injections within a shorter timeframe desensitize the water intake response normally observed after Ang II injection (Quirk et al., 1988; Torsoni et al., 2004; Vento and Daniels, 2010a; Vento and Daniels, 2012; Vento et al., 2012). Interestingly, the behavioral desensitization caused by acute repeated injections of Ang II is selective to the water intake effects of the peptide, with no observed desensitization of saline intake (Vento and Daniels, 2010a). This is potentially informative because previous studies using cell culture models suggested that the mechanism of desensitization relied heavily on receptor internalization (Hein et al., 1997; Mehta and Griendling, 2007; Thomas et al., 1998); however, this internalization would likely affect intake of both saline and water, since the receptors would no longer be able to bind extracellular Ang II.
Another mechanism of desensitization involves the dissociation of G proteins from G protein-coupled receptors in response to agonist binding (Bonde et al., 2010; Crane et al., 1982; Poitras et al., 1998; Speth and Kim, 1990). In such cases not only is there a loss of connectivity with the G protein transducer, there is also a reduction in agonist binding affinity (Crane et al., 1982; Glossmann et al., 1974; Rodbell et al., 1971; Speth and Kim, 1990).
By examining the binding of an agonist angiotensin analog 125I-Sar1 Ang II, versus a classical antagonist angiotensin analog, 125I-Sar1,Ile8 Ang II, it should be possible to determine whether the desensitization of the dipsogenic response to intracerebroventricular (ICV) Ang II (Vento and Daniels, 2010a) occurs via uncoupling of the G protein (decreased 125I-Sar1 Ang II binding with no change in 125I-Sar1,Ile8 Ang II binding) or a G protein-independent mechanism. Possibilities for G protein-independent mechanisms include receptor internalization, in which case both the agonist and antagonist radioligand binding should decrease, or a decrease in signaling pathways downstream from the G protein, in which case radioligand binding may not change. The present studies used this strategy to test the hypothesis that changes in G protein coupling are responsible for the behavioral effects of repeated injections of Ang II.
2. Results
A two-way repeated measures ANOVA was run to determine if the repeated exposures to Ang II selectively reduced the binding affinity of the agonist radioligand in hypothalamic tissue membranes (Table 1). As shown in Figure 1A, there was no reduction in radioligand binding affinity for either the agonist or antagonist radioligand with repeated Ang II exposure (p>0.05). There was no significant interaction between treatment and radioligand binding affinities (p>0.05). Binding affinity for 125I-Sar1 Ang II was significantly lower than for 125I-Sar1, Ile8 Ang II independent of treatment (p=0.0149).
Table 1.
Statistical Analysis of Radioligand Binding Assays of Hypothalami from Angiotensin II Desensitized and Control Brains.
| Condition | Kd | Bmax | Bound |
|---|---|---|---|
| SAR Control | 3.94 ± 1.2 | 823 ± 220 | 100 ± 8.3 |
| SAR Desensitized | 1.93 ± 0.55 | 476 ± 120 | 96 ± 5.6 |
| SI Control | 1.21 ± 0.23 | 425 ± 75 | 121 ± 10 |
| SI Desensitized | 0.96 ± 0.16 | 360 ± 60 | 119 ± 8.1 |
|
| |||
| F values | |||
| Treatment | F1,10 = 3.35 | F1,10 = 5.55 | F1,10 = 0.20 |
| Radioligand | F1,10 = 8.62 | F1,10 = 3.39 | F1,10 = 16.6 |
| Interaction | F1,10 = 2.10 | F1,10 = 1.52 | F1,10 = 0.09 |
|
| |||
| P values | |||
| Treatment | 0.0973 | 0.0955 | 0.6668 |
| Radioligand | 0.0149 | 0.0403 | 0.0022 |
| Interaction | 0.1784 | 0.2461 | 0.769 |
Bolded values are statistically significant (p < 0.05). SAR, 125I-Sar1 Ang II; SI, 125I-Sar1,Ile8 Ang II.
Figure 1. Comparison of agonist radioligand (125I-Sar1 Ang II) and antagonist radioligand (125I-Sar1,Ile8 Ang II) binding to hypothalamic membranes from “desensitized” and “non-desensitized” rat brains.
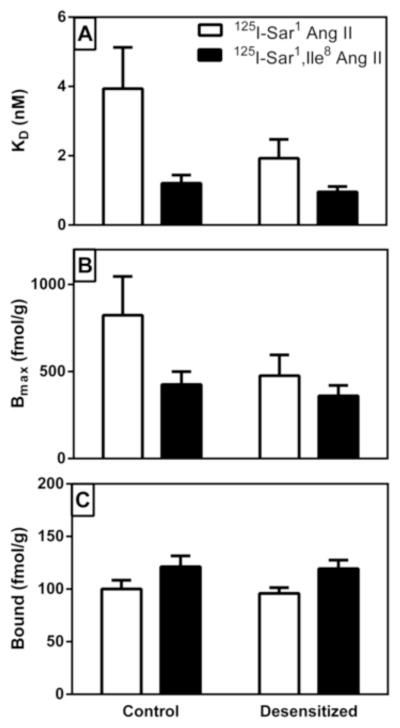
Panel A describes the average dissociation constant (KD) of the radioligands for binding to the hypothalamic membranes for the two different treatment groups. Panel B describes maximal binding (BMAX) of each of the radioligands to the two different groups. Panel C describes the derived values for radioligand binding at 500 pM concentration (to be representative of physiological levels of Ang II that the receptors might be exposed to) based on the average KD and Bmax values for each radioligand and treatment group. Values shown are mean ± S.E.M. n = 11.
Bmax values for radioligand binding in the hypothalamic tissue membranes are shown in Figure 1B. Although the mean decrease in Bmax in the desensitized group was approximately 33%, this was not statistically significant using a two-way repeated measures ANOVA (p>0.05). There was a significant main effect of radioligand, Bmax values for 125I-Sar1 Ang II were 65% greater than for 125I-Sar1, Ile8 Ang II (p=0.04), but there was no significant interaction between treatment and radioligand (p=0.25).
Further analysis of the radioligand binding data to assess the relationship between Kd and Bmax revealed strong positive correlations between Kd and Bmax values: R2 = 0.93, 0.76, 0.96, 0.91, (all p<0.001) for non-desensitized 125I-Sar1 Ang II, non-desensitized 125I-Sar1, Ile8 Ang II, desensitized 125I-Sar1 Ang II, and desensitized 125I-Sar1, Ile8 Ang II, respectively. To better assess differences in receptor occupancy at a physiological concentration, a two-way ANOVA comparison was made for calculated binding values at a radioligand concentration of 500 pM. As shown in Figure 1C, there was no difference in the derived radioligand binding values at 500 pM between the two treatment groups (p=0.67). Once again, there was no significant interaction between treatment and radioligand (p=0.77). The derivation indicated that 125I-Sar1 Ang II binding was lower than 125I-Sar1, Ile8 Ang II binding (p=0.0022), reflecting the lower binding affinity of 125I-Sar1 Ang II for the AT1 receptor.
Analysis of radioligand binding to the SFO, PVN, and MnPO was determined using quantitative densitometric in vitro receptor autoradiography. Representative images of radioligand binding to brain sections containing the SFO and PVN and corresponding thionin stained brain sections are shown in Figure 2 and supplementary material Figure S1. Representative images of radioligand binding to brain sections containing the MnPO and corresponding thionin stained brain sections are shown in Figure 3. Two-way ANOVA with repeated measures for radioligand was used to analyze recorded values for density of radioligand binding to AT1 receptors, area delineated, and AT1 receptor density times area in the PVN, SFO, and MnPO (Table 2).
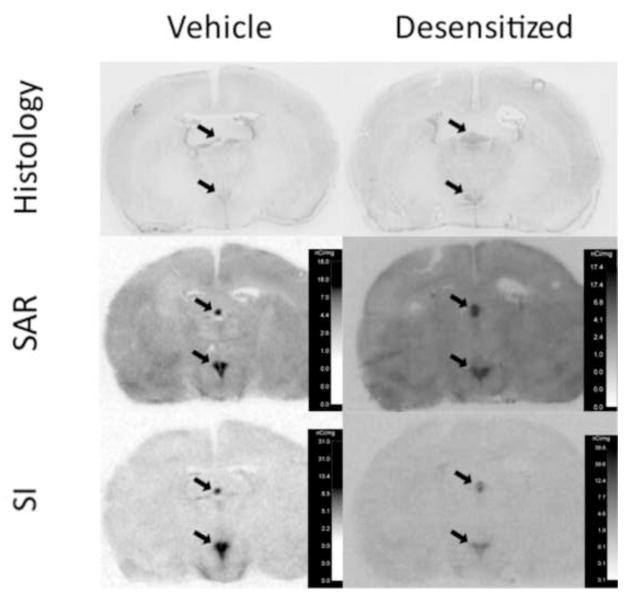
Figure 2. Representative images of radioligand binding to coronal sections of rat forebrain at the anterior-posterior axis encompassing the subfornical organ and paraventricular nucleus of the hypothalamus, ~ 1.5 mm caudal to Bregma.
Top panels indicate thionin-stained sections from a vehicle treated and “desensitized” rat brain adjacent to brain sections incubated with radioligand. Middle and bottom panels show film images of 125I-sar1 Ang II (SAR) and 125I-sar1,Ile8 Ang II (SI) binding, respectively, to AT1 receptors of a vehicle treated (left panels) and “desensitized” (right panels) rat brain. Slides used for receptor autoradiography were incubated with 10 μM PD123319 to saturate AT2 receptors. Calibration scales on each film image were derived from the increase in film exposure caused by calibration standards with increasing known amounts of 125I. The subfornical organ and paraventricular nucleus of the hypothalamus are clearly apparent as intensely labeled midline structures represented in the dorsal and ventral regions of the brain sections, respectively.
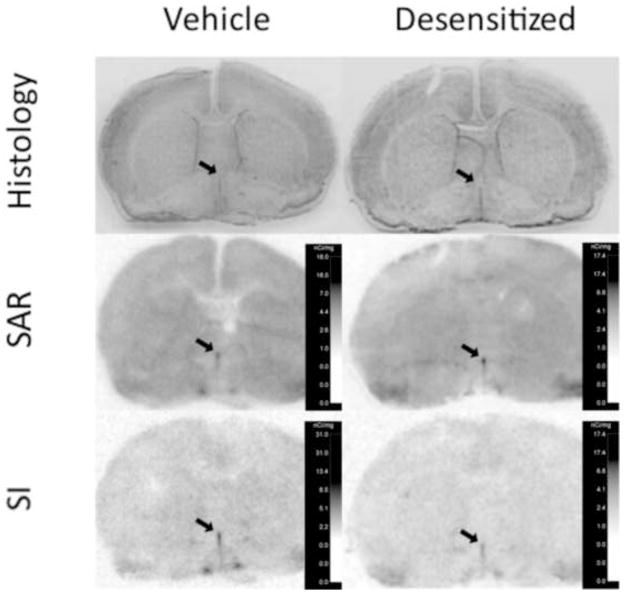
Figure 3. Representative images of radioligand binding to coronal sections of rat forebrain at ~ Bregma, showing the median preoptic nucleus just anterior to the crossing of the anterior commissure.
Top panels indicate thionin-stained sections from a vehicle treated and “desensitized” rat brain adjacent to brain sections incubated with radioligand. Middle and bottom panels show film images of 125I-sar1 Ang II (SAR) and 125I-sar1,Ile8 Ang II (SI) binding, respectively, to AT1 receptors of a vehicle treated (left panels) and “desensitized” (right panels) rat brain. Slides used for receptor autoradiography were incubated with 10 μM PD123319 to saturate AT2 receptors. Calibration scales on each film image were derived from the increase in film exposure caused by calibration standards with increasing known amounts of 125I. Arrows point to the median preoptic nucleus.
Table 2.
Statistical Analysis of Densitometric Readings From Receptor Autoradiography of Angiotensin II Desensitized and Control Brains.
| Condition | Binding Density | Area of Binding Assayed (mm2) | Density times area | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| SFO | PVN | MnPO | SFO | PVN | MnPO | SFO | PVN | MnPO | |
| SAR Control | 7.65 ± 0.56 | 6.49 ± 0.63 | 2.11 ± 0.07 | 0.190 ± 0.023 | 0.446 ± 0.039 | 0.265 ± 0.007 | 1.46 ± 0.21 | 2.82 ± 0.18 | 0.561 ± 0.031 |
| SAR Desensitized | 7.38 ± 0.71 | 5.00 ± 0.78 | 1.70 ± 0.17 | 0.195 ± 0.026 | 0.410 ± 0.016 | 0.271 ± 0.004 | 1.44 ± 0.25 | 2.03 ± 0.31 | 0.460 ± 0.048 |
| SI Control | 6.11 ± 0.57 | 6.48 ± 0.32 | 2.01 ± 0.21 | 0.235 ± 0.038 | 0.337 ± 0.031 | 0.267 ± 0.005 | 1.44 ± 0.26 | 2.19 ± 0.25 | 0.534 ± 0.054 |
| SI Desensitized | 4.78 ± 0.42 | 4.92 ± 0.77 | 1.63 ± 0.10 | 0.259 ± 0.016 | 0.337 ± 0.043 | 0.276 ± 0.001 | 1.22 ± 0.07 | 1.54 ± 0.13 | 0.449 ± 0.026 |
|
| |||||||||
| F values | |||||||||
| Treatment | F1,8 = 2.70 | F1,8 = 4.303 | F1,8 = 5.380 | F1,8 = 0.221 | F1,8 = 0.175 | F1,8 = 2.360 | F1,8 = 0.220 | F1,8 = 7.85 | F1,8 = 3.520 |
| Radioligand | F1,8 = 10.2 | F1,8 = 0.008 | F1,8 = 0.522 | F1,8 = 7.460 | F1,8 = 19.02 | F1,8 = 0.568 | F1,8 = 0.644 | F1,8 = 8.06 | F1,8 = 0.355 |
| Interaction | F1,8 = 0.67 | F1,8 = 0.004 | F1,8 = 0.017 | F1,8 = 0.194 | F1,8 = 0.696 | F1,8 = 0.203 | F1,8 = 0.418 | F1,8 = 0.11 | F1,8 = 0.062 |
|
| |||||||||
| P values | |||||||||
| Treatment | 0.139 | 0.072 | 0.049 | 0.651 | 0.687 | 0.160 | 0.652 | 0.023 | 0.098 |
| Radioligand | 0.013 | 0.933 | 0.490 | 0.026 | 0.002 | 0.470 | 0.446 | 0.022 | 0.570 |
| Interaction | 0.437 | 0.950 | 0.900 | 0.671 | 0.428 | 0.660 | 0.536 | 0.750 | 0.810 |
Bolded values are statistically significant (p < 0.05). SAR, 125I-Sar1 Ang II; SI, 125I-Sar1,Ile8 Ang II.
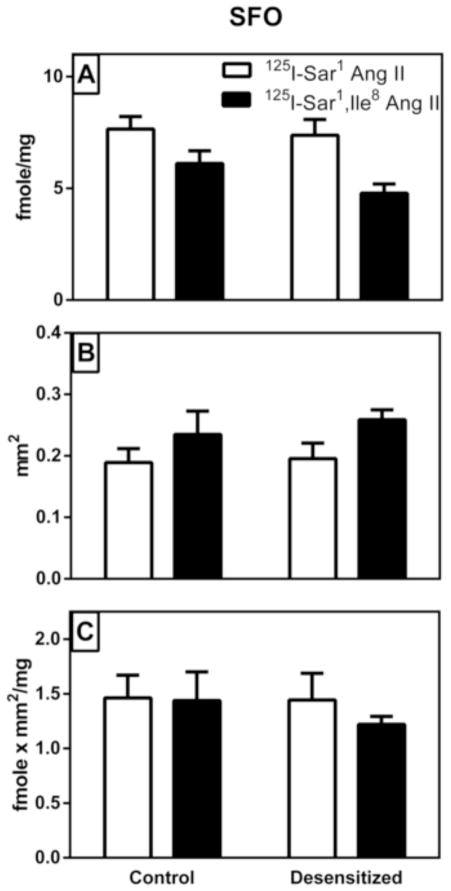
As shown in figure 4A, there was no treatment-related change in radioligand binding density in the SFO (p>0.05). We did not find a significant interaction between radioligand and treatment (Table 2), but there was 37% higher binding density of 125I-Sar1 Ang II compared to 125I-Sar1, Ile8 Ang II resulting in a statistically significant main effect of radioligand (p=0.013). As shown in figure 4B, the area delineated as SFO with 125I-Sar1 Ang II was 22% smaller than that delineated for 125I-Sar1, Ile8 Ang II (p=0.026). However, there was no significant interaction between treatment and radioligand, nor was there any significant difference in the area delineated as SFO in the control vs desensitized group (Table 2). As shown in Figure 4C, there was no significant difference in density times area between treatments or between radioligands, nor was there a significant interaction between treatment and radioligand (Table 2).
Figure 4. Graphical representation of specific AT1 receptor binding to the subfornical organ (SFO) of vehicle treated control and “desensitized” rat brains.
Panel A shows the density of binding within the circumscribed area defined as the SFO. Panel B shows the area that was circumscribed as the SFO where there was a high expression of AT1 receptor binding. Panel C shows a density times area analysis of the SFO. As described in Table 2, there were statistically significant changes in binding density and area of binding assayed between the agonist radioligand (SAR) compared with the antagonist radioligand (SI) regardless of treatment. However when density times area values were compared there were no statistically significant differences between radioligands. Values are mean ± S.E.M. n = 5
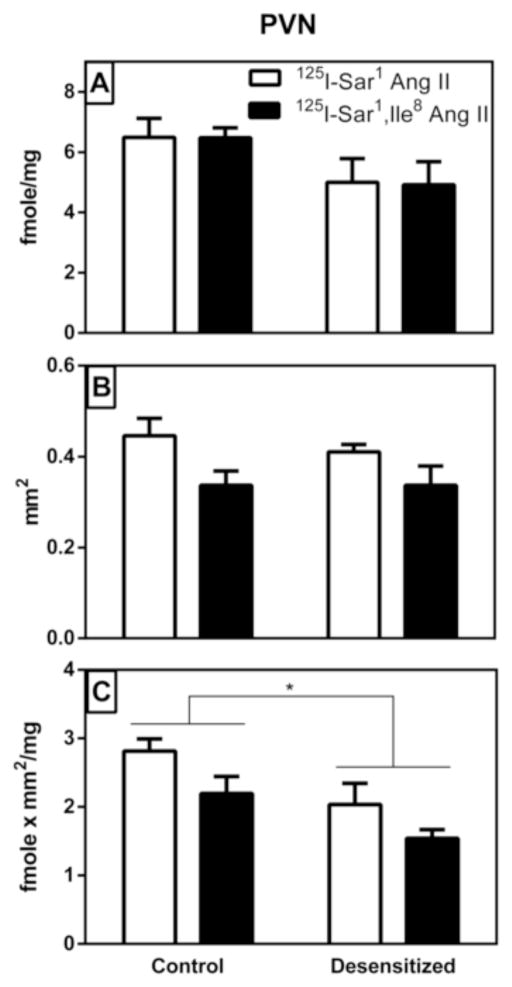
As shown in Figure 5A, there was a 24% reduction in radioligand binding density in the PVN of the desensitized group, but this was not statistically significant (p>0.05, Table 2). There was no significant difference in binding density between the two radioligands, nor was there any interaction between treatment and radioligand. As shown in Figure 5B, there was no significant difference in the area delineated as PVN in the control vs desensitized group (p>0.05). The area delineated as PVN with 125I-Sar1 Ang II was 27% greater than that delineated with 125I-Sar1, Ile8 Ang II (p=0.0024), again, with no significant interaction between treatment and radioligand (Table 2). As shown in Figure 5C, there was a statistically significant (p=0.023) 29% decrease in density times area (fmole/g x mm2) in the PVN of the desensitized group, once again, with no significant interaction between treatment and radioligand (Table 2). There was also a significant (p=0.022) 30% increase in density times area of 125I-Sar1 Ang II compared with that of 125I-Sar1, Ile8 Ang II in the PVN.
Figure 5. Graphical representation of specific AT1 receptor binding to the paraventricular nucleus of the hypothalamus (PVN) of vehicle treated control and “desensitized” rat brains.
Panel A shows the density of binding within the circumscribed area defined as the PVN. Panel B shows the area that was circumscribed as the PVN where there was a high expression of AT1 receptor binding. Panel C shows a density times area analysis of the PVN. As described in Table 2, there were statistically significant differences in area of binding assayed and density times area between radioligands regardless of treatment as well as in density times area measurements between treatment groups. Values are mean ± S.E.M. n = 5. * p<0.05.
As shown in Figure 6A, there was a significant (p=0.049) 19% decrease in radioligand binding density in the MnPO of the desensitized group compared with control with no significant interaction between treatment and radioligand (Table 2). There was no significant difference in binding between radioligands (p>0.05). As shown in Figure 6B, there was no significant difference in the area delineated in the control vs desensitized groups (p>0.05) and no significant interaction between treatment and radioligand (Table 2). There was also no significant difference in area between radioligands (Table 2). As shown in Figure 6C, when density times area in the control vs desensitized groups were compared, the decrease in the desensitized group was no longer significant (p>0.05). Once again, there was no significant interaction between treatment and radioligand in the MnPO and no significant difference between radioligands (Table 2).
Figure 6. Graphical representation of specific AT1 receptor binding to the median preoptic nucleus (MnPO) of vehicle treated control and “desensitized” rat brains.
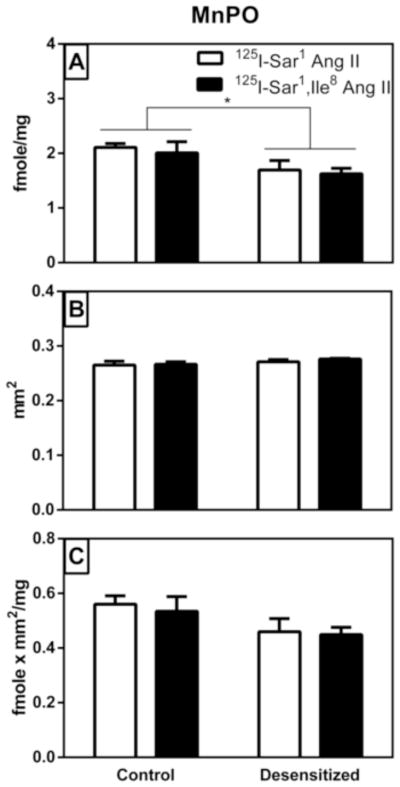
Panel A shows the density of binding within the circumscribed area defined as the MnPO. Panel B shows the area that was circumscribed as the MnPO where there was a high expression of AT1 receptor binding. Panel C shows a density times area analysis of the MnPO. As described in Table 2, there was a statistically significant difference in binding density between treatment groups. Values are mean ± S.E.M. n = 5. * p<0.05.
3. Discussion
Receptor desensitization appears to be a critical mechanism for the regulation of a variety of biological functions. With respect to Ang II, receptor mutation that renders the receptor unable to desensitize is associated with a variety of deleterious effects including renal and cardiac fibrosis and hypertension (Billet et al., 2007). Our previous studies have developed a model of behavioral desensitization that was hypothesized to reflect a receptor-mediated event involving changes in G protein coupling (Vento and Daniels, 2010b). The present studies found no evidence for differential changes in agonist versus antagonist radioligand binding with acute repeated injections of a large dose of Ang II (desensitization protocol), thereby failing to support the original hypothesis. There was, however, evidence for a reduction in AT1 receptor binding in two brain regions that are responsive to ICV Ang II: the PVN and the MnPO. However, membrane homogenate binding assays from the hypothalamus did not detect a statistically significant reduction in the concentration of receptors (Bmax) as a function of the desensitization protocol. Although our previous studies using this approach to desensitization have focused on the drinking response to Ang II (Vento and Daniels, 2010b; Vento and Daniels, 2012; Vento et al., 2012; Vento and Daniels, 2014), other effects of Ang II have received less attention. Nevertheless, the present findings may provide important insight for future studies of other consequences of the desensitization of Ang II and perhaps for studies of other receptor systems.
Of the three brain areas examined autoradiographically, the PVN showed a statistically significant reduction when density of binding times area was determined, while the MnPO showed a statistically significant reduction when density only was determined. Thus, these data support the hypothesis that acute repeated ICV injections of Ang II reduce the dipsogenic potency of Ang II by decreasing AT1 receptor availability. The extent of the reduction in receptor binding is concomitant with the extent of the behavioral response of reduced water drinking in response to Ang II following the same desensitization protocol (Vento and Daniels, 2014).
The present studies were designed to test the hypothesis that the decreased ability of Ang II to cause thirst after repeated injections of the peptide results from G protein uncoupling, rather than receptor internalization. Previous studies have shown divergent signaling requirements of Ang II receptors for stimulation of water and saline intake, with G protein-mediated signaling being the most critical for the water intake effects of Ang II (Daniels et al., 2005; Daniels et al., 2007; Daniels et al., 2009; Daniels, 2010) and G protein-independent signaling being most critical for stimulation of saline intake. Given that the behavioral effects of repeated injections of Ang II reduce its ability to stimulate water intake without altering its ability to stimulate saline intake (Vento and Daniels, 2010a), it seemed reasonable to hypothesize that a selective effect on G protein action plays a role in the observed tachyphylaxis.
In vitro studies using stable GTP analogs demonstrate that dissociation of the G protein from the β-adrenergic receptors reduces G protein-coupled receptor agonist binding affinity (Lefkowitz et al., 1976; Maguire et al., 1976). This effect also has been observed in rat adrenal (Glossmann et al., 1974) and liver angiotensin receptors (Crane et al., 1982) which are known to express the AT1 subtype exclusively (Speth and Kim, 1990). Accordingly, if repeated injections of Ang II lead to a similar G protein uncoupling, it would shift the AT1 receptor to a low affinity agonist binding conformation, which would further compromise the ability of the AT1 receptor to cause thirst. However, if the ability of Ang II to bind to the AT1 receptor is diminished, it should also decrease the salt appetite response. The present studies found no interaction between the treatment and the radioligand, and, therefore, do not support selective effects on G protein signaling as a mechanism of the behavioral changes observed. In fact, in the membrane homogenate studies, we observed what appeared to be increased binding affinity after repeated Ang II administration, although this perceived difference was not statistically significant. As such, we conclude that G protein uncoupling is not likely to be responsible for the observed behavioral effects of repeated injections of Ang II. Of note an AT1 receptor in which the DRY motif at the cytoplasmic junction of the third transmembrane spanning domain is mutated to AAY or ALY, preventing it from binding G proteins, binds Ang II with an affinity similar to that of wild-type AT1 receptors (Feng et al., 2005; Wei et al., 2003). This observation challenges the necessity of G protein binding to the G protein-coupled receptor for high affinity agonist binding.
There are other explanations for the occurrence of desensitization of the thirst response without any effect on saline intake. First, it is possible that the different effect on water and saline intakes reflects different baseline sensitivities to Ang II. Indeed, a direct dose-response comparison reported previously suggests that the minimum dose of ICV Ang II needed to generate saline intake is less than that needed to produce water intake (Daniels et al., 2009). Accordingly, it is possible that fewer receptors are needed for the saline intake response and, therefore, reducing the number available would have a greater impact on the water intake response. Second, it is possible that the signaling pathway of the AT1 receptor that mediates water intake is compromised; an event that can occur independently of expression of cell surface receptors and G protein coupling. Expression of SOCS-3 (suppressor of cytokine signaling-3) is reported to be necessary for Ang II-induced desensitization of the water intake response, and this relies on negative feedback through the JAK/STAT signaling pathway (Torsoni et al., 2004). Our lab has previously shown that MAP kinase is similarly required for behavioral desensitization, and there is evidence for MAP kinase activation of JAK/STAT signaling after Ang II (Bhat and Baker, 1997; Kodama et al., 1998). Accordingly, this has led to the working hypothesis that Ang II-induced activation of MAPK can potentially stimulate SOCS-3 expression through activation of the JAK/STAT pathway. SOCS-3 may then feed back to inhibit further JAK/STAT signaling causing functional changes in Ang II responsiveness, specifically the thirst response mediated by the AT1 receptor. The saline intake response that does not desensitize (Vento and Daniels, 2010a) could be mediated by a different pathway that is not inhibited by SOCS-3. Further research is required to test these possiblities and to better clarify how these intracellular signaling cascades interact.
4. Conclusion
The desensitization protocol involving repeated administration of Ang II had limited effects on radioligand binding to AT1 receptors in brain membrane homogenates. Radioligand binding to specific brain nuclei assessed autoradiographically was moderately reduced, but this reduction only attained statistical significance in the PVN and MnPO. The lack of an interaction between radioligands (agonist versus antagonist) and treatments (control versus desensitized) suggests that the desensitization protocol did not cause a conformational change in receptor structure to effect a selective reduction in agonist binding affinity.
Desensitization of G protein-coupled receptors has been shown to occur either by dissociation of the G protein away from the receptor with subsequent loss of signaling capability, or by internalization via endocytosis. In the case of dissociation of the G protein from its receptor, there should be reduced agonist, but not antagonist, binding affinity. In the case of receptor internalization there is a non-selective reduction of both agonist and antagonist binding with no change in binding affinities. The radioligand binding data is consistent with the latter scenario, suggesting that the water intake response to Ang II is more dependent on the number of AT1 receptors than the saline intake response. However, alterations in downstream signaling mechanisms of the G protein coupled response cannot be eliminated as the mechanism(s) of desensitization.
5. Experimental Procedures
5.1 Animals
Male Sprague Dawley rats (175–199g upon arrival from breeder) were obtained from Harlan Laboratories (Indianapolis, IN) and were housed individually in hanging steel wire-mesh cages in a temperature- and humidity-controlled room. Rats were given ad libitum access to food and water unless otherwise stated. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo and Nova Southeastern University.
5.2 Surgery
No fewer than 5 days after arrival from breeder, rats were anesthetized by intramuscular injection of a combination of ketamine (70 mg/kg) and xylazine (5 mg/kg) and their heads were fixed in a stereotaxic apparatus. A small incision was made in the scalp and burr holes were drilled in the skull. A guide cannula (33 gauge, Plastics One, Roanoke, VA) aimed at the lateral cerebral ventricle (coordinates: 0.9 mm posterior to bregma, 1.4 mm lateral to the mid-line, 1.8 mm ventral to the dura) was implanted and affixed to the skull using bones screws and dental cement. Carprofen (5mg/kg; s.c.) was given after surgery to minimize pain and swelling. Rats were given no fewer than 5 days to recover from surgery before testing began. Proper cannula placement was assessed by administering a single injection of Ang II (10ng/1μl; Biochem Bioscience Inc., King of Prussia, PA) through the cannula aimed at the lateral ventricle. Only rats that consumed at least 6 ml of water in the 30 min after Ang II injection were included in subsequent experiments.
5.3 Drug injections and treatment protocol
All icv injections were made through an injector cannula (26 gauge, Plastics One, Roanoke, VA) using a 2 μl Hamilton syringe (Hamilton Company, Reno, NV) attached to water-filled PE 50 tubing. The injector was left in place for approximately 30 sec after injection to allow for diffusion of drug. Rats were given a treatment regimen comprising three injections of either Ang II (300 ng/1 μl in TBS) or vehicle (1 μl TBS), with each injection separated by 20 min. Food and water were removed immediately prior to the first treatment regimen injection. Eighteen minutes after the final treatment regimen injection, rats were anesthetized by inhaled isoflurane, and 2 min later (20 min after final treatment regimen injection), rats were killed by decapitation, and the brains were rapidly removed and frozen in 2-methylbutane on dry ice. Brains were stored at −80° C for later analyses.
5.4 Materials
125I-Sar1 Ang II and 125I-Sar1,Ile8 Ang II were prepared using the chloramine T procedure (Hunter and Greenwood, 1962) with HPLC purification to theoretical specific activity (2175 Ci/mmole) as described (Speth and Harding, 2001). Ang II was obtained from Phoenix Pharmaceuticals or American Peptides; PD123319 from Tocris; phenylmethylsulfonyl fluoride (PMSF), O-phenanthroline, EDTA, NaCl, and bacitracin were obtained from Sigma Chemical Company or Fisher Scientific.
5.5 Radioligand binding assays
The hypothalamus was dissected from the frozen brain as described elsewhere (Glowinski and Iversen, 1966). The hypothalamus was weighed, placed in 25 ml of ice-cold hypotonic buffer (20 mM NaPO4, pH 7.1–7.2, homogenized (Tissumizer) for 10 sec, centrifuged at 4° C for 20 min at 48,000 × g. The supernatant was discarded and the membrane pellet was resuspended in a buffer containing 150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, and 50 mM NaPO4, pH 7.1 – 7.2, rehomogenized and recentrifuged as before. The supernatant was again discarded and the membrane pellet was resuspended by homogenization in 9–23 volumes (w/v) of the previous buffer. PD123319 (10 μM, to saturate AT2 receptors), PMSF (100 μM) and O-phenanthroline (100 μM) were present in the final tissue homogenate.
Radioligand binding assays were carried out at ~ 23° C for one hour in a volume of 100 μl with 6 different concentrations of radioligand ranging from 0.2 to 4 nM in the presence or absence of 3 μM Ang II to define non-specific and total binding so as to derive a value of specific binding to AT1 receptors. Membrane-bound radioligand was collected on GF/B filters using a Brandel Cell Harvester and the filters were assayed for iodine-125 in a gamma counter. The KD and Bmax values were derived from the specific bound and free radioligand concentration using a one-site saturation curve-fitting algorithm (Prism, Graphpad Software).
Brain sectioning and receptor autoradiography were carried out as described previously (Speth et al., 1999) using 500 pM concentrations of either 125I-Sar1 Ang II or 125I-Sar1,Ile8 Ang II in the presence or absence of 3 μM Ang II to define non-specific binding, except that 10 μM PD123319 was present to saturate AT2 receptors, such that only AT1 receptors were able to bind the radioligands. Densitometric analysis of radioligand binding to rat brain regions was carried out on scanned images of the autoradiography films using MCID (Interfocus Imaging, Ltd.). Radioactive exposure was calibrated for using a set of iodine-125 standards (ARI 0133, American Radiolabeled Chemicals).
To assess radioligand binding in the paraventricular hypothalamus (PVN) and subfornical organ (SFO), a freehand line circumscribing the region of high binding density corresponding to the brain region as seen in an adjacent thionin stained section was drawn. For measurement of radioligand binding to the median preoptic nucleus (MnPO), a rectangular sampling tool corresponding to the histologically identified MnPO at Bregma was used. The circumscribed area was assessed densitometrically for average optical density and scan area. Four (PVN, MnPO) or three (SFO) successive brain sections were measured for each brain for total binding and non-specific binding. The average specific binding density, scan area, and specific binding times area values were determined and recorded for each brain. Measurements of radioligand binding were determined without knowledge of the treatment groups to minimize subjective error.
5.6 Statistical analysis
Comparison of Bmax (fmoles/mg initial wet weight) and Kd (unit) values between groups were made using repeated measures two-way analysis of variance (ANOVA) for radioligand and treatment (Ang II or vehicle). Pairing was made for radioligand and treatment since the membrane homogenate for each brain was evaluated with both 125I-Sar1 Ang II and 125I-Sar1, Ile8 Ang II. One desensitized and one control brain were analyzed at the same time. Comparisons of radioligand binding to the PVN, MnPO and SFO were also made using a repeated measures two-way ANOVA for radioligand. Pairing was made for radioligand since adjacent sections for each brain were evaluated with both 125I-Sar1 Ang II and 125I-Sar1, Ile8 Ang II. Values are presented as mean ± S.E.M. Significance level was set at p<0.05.
Supplementary Material
Highlights.
Brain AT1 agonist and antagonist binding was reduced by repeated ICV Ang II treatment
The reduced binding correlates with reduced water intake to ICV Ang II
The reduced binding does not correlate with saline intake to ICV Ang II
Acknowledgments
This research was supported by NIH grants HL-091911 (DD) and HL-113905 (RCS), and Nova Southeastern University Chancellor’s Faculty Research Development Grant (RCS, DD, LG-R, JS). We thank Malaika Jean-Baptiste for graphical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhat GJ, Baker KM. Angiotensin II stimulates rapid serine phosphorylation of transcription factor Stat3. Mol Cell Biochem. 1997;170:171–176. doi: 10.1023/a:1006865721939. [DOI] [PubMed] [Google Scholar]
- Billet S, et al. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–25. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde MM, et al. Biased signaling of the angiotensin II type 1 receptor can be mediated through distinct mechanisms. PLoS One. 2010;5:e14135. doi: 10.1371/journal.pone.0014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RW, et al. Arousal of a specific and persistent sodium appetite in the rat with continuous intracerebroventricular infusion of angiotensin II. J Physiol. 1980;301:365–382. doi: 10.1113/jphysiol.1980.sp013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JK, Campanile CP, Garrison JC. The hepatic angiotensin II receptor II. Effect of guanine nucleotides and interaction with cyclic AMP production. J Biol Chem. 1982;257:4959–4965. [PubMed] [Google Scholar]
- Daniels D, et al. Divergent behavioral roles of angiotensin receptor intracellular signaling cascades. Endocrinology. 2005;146:5552–5560. doi: 10.1210/en.2005-0774. [DOI] [PubMed] [Google Scholar]
- Daniels D, Yee DK, Fluharty SJ. Angiotensin II receptor signalling. Exp Physiol. 2007;92:523–7. doi: 10.1113/expphysiol.2006.036897. [DOI] [PubMed] [Google Scholar]
- Daniels D, et al. Angiotensin II stimulates water and NaCl intake through separate cell signalling pathways in rats. Exp Physiol. 2009;94:130–137. doi: 10.1113/expphysiol.2008.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D. Alan [corrected] N. Epstein award: Intracellular signaling and ingestive behaviors. Physiol Behav. 2010;100:496–502. doi: 10.1016/j.physbeh.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YH, et al. Unconventional homologous internalization of the angiotensin II type-1 receptor induced by G-protein-independent signals. Hypertension. 2005;46:419–25. doi: 10.1161/01.HYP.0000172621.68061.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H, Baukal A, Catt KJ. Angiotensin II receptors in bovine adrenal cortex. J Biol Chem. 1974;249:664–666. [PubMed] [Google Scholar]
- Glowinski J, Iversen LI. Regional studies of catecholamines in the rat brain. I the disposition of (3 H)norepinephrine, ( 3 H)dopamine, and ( 3 H)DOPA in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Hein L, et al. Intracellular trafficking of angiotensin II and its AT(1) and AT(2) receptors: Evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- Hunter WM, Greenwood FC. Preparation of iodine-131 labeled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kodama H, et al. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ Res. 1998;82:244–50. doi: 10.1161/01.res.82.2.244. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Mullikin D, Caron MG. Regulation of beta-adrenergic receptors by guanyl-5′-yl imidodiphosphate and other purine nucleotides. J Biol Chem. 1976;251:4686–92. [PubMed] [Google Scholar]
- Maguire ME, Van Arsdale PM, Gilman AG. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976;12:335–339. [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Moellenhoff E, et al. Effect of repetitive icv injections of ANG II on c-Fos and AT(1)-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1095–R1104. doi: 10.1152/ajpregu.2001.280.4.R1095. [DOI] [PubMed] [Google Scholar]
- Pereira DT, Menani JV, De Luca LA., Jr FURO/CAP: a protocol for sodium intake sensitization. Physiol Behav. 2010;99:472–81. doi: 10.1016/j.physbeh.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Poitras M, et al. Effect of uncoupling agents on AT(1) receptor affinity for antagonist analogs of angiotensin II. Receptors & Channels. 1998;6:65–72. [PubMed] [Google Scholar]
- Quirk WS, Wright JW, Harding JW. Tachyphylaxis of dipsogenic activity to intracerebroventricular administration of angiotensins. Brain Res. 1988;452:73–8. doi: 10.1016/0006-8993(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Rodbell M, et al. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. Journal of Biological Chemistry. 1971;246:1872–1876. [PubMed] [Google Scholar]
- Speth RC, Kim KH. Discrimination of two angiotensin II receptor subtypes with a selective analogue of angiotensin II, p-aminophenylalanine 6 angiotensin II. Biochemical And Biophysical Research Communications. 1990;169:997–1006. doi: 10.1016/0006-291x(90)91993-3. [DOI] [PubMed] [Google Scholar]
- Speth RC, et al. A comparison of brain angiotensin II receptors during lactation and diestrus of the estrous cycle in the rat. Am J Physiol. 1999;277:R904–R909. doi: 10.1152/ajpregu.1999.277.3.R904. [DOI] [PubMed] [Google Scholar]
- Speth RC, Harding JW. Radiolabeling of angiotensin peptides. Angiotensin Protocols. In: Wang DH, editor. Methods in Molecular Medicine. Vol. 51. Humana Press; Totowa NJ: 2001. pp. 275–295. [DOI] [PubMed] [Google Scholar]
- Thomas WG, et al. Phosphorylation of the angiotensin II (AT1A) receptor carboxyl terminus: a role in receptor endocytosis. Mol Endocrinol. 1998;12:1513–24. doi: 10.1210/mend.12.10.0179. [DOI] [PubMed] [Google Scholar]
- Torsoni MA, et al. Angiotensin II (AngII) induces the expression of suppressor of cytokine signaling (SOCS)-3 in rat hypothalamus - a mechanism for desensitization of AngII signaling. J Endocrinol. 2004;181:117–28. doi: 10.1677/joe.0.1810117. [DOI] [PubMed] [Google Scholar]
- Vento PJ, Daniels D. Repeated administration of angiotensin II reduces its dipsogenic effect without affecting saline intake. Exp Physiol. 2010a;95:736–745. doi: 10.1113/expphysiol.2010.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento PJ, Daniels D. Repeated administration of angiotensin II reduces its dipsogenic effect without affecting saline intake. Exp Physiol. 2010b;95:736–45. doi: 10.1113/expphysiol.2010.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento PJ, Daniels D. Mitogen-activated protein kinase is required for the behavioural desensitization that occurs after repeated injections of angiotensin II. Exp Physiol. 2012;97:1305–14. doi: 10.1113/expphysiol.2012.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento PJ, Myers KP, Daniels D. Investigation into the specificity of angiotensin II-induced behavioral desensitization. Physiol Behav. 2012;105:1076–81. doi: 10.1016/j.physbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento PJ, Daniels D. The anteroventral third ventricle region is critical for the behavioral desensitization caused by repeated injections of angiotensin II. Behav Brain Res. 2014;258:27–33. doi: 10.1016/j.bbr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.