Abstract
Background
Olaparib is an oral poly(ADP-ribose) polymerase inhibitor and cediranib is an oral anti-angiogenic with activity against VEGFR-1, 2, and 3. Both agents have antitumor activity in women with recurrent ovarian cancer, and the combination of these agents was active and had manageable toxicities in a Phase 1 trial. We asked whether the combination of cediranib and olaparib could improve progression-free survival compared to olaparib monotherapy in women with recurrent platinum-sensitive ovarian cancer.
Methods
We conducted a randomized, open-label, phase 2 study to evaluate the activity of olaparib monotherapy compared with combination cediranib and olaparib in women with ovarian cancer with measurable platinum-sensitive, relapsed, high-grade serous or endometrioid disease or those with deleterious germline BRCA1/2 mutations (gBRCAm). Patients were randomized using permuted blocks within stratum defined by gBRCA status and prior anti-angiogenic therapy to receive olaparib capsules 400mg twice daily or the combination at the recommended phase 2 dose of cediranib 30mg daily and olaparib capsules 200mg twice daily. The primary endpoint was progression-free survival (PFS) analyzed under intention to treat. The trial is registered with ClinicalTrials.gov, NCT01116648. The Phase 2 portion of the trial reported here is no longer accruing patients.
Findings
Forty-six of 90 randomized patients received olaparib alone, and 44 received cediranib/olaparib. Median PFS was significantly longer with cediranib/olaparib (17.7 vs. 9.0 mos, HR 0.42; p = 0.005). Grade 3 and 4 adverse events were more common with cediranib/olaparib, including fatigue (12 vs. 5), diarrhea (10 vs. 0), and hypertension (18 vs. 0). Subset analysis within stratum defined by BRCA1/2 status demonstrated activity of cediranib/olaparib in both gBRCAm and gBRCAwt/u (wild-type/unknown) patients. Significant improvement in PFS occurred in gBRCAwt/u women receiving cediranib/olaparib (16.5 vs. 5.7 mos, p = 0.008) with a smaller trend towards increased PFS in gBRCAm patients (19.4 vs. 16.5 mos, p = 0.16).
Interpretation
The combination of cediranib and olaparib significantly extended PFS by 8.7 months compared to olaparib alone in recurrent platinum-sensitive ovarian cancer. The activity observed with this oral combinaton in both gBRCAmt and gBRCAwt/u patients is encouraging and should be further explored as a potential alternative to cytotoxic chemotherapy. Given the side effect profile, such explorations should include assessments on quality of life and patient-reported outcomes to understand the effects of an ongoing oral regimen to that of intermittent chemotherapy.
Ovarian cancer is the leading cause of death from gynecologic malignancy in the United States, with approximately 22,000 new diagnoses and 14,000 deaths per year (1). Although response rates to initial treatment are high, most patients will experience disease relapse (2). New therapeutic directions that increase cure rate, prolong life, and do so in the most tolerable fashion are needed; targeted therapeutics have promise to fill that need.
Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) are a promising class of drugs that exhibit activity against ovarian cancer. Inhibition of PARP by these drugs leads to the accumulation of double-stranded DNA breaks that cannot be accurately repaired, resulting in synthetic lethality in cells that are deficient in homologous recombination (3, 4). Homologous recombination deficiency has been described in up to 50% of patients with high-grade serous ovarian cancers (HGSOC) with approximately 17% of newly diagnosed women having deleterious germline BRCA1/2 mutations (gBRCAm) or other defects in the homologous recombination pathway (5, 6). Clinical development of PARP inhibition in ovarian cancer is of particular interest.
Olaparib is a potent, orally-available PARPi, with documented activity against active disease in gBRCAm-associated ovarian cancer and breast cancer seen in both Phase 1 (7, 8) and Phase 2 (9–11) studies. Activity has been demonstrated in germline wild-type BRCA1/2 (gBRCAwt) ovarian cancer, with a response rate of 24% (10). In addition, remarkable benefit was demonstrated in a randomized Phase 2 study of olaparib versus placebo administered as maintenance after completion of platinum-based chemotherapy to women with platinum-sensitive HGSOC. The median progression-free survival (PFS) increased from 4.8 months to 8.4 months in the cohort as a whole (HR 0.35, p = 0.001) (12). This difference was more striking in a group including gBRCAm patients and those with somatic BRCA1/2 mutations, in which median PFS was increased from 4.3 months to 11.2 months (HR 0.18, p < 0.00001) (13).
Angiogenesis inhibitors are active in recurrent ovarian cancer (14, 15). Cediranib is an oral ATP-competitive tyrosine kinase inhibitor of vascular endothelial growth factor receptors (VEGFR)-1/2/3 that has produced a 17% response rate in relapsed ovarian cancer (16). Ledermann et al. reported on a Phase 3 study in women with platinum-sensitive recurrent ovarian cancer wherein combining cediranib together with platinum-based chemotherapy followed by cediranib maintenance therapy significantly increased both PFS and overall survival (OS) over platinum-based chemotherapy alone (17).
Angiogenesis inhibitors combined with PARPi demonstrated supra-additive effects in preclinical studies. In vivo anti-angiogenic activity has been observed with PARPi and in PARP-1 knockout mice (18). Downregulation of homologous recombination repair genes, such as BRCA1 and RAD51, occurs under hypoxia, with enhancement of PARPi sensitivity in the hypoxic setting (19, 20). A recent study also demonstrated that VEGFR3 inhibition results in downregulation of both BRCA1 and BRCA2 in cancer cells (21). We observed pre-clinical synergy between olaparib and cediranib in inhibiting both ovarian cancer cell invasion and microvascular endothelial cell tube formation in vitro (unpublished data). Promising preliminary activity against recurrent ovarian cancer was seen in our Phase 1 dose-finding trial of the combination of cediranib and olaparib, which yielded an objective response rate of 44% (22).
In this study, we therefore evaluated the efficacy and toxicity of the combination of cediranib and olaparib compared to olaparib alone in platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancers.
PATIENTS AND METHODS
Study Design and Oversight
This randomized, open-label, phase 2 trial was performed at nine participating US institutions. Oversight of the study was provided by the Dana-Farber / Harvard Cancer Center (DFHCC) Data and Safety Monitoring Board which reviewed the study progress twice yearly. Patients were registered and randomized by the DFHCC Quality Assurance Office for Clinical Trials (QACT) in a 1:1 ratio to olaparib capsule monotherapy 400mg orally twice daily or a previously established recommended Phase 2 dose of cediranib 30mg orally daily with olaparib capsules 200mg orally twice daily (22), using permuted blocks within stratum defined by gBRCAm status (vs. gBRCAwt vs. germline BRCA1/2 unknown [gBRCAu]) and by receipt of prior anti-angiogenic therapy in the first-line setting. Patients continued on study treatment until disease progression, defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines; meeting criteria for discontinuation (grade 3 or 4 study-related events that did not resolve to grade 1 or less within 14 days, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events [CTCAE], version 4.0); or treatment discontinuation for other reasons (patient withdrawal, physician discretion). Dosing could be interrupted for up to 14 days for study-related toxicities. For patients on olaparib monotherapy, two dose reductions of olaparib were allowed, to 200mg twice daily and 100mg twice daily. For patients on the cediranib/olaparib combination arm, dose reductions of cediranib to 20mg and 15mg daily were allowed, and olaparib dose reductions to 150mg twice daily and 100mg twice daily were allowed. Patients in the combination arm could have dosing interruptions or reductions of either drug independently of one another, pending on the attribution of the toxicity. Patients on the olaparib monotherapy arm were not permitted to cross over to combination cediranib/olaparib after disease progression.
Patient Eligibility
Eligibility criteria included age ≥18 years, ECOG performance status 0 or 1, estimated life expectancy >6 months, and recurrent high-grade serous or endometrioid epithelial ovarian, fallopian tube, or primary peritoneal cancer. gBRCAm patients with any high-grade histology were considered eligible. Patients were required to have platinum-sensitive disease, defined as disease recurrence >6 months after the last receipt of platinum and no evidence of progression on a prior platinum-containing regimen. Patients could have received an unlimited number of prior platinum-based chemotherapies, but no more than one non-platinum therapy in the recurrent setting. Patients were also required to have measurable disease by RECIST 1.1 criteria. Prior antiangiogenic agents were permitted only in the front-line (including maintenance) setting. Patients were excluded if they had previously received a PARPi. Prior hormonal treatment was allowed and not considered as a line of non-platinum therapy. Ability to comply with an oral medication regimen was required. Patients had to have normal organ and marrow function (as demonstrated by absolute neutrophil count ≥1500/mcL; platelets ≥100,000/mcL, hemoglobin > 9g/dL; total bilirubin within 1.5× the institutional upper limit of normal [ULN]; aspartate aminotransferase and alanine aminotransferase ≤ 2.5× ULN; creatinine ≤ ULN or a creatinine clearance ≥60mL/min/1.73m2 for subjects with creatinine levels > ULN; ≤1+ proteinuria on two consecutive dipsticks taken no less than 1 week apart, <1gm protein on 24-hour urine collection, or a urine protein:creatinine ration <1; troponin T or I within institutional limits; international normalized ratio and activated partial thromboplastin time ≤1.25x ULN), controlled hypertension (<140/90) on no more than 3 anti-hypertensive agents, and no history of hypertensive crisis, abdominal fistula or perforation, signs or symptoms of bowel obstruction within the last 3 months, or major surgical procedure within the prior 28 days. The study protocol was approved by the institutional review board at each participating site, and written informed consent was obtained from all participating patients.
Safety and Toxicity Evaluations
Serum chemistries, hematologic studies, and assessment for proteinuria were performed every two weeks for the first eight weeks and subsequently every four weeks. All patients receiving cediranib were provided with a blood pressure cuff and were required to take and document their blood pressure twice daily. Patients were instructed to contact the study staff immediately for any blood pressure reading above 140/90. Additionally, all patients received weekly phone calls from study staff to review any ongoing toxicities for the first 8 weeks of study treatment.
Study Assessments and Endpoints
The primary endpoint of the study was PFS, as assessed by the site investigator. Assessments for tumor change were conducted by imaging every 8 +/−1 weeks by CT or MRI. PFS was defined as time from randomization to radiographic progression by RECIST 1.1 criteria or death from any cause. Patients who were alive without evidence of progression at the time of the reported analysis or were removed from the study due to toxicity, withdrawal of consent, or other cause were censored at the time of last disease assessment. Secondary endpoints included objective response rate (ORR), defined per the best confirmed RECIST response; toxicity, as graded by CTCAE version 4.0 criteria, and OS. Compliance was calculated by the ratio of the total administered dose to the total prescribed dose, as assessed through an independent National Cancer Institute-sponsored audit.
Statistical Analysis
The primary objective was to assess the efficacy of the combination of cediranib and olaparib compared to olaparib alone, and was evaluated under a stratified log-rank test using a one-sided type I error of 0.10. Target accrual was 90 patients, expecting 82 (90%) to be evaluable. This provided 86% power to detect a hazard ratio (HR) of 0.57 at 71 PFS events, and 80% power to see an improvement in response from 20% to 50%. A single interim analysis was planned at 36 PFS events to stop for futility for an estimated HR ≥ 1.0. Activity was based upon intention-to-treat, and safety analyses included all patients who received at least one dose of study medication. Time-to-event endpoints were summarized by Kaplan Meier estimates, and contrasts of response rates and toxicity used Fisher’s exact tests. An unplanned exploratory analysis was conducted in gBRCAm and gBRCAwt/u subgroups. All analyses were conducted in the intent-to-treat manner according to randomized treatment assignment. Estimates are reported with 95% confidence intervals, and all statistical analyses were performed using SAS v9.2 (Cary, NC). This trial is registered with ClinicalTrials.gov, number NCT01116648.
Role of the Funding Source
The study was designed by the first and last authors in collaboration with the study sponsor, the National Cancer Institute / Cancer Therapy Evaluation Program (NCI/CTEP). The study sponsor had no role in the collection, analysis, or interpretation of data and was not involved in the writing of the report. Study drug was provided by AstraZeneca through a cooperative research and development agreement (CRADA) with the NCI. Data collection and analysis were performed by the lead study site. JFL, WTB, and WL had access to the raw data of the study. The corresponding author had full access to all of the data and had final responsibility to submit for publication.
RESULTS
Patient Characteristics
Between October 2011 and June 2013, 90 patients were enrolled to the study; 46 were randomized to olaparib and 44 to cediranib/olaparib (CONSORT diagram, Figure 1). The treatment groups were generally balanced across baseline characteristics, although 63% of patients in the olaparib arm had received >1 prior line of therapy compared to 41% in the combination arm (p = 0.06), and there was a trend towards slightly higher baseline CA125 levels in the olaparib arm (Table 1). Forty-seven women were gBRCAm, of whom 24 were randomized to olaparib monotherapy and 23 to combination treatment.
Figure 1.
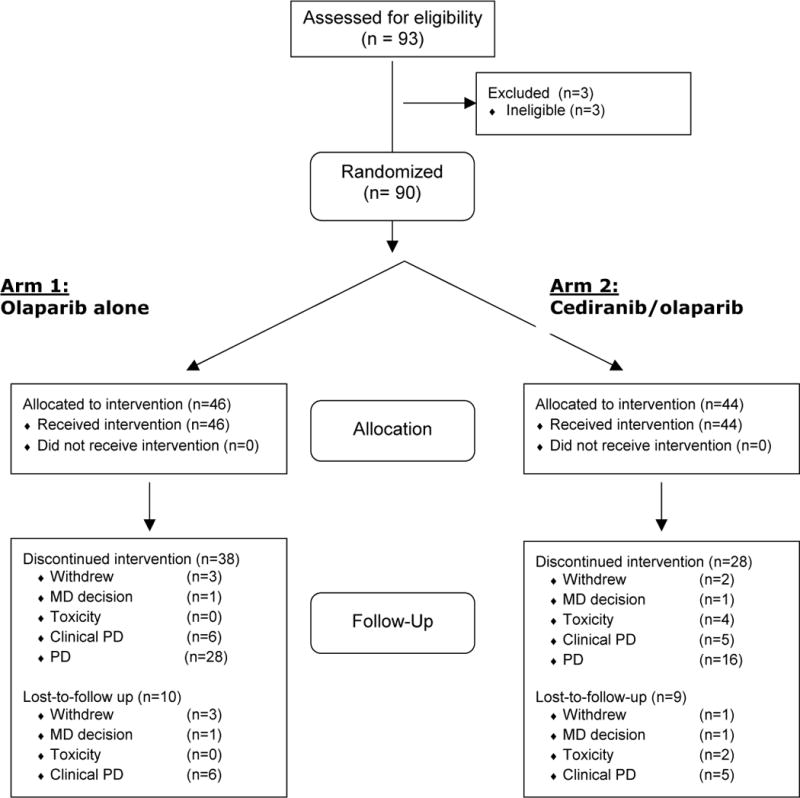
CONSORT diagram
Table 1.
Patient Baseline Characteristics
| Olaparib (N=46) | Cediranib/olaparib (N=44) | p-value | |
|---|---|---|---|
| Age, median (range) | 58.1 (32.7–81.9) | 57.8 (41.9–85.6) | 0.33 |
| ECOG performance status 0 1 |
34 (73.9%) 12 (26.1%) |
31 (70.5%) 13 (29.5%) |
0.82 |
|
BRCA mutation status Mutation carrier Non-carrier Unknown |
24 (52.2%) 11 (23.9%) 11 (23.9%) |
23 (52.3%) 12 (27.3%) 9 (20.5%) |
0.92 |
| Prior anti-angiogenic therapy No Yes |
40 (87.0%) 6 (13.0%) |
38 (86.4%) 6 (13.6%) |
1.00 |
| Prior platinum-free interval 6–12 months >12 months |
26 (56.5%) 20 (43.5%) |
23 (52.3%) 21 (47.7%) |
0.83 |
| Number of prior lines 1 2 3+ |
17 (37.0%) 18 (39.1%) 11 (23.9%) |
26 (59.1%) 10 (22.7%) 8 (18.2%) |
0.11 |
| Baseline CA125 (range) | 115.3 (10.9–11,512.0) | 68.0 (4.0–1,351.0) | 0.08 |
P-values are from Fisher’s exact test for categorical factors and Wilcoxon rank sum test for continuous factors.
Activity
Interim analysis occurred in November 2013 at 50% of expected events, at which time the DFHCC DSMB recommended unblinding of study analysis and release of data. The cutoff date for the primary analysis was March 31, 2014, when a total of 47 PFS events had been observed (yielding 74% power to detect HR = 0.75) and the median length of follow-up in surviving patients was 16.6 months (IQR: 12.1–22.2). Median (95%CI) PFS by arm was 9.0 (5.7–16.5) and 17.7 (14.7-Inf) months for the olaparib and cediranib/olaparib arms, respectively (HR 0.418, 95% CI 0.229–0.763, p = 0.005; Figure 2).
ORR, a secondary objective, was 47.8% with single agent olaparib compared to 79.6% with cediranib/olaparib (OR = 4.24, 95% CI 1.53–12.22, p = 0.002). Best overall responses on single agent olaparib included 2 complete responses (CR), 20 partial responses (PR), 19 stable disease (SD), and 1 progressive disease (PD). Responses on the combination arm included 5 CR, 30 PR, 8 SD, and no primary progression events. Six CRs occurred in gBRCAm women; 1 CR occurred in a gBRCAwt woman on the combination arm. Overall survival data are not mature; a total of sixteen deaths have occurred, 10 with olaparib monotherapy and 6 with cediranib/olaparib. The rate of overall survival at 24 months was 0.65 (95% CI 0.42–0.81) in the olaparib arm and 0.81 (95% CI 0.60–0.91) in the combination arm.
A post-hoc exploratory analysis for PFS and response rate was performed in subgroups divided by gBRCAm (n=47) vs. gBRCAwt/u (n=43) status (Figure 3). Increased activity of the cediranib/olaparib combination compared to olaparib monotherapy was observed in the gBRCAwt/u patients with an increase in median (95% CI) PFS from 5.7 (5.3–11.2) to 16.5 (10.8-Inf) months (HR 0.32, p = 0.008) and an increase in ORR from 32% to 76% (p = 0.006). In gBRCAm patients, there was a lesser trend towards increased activity with the combination, with increased PFS from 16.5 (7.5–20.1) to 19.4 (14.7-Inf) months (HR = 0.55, p=0.16) and increased ORR from 63% to 84% (p=0.19).
Safety and Compliance
Eighty-seven of the 90 patients enrolled to the study experienced at least one drug-related adverse event (AE). Drug-related AEs were more common in the cediranib/olaparib arm, with 70% of patients experiencing a Grade 3 or higher event. Drug-related grade 2 or higher non-hematologic AEs with an incidence of at least 10% in either arm and overall hematologic AEs are shown in Table 2. Grade 3 or higher AEs occurring more frequently in the cediranib/olaparib arm included diarrhea (23% vs. 0%, p = 0.0004), fatigue (27% vs. 11%, p = 0.06), and hypertension (41% vs. 0%, p<0.0001). Grade 3 hypertension is defined by CTCAE as requiring two or more drugs for hypertension control; 34% of patients in the cediranib/olaparib arm had baseline hypertension. Two grade 4 events occurred, both on the cediranib/olaparib arm; one was hypertensive crisis during the 1st cycle of therapy in a patient who was non-compliant with anti-hypertensive medications and was in part study-related. The second case was myelodysplastic syndrome (MDS) occurring during the 14th cycle of therapy, possibly study-related.
Table 2.
Treatment-related Adverse Events
| Adverse Event | Olaparib alone (N = 46) | Cediranib/Olaparib (N = 44) | ||||||
|---|---|---|---|---|---|---|---|---|
| Maximum Grade | Maximum Grade | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Non-Hematologic | ||||||||
| Hypertension | – | – | – | – | 2 (5) | 15 (34) | 17 (39) | 1 (2) |
| Diarrhea | 1 (2) | – | – | – | 11 (25) | 20 (45) | 10 (23) | – |
| Fatigue | 14 (30) | 7 (15) | 5 (11) | – | 14 (32) | 12 (27) | 12 (27) | – |
| Nausea | 22 (50) | 12 (26) | – | – | 23 (52) | 7 (16) | 2 (5) | – |
| Headache | 4 (9) | – | – | – | 13 (30) | 4 (9) | 2 (5) | – |
| Hypothyroidism | – | 1 (2) | – | – | 1 (2) | 6 (14) | – | – |
| Hematologic | ||||||||
| Anemia | 6 (13) | 2 (4) | – | – | 6 (14) | 1 (2) | – | – |
| Neutrophil count decreased | 1 (2) | 4 (9) | – | – | 1 (2) | 2 (5) | – | – |
| WBC decreased | 1 (2) | 3 (7) | – | – | 3 (7) | 2 (5) | – | – |
| Platelet decreased | 3 (7) | – | – | – | 5 (11) | 1 (2) | – | – |
Compliance with the planned study treatment was evaluated in 51 of 90 patients, and the observed rate was 97%, inclusive of dose modifications. Among all patients, more dose reductions (77% vs. 24%) occurred on the cediranib/olaparib arm. All patients in the cediranib/olaparib arm with a dose reduction had at least one dose reduction of cediranib; 23% of patients in the cediranib/olaparib arm also had a dose reduction of olaparib. Four patients were withdrawn from study treatment for toxicity, all on the cediranib/olaparib arm for 1 event each of MDS, persistent weight loss, recurrent avascular necrosis of the hips, and new rectovaginal fistula. Other reasons for discontinuation of study treatment without a PFS event were balanced between arms, including withdrawal of consent (3 olaparib, 2 cediranib/olaparib), investigator decision (1 in each arm), and clinical progression (6 olaparib, 5 cediranib/olaparib). There were no treatment-related deaths.
DISCUSSION
This is the first Phase 2 study to evaluate the efficacy of a PARPi/anti-angiogenic combination. In this study, the combination of cediranib and olaparib significantly improved median PFS and ORR over olaparib monotherapy in women with platinum-sensitive HGSOC, with an 8.7 month improvement in median PFS and a 32% increase in ORR.
These results suggest that the combination of a PARPi and an anti-angiogenic may synergize to result in increased activity in patients with platinum-sensitive HGSOC, as compared to either agent alone, consistent with hypotheses generated by pre-clinical data,. The median PFS of single-agent olaparib in this study was 9.0 months. There are limited data of PFS and RR in a similar population to this trial. Phase 2 studies that have included olaparib monotherapy have reported median PFS intervals of between 6.4 and 8.8 months, although it should be noted that these trial populations have differed significantly from the patients enrolled in this trial (10, 23). However, the similarity of the observed PFS to other Phase 2 olaparib monotherapy results suggests that the improved clinical activity of cediranib/olaparib observed in this trial is less likely to be secondary to underperformance of the olaparib only arm. Although this trial does not include a single-agent cediranib arm, the median PFS reported (both in the overall study population and in the platinum-sensitive population) in a Phase 2 study of single-agent cediranib in recurrent ovarian cancer was 5.2 months (16). A second Phase 2 study preliminarily reported an overall 4.1 month median time to progression (24). Taken together, these two Phase 2 studies of single-agent cediranib also suggest a degree of activity in recurrent ovarian cancer that is less than that observed with the combination of cediranib and olaparib in this study.
Fatigue, diarrhea, and hypertension were the most common grade 3 or higher AEs which occurred with greater frequency in the cediranib/olaparib combination arm. This was consistent with the results seen in the Phase 1 study (22). Although hypertension was the most common grade 3 or higher AE, the CTCAE grading reflects the number of blood pressure medications needed for management; for patients on the study, blood pressure elevations were generally asymptomatic, except for a case of grade 4 hypertension in the setting of non-compliance with anti-hypertensive medications. Fatigue and diarrhea were the most notable symptoms in the combination cediranib/olaparib arm, and dose reductions were required in over three quarters of patients. Formal quality of life and patient-reported outcomes were not collected on this study, and therefore, we were not able to quantify the impact of these side effects on the patient’s overall experience. However, patient-reported outcomes should be measured in any future studies of this combination. In general, patients were able to remain on study with close management and dose reductions as necessary, and withdrawal from study participation did not differ significantly between the two arms. Nonetheless, the observed toxicities with the cediranib/olaparib combination emphasize the necessity for aggressive symptom management, including careful monitoring and management of blood pressure and patient education, and for examination of patient-related outcomes and preferences in future explorations of the cediranib/olaparib combination. In comparison, significant toxicities with platinum-based doublets have most frequently included grade 3 or higher hematologic toxicities, reported in 20 to 80% of patients, as well as grade 2 or higher non-hematologic toxicities such as fatigue, nausea, and neuropathy (25–28). Of note, although no bowel perforation events were observed, this trial specifically excluded patients with signs or symptoms of bowel obstruction within the prior 3 months.
Limitations of this study include that it is a Phase 2 study with a limited sample size and was powered for PFS and not OS. Additionally, there was a trend towards increased prior therapy and higher baseline CA125 in the olaparib only arm; however, a multivariate model incorporating these variables as well as the stratification factors demonstrated a continued statistically significant PFS benefit to the combination arm (Supplemental Table 1). Despite these limitations, the results of this study suggest a cediranib and olaparib combination should be studied further in ovarian cancer. One possible setting might be the consideration of cediranib/olaparib as an alternative to chemotherapy in platinum-sensitive disease. Standard recommendations for treatment of recurrent platinum-sensitive disease, typically defined as recurrence within 6 months of last receipt of platinum, include use of additional platinum-based chemotherapy (29). Randomized Phase 3 clinical trials have demonstrated that platinum-based doublets such as carboplatin and paclitaxel, carboplatin and gemcitabine, or carboplatin and PLD are effective therapies in this setting, with median PFS ranging in the 8 to 13 month range (25–28). However, retreatment with platinum-based chemotherapy poses certain risks, including exacerbation of residual neuropathy from initial treatment, enhanced myelosuppression, or onset of hypersensitivity reactions to platinum. Moon and colleagues identified a higher frequency of platinum hypersensitivity reactions at a lower net exposure in gBRCAm patients (30). Use of the cediranib/olaparib combination in lieu of platinum-containing chemotherapy would potentially reduce this risk while prolonging the platinum-free interval. As the point estimate of median PFS in this study compares favorably to that seen with platinum-based therapy, a comparison of the cediranib/olaparib regimen as an alternative to platinum-based chemotherapy would be of potential interest. Similarly, given the improvement in PFS observed with both cediranib maintenance therapy (following platinum-based chemotherapy in combination with cediranib) and olaparib maintenance therapy in the setting of recurrent platinum-sensitive ovarian cancer (12, 17), exploration of the cediranib/olaparib combination as maintenance therapy would also be of interest.
The observation that patients without a known deleterious germline BRCA mutation had a greater benefit from the combination of cediranib and olaparib compared to olaparib alone raises some interesting questions. As might be expected, patients with gBRCAwt/u status appeared to have a reduced PFS with olaparib monotherapy compared to gBRCAmt patients (5.7 vs. 16.5 mos), but derived a significantly increased comparative PFS benefit with the addition of cediranib. This unplanned exploratory analysis suggests that the cediranib/olaparib combination may provide significant marginal benefit even in the absence of BRCA1/2 loss. This difference may reflect greater synergism between these two drugs in the setting of more homologous recombination proficient tumors or hypoxia. These subset analyses should be interpreted with caution as the number of patients is small, and especially as the 16.5 month PFS observed on olaparib only for gBRCAmt patients may represent an overperformance of this arm. However, the magnitude of observed benefit provides a rationale for further exploration and validation of the activity of this combination in the gBRCAwt/u populations.
In conclusion, combination cediranib and olaparib had significantly increased clinical activity, as measured by PFS and ORR, compared to olaparib alone in platinum-sensitive HGSOC. The activity of the combination in this setting supports further exploration of cediranib and olaparib together in ovarian cancer.
Supplementary Material
Research in Context.
Systematic Review
To identify prior experience with combination of antiangiogenics and PARP inhibitors, we searched PubMed and abstracts presented at recent international clinical oncology meetings. Search terms included “anti-angiogenics”, “PARP inhibitors”, and “ovarian,” without date or language limits. Careful review of identified articles and abstracts demonstrated that preclinical data suggested possible activity for a combination of these agents, although clinical experience with anti-angiogenic/PARP inhibitor combinations was limited to two Phase 1 trials.
Interpretation
To our knowledge, this is the first Phase 2 study to explore the activity of an anti-angiogenic/PARP-inhibitor combination. In this trial, the combination of cediranib and olaparib significantly improved progression-free survival and overall response rate compared to olaparib monotherapy in a population of women with platinum-sensitive recurrent high-grade serous or endometrioid ovarian cancer. Of note, in exploratory analyses, the activity of the cediranib/olaprib combination appeared to be robust in both BRCA mutated and BRCA wild-type or unknown populations. Significant side effects were seen with the cediranib/olaparib combination, resulting in dose-reductions in over three quarters of patients. Therefore, an important consideration in further exploration of this oral combination will include measures of quality of life and patient-reported outcomes. Nonetheless, the observed progression-free survival of 17.7 months on the cediranib/olaparib combination arm compares favorably to that seen with standard-of-care chemotherapy in this setting, and raises the question of whether the cediranib/olaparib combination might be a potential alternative to chemotherapy, should these findings be confirmed in a Phase 3 setting.
Acknowledgments
This study was sponsored by the Cancer Therapy Evaluation Program of the National Cancer Institute and supported by an American Recovery and Reinvestment Act (ARRA) grant through the National Institutes of Health (3 U01 CA062490-16S2). WTB was supported by the CJL Foundation. J-ML/ECK were supported by the Intramural Program of the Center for Cancer Research (CCR), and the CCR and Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, USA, respectively. The funding source had no role in the conduct, data collection, or analysis of the study.
Funding:
American Recovery and Reinvestment Act grant through the National Institutes of Health (NIH) (3 U01 CA062490-16S2; Intramural Program of the Center for Cancer Research; and the Division of Cancer Treatment and Diagnosis, National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare they have no conflicts of interest.
Contributors
JFL, EW, ECK, PI, and UAM were involved in the design and development of the study. JFL, MB, J-ML, RB, GFF, BJR, MKB, SN, JH, PQ, CW, LO, and ECK were responsible for patient inclusion and data collection. Statistical design and analyses were performed by WTB, WL, and HL. All authors participated in the review and writing of manuscript and gave final approval to submit for publication.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014 Jan-Feb;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014 Apr 17; doi: 10.1016/S0140-6736(13)62146-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005 Apr 14;434(7035):913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 4.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005 Apr 14;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011 Jun 30;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S America. 2011 Nov 1;108(44):18032–7. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New Engl J Med. 2009 Jul 9;361(2):123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 8.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010 May 20;28(15):2512–9. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 9.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010 Jul 24;376(9737):245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 10.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011 Sep;12(9):852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 11.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010 Jul 24;376(9737):235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 12.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. New Engl J Med. 2012 Apr 12;366(15):1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 13.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014 Jul;15(8):852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 14.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007 Nov 20;25(33):5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 15.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007 Nov 20;25(33):5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 16.Matulonis UA, Berlin S, Ivy P, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009 Nov 20;27(33):5601–6. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledermann J, Perren TJ, Raja FA, et al. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: Results of the ICON6 trial. European Cancer Congress. 2013;2013 Abstr 10. [Google Scholar]
- 18.Tentori L, Lacal PM, Muzi A, et al. Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur J Cancer. 2007 Sep;43(14):2124–33. doi: 10.1016/j.ejca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Bindra RS, Gibson SL, Meng A, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005 Dec 15;65(24):11597–604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 20.Bindra RS, Schaffer PJ, Meng A, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Bio. 2004 Oct;24(19):8504–18. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. VEGFR3 Inhibition Chemosensitizes Ovarian Cancer Stemlike Cells through Down-Regulation of BRCA1 and BRCA2. Neoplasia. 2014 Apr;16(4):343–53 e2. doi: 10.1016/j.neo.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JF, Tolaney SM, Birrer M, et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013 Sep;49(14):2972–8. doi: 10.1016/j.ejca.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012 Feb 1;30(4):372–9. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 24.Hirte H, Vidal L, Fleming GF, et al. A phase II study of cediranib (AZD2171) in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: Final results of a PMH, Chicago, and California consortia trial. J Clin Oncol. 2008;26(15S) doi: 10.1016/j.ygyno.2015.04.009. Abstr 5521. [DOI] [PubMed] [Google Scholar]
- 25.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012 Jun 10;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003 Jun 21;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 27.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006 Oct 10;24(29):4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 28.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010 Jul 10;28(20):3323–9. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. NCCN Guidelines: Ovarian Cancer. Available from http://www.nccn.org. Last accessed June 10, 2014.
- 30.Moon DH, Lee JM, Noonan AM, et al. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br J Cancer. 2013 Aug 20;109(4):1072–8. doi: 10.1038/bjc.2013.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


