Abstract
Van den Ende–Gupta Syndrome (VDEGS) is an autosomal recessive disorder characterized by blepharophimosis, distinctive nose, hypoplastic maxilla, and skeletal abnormalities. Using homozygosity mapping in four VDEGS patients from three consanguineous families, Anastacio et al. [Anastacio et al. (2010); Am J Hum Genet 87:553–559] identified homozygous mutations in SCARF2, located at 22q11.2. Bedeschi et al. [2011] described a VDEGS patient with sclerocornea and cataracts with compound heterozygosity for the common 22q11.2 microdeletion and a hemizygous SCARF2 mutation. Because sclerocornea had been described in DiGeorge-velo-cardio-facial syndrome but not in VDEGS, they suggested that the ocular abnormalities were caused by the 22q11.2 microdeletion. We report on a 23-year-old male who presented with bilateral sclerocornea and the VDGEGS phenotype who was subsequently found to be homozygous for a 17 bp deletion in exon 4 of SCARF2. The occurrence of bilateral sclerocornea in our patient together with that of Bedeschi et al., suggests that the full VDEGS phenotype may include sclerocornea resulting from homozygosity or compound heterozygosity for loss of function variants in SCARF2.
Keywords: Van den Ende–Gupta syndrome, sclerocornea, SCARF2, SCARF1
INTRODUCTION
Van den Ende–Gupta syndrome (VDEGS) (OMIM 600920) is a rare, autosomal recessive disorder characterized by blepharophimosis, a characteristic nose, hypoplastic maxilla with or without cleft palate, everted lower lip, prominent ears, arachnodactyly, camptodactyly, self-limited congenital joint contractures, and skeletal abnormalities (hooked clavicles, gracile ribs, long slender bones of hands and feet, mild bowing of long bones). Growth and psychomotor development are normal [Gupta et al., 1995; Phadke et al., 1998; Schweitzer et al., 2003; Guerra et al., 2005; Carr et al., 2007; Ali et al., 2010]. To date, at least 28 patients in 19 families have been described (12 males and 16 females [Gupta et al., 1995; Phadke et al., 1998; Schweitzer et al., 2003; Guerra et al., 2005; Carr et al., 2007; Leal and Silva, 2009; Ali et al., 2010; Anastasio et al., 2010; Bedeschi et al., 2010; Patel et al., 2013]). The inheritance pattern is autosomal recessive with frequent parental consanguinity and recurrence of the disorder in sibs of unaffected parents. One exception is a kindred with three affected individuals, two brothers, and their half-sister, consistent with autosomal dominant inheritance with gonadal mosaicism [Leal and Silva, 2009]. Using homozygosity mapping in three consanguineous Qatari families, Anastasio et al. [2010] identified homozygous mutations in SCARF2 (OMIM 613619), an 11 exon gene located at 22q11.21, in four VDEGS patients.
PATIENT AND METHODS
The propositus, his unaffected parents, sister and brother were submitted to the Baylor–Hopkins Center for Mendelian Genomics (BHCMG) study through the PhenoDB online submission portal [Hamosh et al., 2013] for consideration of whole exome sequencing. Local approval for this study was provided by the Johns Hopkins Institutional Review Board, and all participants signed the appropriate informed consent form.
The propositus is a 23-year-old male who was born at 37 weeks from a G3P3 27-year-old mother and a 35-year-old father, his birth weight was 2,900 g. The first cousin parents were healthy and from a small city in rural Brazil. The patient has an unaffected sister and brother. The mother denied any illnesses or drug exposure during the pregnancy. At birth, the patient was noted to have facial anomalies including widely spaced eyes, bilateral corneal opacity, and low set and posterior rotated ears. Slender fingers, bilateral adduction of his thumbs, and camptodactyly were also present. At 4 months his length was 62.5 cm (between the 3rd and 10th centiles), weight was 4,960 g (<3rd centile) and head circumference was 40.5 cm (−2 SD). An ophthalmologic evaluation revealed bilateral sclerocornea, nystagmus, and strabismus. At 5 years his weight and height were at the 10th centile and his head circumference was at −1 SD. His facial dysmorphism was more prominent with a high and broad forehead, widely spaced eyes, short palpebral fissures, rethrognathia, underdeveloped ala nasi, low hanging columella, protruding ears, everted lower lip, pectus excavatum, slender fingers, camptodactyly, bilateral adducted thumbs, hammertoe, and overlapping toes (Fig. 1). His language development was normal but major motor development was delayed: he sat at 8 months and walked at 36 months. At age 5 years, he had mild motor developmental delay but with normal language and cognitive function.
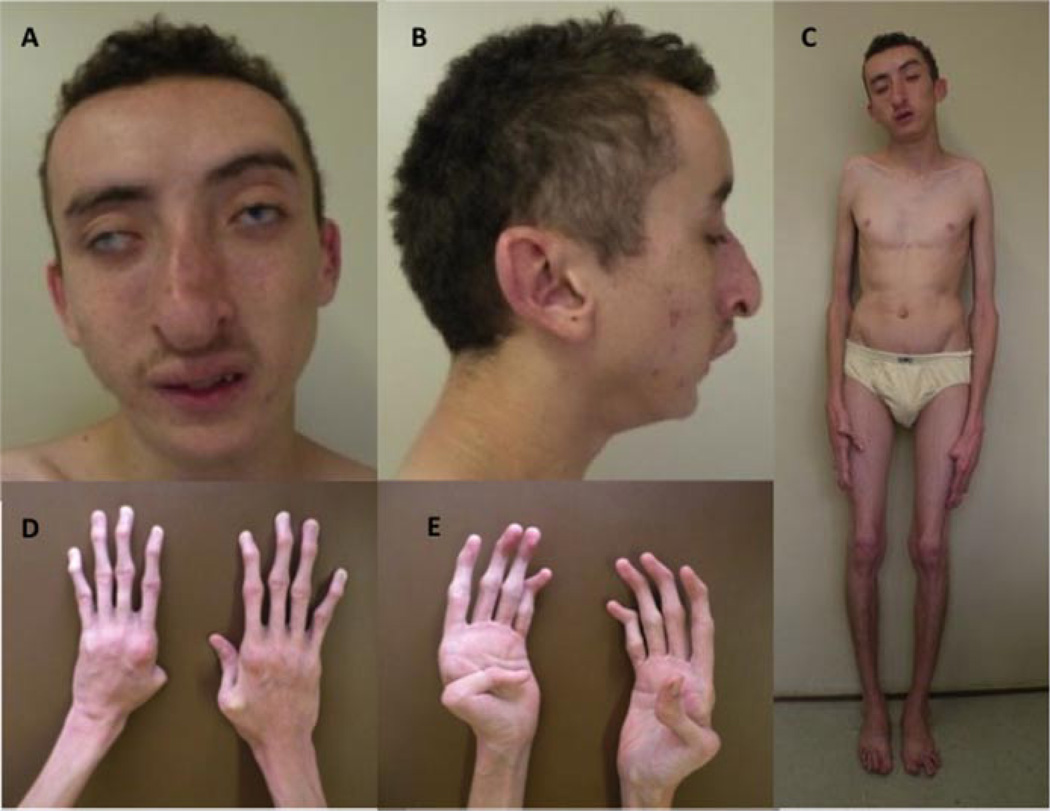
FIG. 1.
Twenty-year-old patient with bilateral sclerocornea, short palpebral fissures, underdeveloped ala nasi, low hanging columella, everted lower lip (A), brachycephaly, low-set ears and increased posterior angulation, rethrognathia (B), dolichostenomelia, genus varus (C), slender fingers, camptodactyly (D), and bilateral adducted thumbs (E).
At 21 years of age, he showed improvement of his foot contractures but worsening of thumb abduction and progression of thoracic scoliosis. His ophthalmologic evaluation revealed poor visual acuity. His sclerocornea had progressed with bilateral opaque corneas. Prosthetic ocular lenses were used for aesthetic effect. A skeletal survey showed retrognathia, mild thickening of the calvarium, sclerosis of mastoid cells, thoraco and thoraco-lumbar scoliosis, gracile ribs, narrow iliac bones, slender long bones, mild bowing of the femurs and fibulae, short ulnas bilaterally not articulating with the radii, long metatarsii, bilateral valgus deformity of the halluces and osteopenia (Fig. 2). A cardiac ultrasound and brain MRI were normal. Chromosome analysis at the 500 band level was normal.
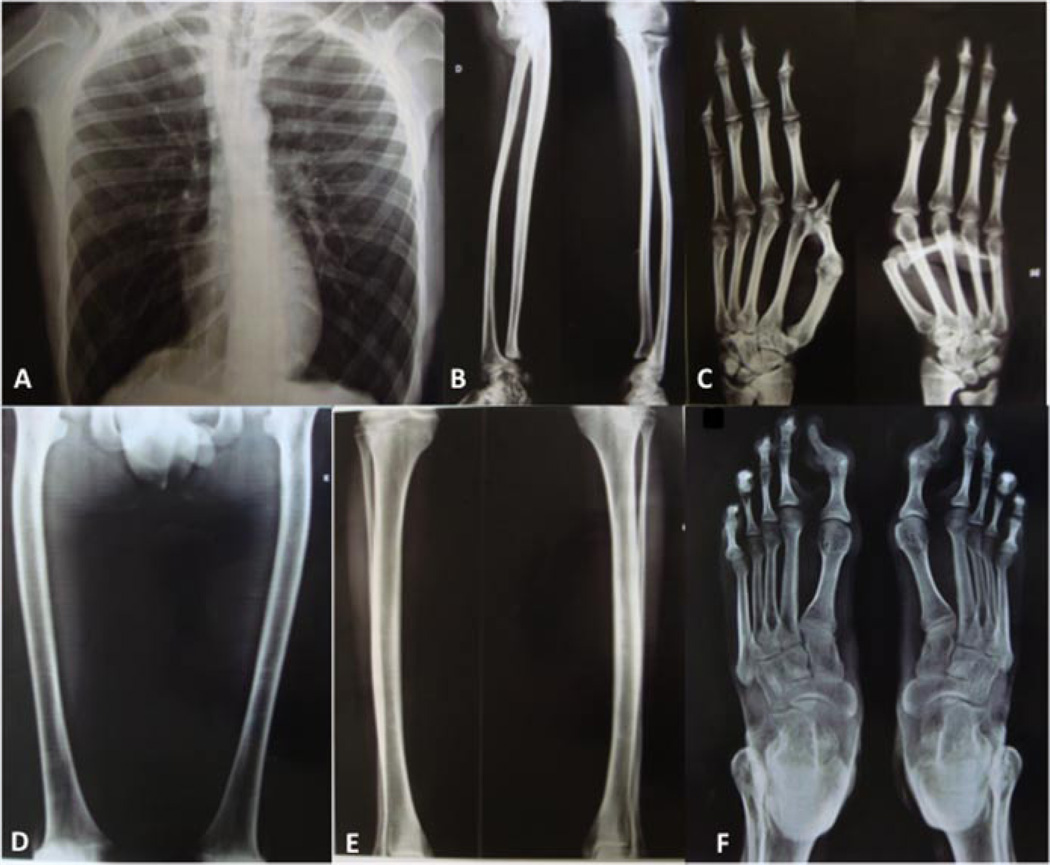
FIG. 2.
Skeletal survey: Scoliosis, slender ribs (A), slender long bones, short ulnas bilaterally not articulated with the radii (B), long metacarpus and phalanges (C), mild bowing of the femurs (D) and fibulae (E), and long metatarsii and bilateral valgus deformity of the halluces (F).
RESULTS
We performed whole exome sequencing on the propositus and his unaffected brother and applied a filtering designed to prioritize conserved, rare functional variants (missense, nonsense, splice site variants, and indels) that were homozygous or in the case of X-linked variants, hemizygous, in the proband but not in his unaffected brother. We identified a single true positive variant, a 17 bp deletion in exon 4 of SCARF2 (c.438_454del17; p.Trp148Alafs*20) that was homozygous and not found in the unaffected brother (see Methods in Supplementary materials). Review of the literature on SCARF2 revealed that loss of function mutations had been described in four patients with VDEGS from unrelated families [Anastacio et al., 2010]. Aside from sclerocornea, the phenotypic features of our patient are entirely consistent with the diagnosis of VDEGS. Next we used PCR and Sanger sequencing and showed that Individual II-1 has only the wild type 212 bp product, individuals I-1, I-2, and II-2 are heterozygotes with both the 212 bp wild type product and the mutant 195 bp product, and the individual II-3 has only the mutant 195 bp product (see Methods in Supplementary materials). Whole genome SNP genotyping detected no CNVs that met our criteria for pathogenicity. We noticed however two large segments of homozygosity of 9.4 Mb (16,055,170–25,464,310 bp) and of 7.8 Mb (29,540,129–37,409,795 bp) on chromosome 22 (see Methods in Supplementary material).
DISCUSSION
Our results and those published previously by two other groups identify LOF variants in SCARF2 as the cause of VDEGS [Anastacio et al., 2010; Bedeschi et al., 2010]. SCARF2 encodes one of two members of the scavenger receptor type F family [Plüddemann et al., 2007]. Located at 22q11.21, SCARF2 has 11 exons spanning 13,273 bp and encodes two isoforms, the longest of which is an 870 amino acid type one integral membrane protein with seven complete and three partial N-terminus, extracellular epidermal growth factor (EGF) domains and an ~400 residue Serine and Proline rich cytoplasmic C-terminus with multiple Serine and Threonine phosphorylation sites. The SCARF2 protein (also known as SREC-II) participates in homophilic and heterophilic (with SCARF1) interactions via its multiple EGF domains and interacts with multiple small molecule ligands including acetylated low density lipoprotein (Ac-LDL). Northern blot analysis detected a SCARF2 transcript of about 3.5 kb in most human tissues examined, with highest expression in eye, gastrointestinal tract, muscle, ovary, and stomach [Ishii et al., 2002; http://research-public.gene.com/Research/genentech/genehubgepis/index.html].
Interestingly, Bedeschi et al. [2010] described a patient with VDEGS and unilateral sclerocornea. Molecular analysis of this patient, a product of nonconsanguineous parents, showed compound heterozygosity for a de novo 22q11.2 microdeletion corresponding to the common DiGeorge/VCFS “3 Mb” deletion which includes SCARF2 and a hemizygous SCARF2 donor splice site acceptor mutation in intron 4 (c.854 + 1G>T) on the maternal allele. Since the sclerocornea had not been reported in any of the 24 patients with VDEGS described previously, but had been observed with a frequency of ~0.5% in patients with 22q.11 deletion syndrome [Binenbaum et al., 2008], the authors concluded that the sclerocornea was a consequence of the 22q11 microdeletion [Bedeschi et al., 2010].
Sclerocornea is a developmental anomaly of the eye in which the normally sharp margin separating the opaque sclera from the clear cornea is blurred to a variable extent. In the most severe form, sclerocornea totalis, the entire cornea is opaque while in milder forms only a segment of the corneal margin is blurred with complete sparing of the central cornea. The severe form is inherited in an autosomal recessive manner (OMIM 269400), while milder forms may segregate as dominant traits (OMIM 181700). Peters anomaly is another developmental defect of the anterior segment in which there is a central corneal leukoma (an opaque white spot), absence of the posterior corneal stroma and Descemet’s membrane, and a variable degree of iris and lenticular attachments to the central aspect of the posterior cornea. Peters anomaly and sclerocornea are diagnoses that are often used to label the clinical picture of total congenital corneal opacification (CCO) [Mataftsi et al., 2010]. Many genes (FOXE3, CYP1B1, SOX2, PITX2, TFAP2A, FOXC1, EYA1, PITX3, FGFR2, PAX6, DCN, KERA, B3GALTL, MAF, RAX, VSX1, SLC4A11) are known to be associated to CCO but the molecular basis of sclerocornea is not known [Binenbaum et al., 2008; Mataftsi et al., 2010].
Aside from sclerocornea, the phenotype of our patient is quite similar to that of previously reported VDEGS patients [Gupta et al., 1995; Phadke et al., 1998; Schweitzer et al., 2003; Guerra et al., 2005; Carr et al., 2007; Leal and Silva, 2009; Ali et al., 2010; Anastasio et al., 2010; Bedeschi et al., 2010; Patel et al., 2013]. The remarkable feature that distinguishes our patient is the bilateral sclerocornea. However, our results, together with those of Bedeschi et al. [2010] suggest the possible involvement of SCARF2 as the cause of sclerocornea in these patients. In which case, the phenotype of VDEGS would be expanded to include sclerocornea. Moreover, our results suggest an alternative explanation for the previously reported 22q11.2 deletion patients with sclerocornea (1 in a series of 240) [Binenbaum et al., 2008]. We suggest that in these patients, the 22q11.2 deletion exposes a hemizygous loss of function variant in SCARF2 on the remaining homolog. Such a model would predict that these patients would also have features of VDEG syndrome. A key variable might be the severity of the loss of function in the remaining allele; complete loss of function would result in some combination of the 22q11.2 deletion syndrome and VDEGS phenotypes, while a more modest loss of function may only add sclerocornea to the 22q11.2 deletion phenotype. Interestingly, Casteels and Devriendt [2005] and Erdoğan et al. [2008] each described a case of 22q11.2 deletion with unilateral Peters anomaly. The patient described by Erdoğan et al. [2008] was a 4-month-old male with facial dysmorphic findings including narrow nose, prominent ears, and arachnodactyly of hands and feet, phenotypic features of VDEGS but not of the 22q11.2 deletion syndrome. We suggest that the patient described by Erdoğan et al. [2008] like the patient described by Bedeschi et al. [2010] has a hemizygous loss of function mutation in the second SCARF2 allele resulting in VDEGS. Unfortunately this patient is deceased and a DNA sample for SCARF2 sequencing is not available. Moreover, we propose that loss of function of SCARF2 may be responsible for the ocular abnormalities in our patient and in the patients described by Erdoğan et al. [2008]. We further suggest that the patients with sclerocornea associated with 22q11.2 syndrome may have a hemizygous loss of function mutations on their remaining SCARF2 allele resulting in sclerocornea. In support of this hypothesis, we did not find mutations in the genes known to be responsible for CCO [Mataftsi et al., 2011] in our patient. At the present time, however, we cannot rule out the possibility that sclerocornea in our patient is caused by recessive or dominant mutations in another gene either not detected in our exome sequencing or not yet know to cause sclerocornea.
Finally, knowing that SCARF2 heterodimerizes with SCARF1 [Ishii et al., 2002], we suggest that sclerocornea might also result from mutations in SCARF1 (17p13.3). We note that Rodrigues et al. [1974] described an individual with sclerocornea and an unbalanced translocation involving monosomy of 17p and trisomy of 10q. The phenotype of this patient included sclerocornea plus several other features possibly related to the 17p and 10q imbalance. Although the breakpoints of the 17p translocation were not defined, it is possible that perturbation of SCARF1 accounts for the sclerocornea in the patient described by Rodrigues et al. [1974].
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge the families for their participation and support and Dr. Dilek Aktas for discussion. Our work was supported in part by the grant from the National Institutes of Health/National Human Genome Research Institute, number 1U54HG006542.
Grant sponsor: National Institutes of Health/National Human Genome Research Institute; Grant number: 1U54HG006542.
Footnotes
WEB RESOURCES
Gepsis: http://research-public.gene.com/Research/genentech/genehub-gepis/index.html
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
Conflict of interest: none.
REFERENCES
- 1.Ali R, Almureikhi M, Al-Musaifri F, Bhat V, Teebi A, Ben-Omran T. Further delineation of the Van den Ende–Gupta syndrome. Am J Med Genet Part A. 2010;152A:3095–3100. doi: 10.1002/ajmg.a.33725. [DOI] [PubMed] [Google Scholar]
- 2.Anastasio N, Ben-Omran T, Teebi A, Ha KCH, Lalonde E, Ali R, Almureikhi M, Der Kaloustian VM, Liu J, Rosenblatt DS, Majewski J, Jerome-Majewska LA. Mutations in SCARF2 are responsible for Van Den Ende–Gupta syndrome. Am J Hum Genet. 2010;87:553–559. doi: 10.1016/j.ajhg.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedeschi MF, Colombo L, Mari F, Hofmann K, Rauch A, Gentilin B, Renieri A, Clerici D. Unmasking of a recessive SCARF2 mutation by a 22q11. 12 de novo deletion in a patient with Van den Ende–Gupta syndrome. Mol Syndromol. 2010;1:239–245. doi: 10.1159/000328135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binenbaum G, McDonald-McGinn DM, Zackai EH, Walker BM, Coleman K, Mach AM, Adam M, Manning M, Alcorn DM, Zabel C, Anderson DR, Forbes BJ. Sclerocornea associated with the chromosome 22q11.2 deletion syndrome. Am J Med Genet Part A. 2008;146A:904–909. doi: 10.1002/ajmg.a.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr CW, Carron JD, Lachman RS, Abdul-Rahman OA. Van Den Ende–Gupta syndrome: Laryngeal abnormalities in two siblings. Am J Med Genet Part A. 2007;143A:2706–2711. doi: 10.1002/ajmg.a.32007. [DOI] [PubMed] [Google Scholar]
- 6.Casteels I, Devriendt K. Unilateral Peters’ anomaly in a patient with DiGeorge syndrome. J Pediatr Ophthalmol Strabismus. 2005;42:311–313. doi: 10.3928/0191-3913-20050901-17. [DOI] [PubMed] [Google Scholar]
- 7.Erdoğan MK, Utine GE, Alanay Y, Aktaş D. Unilateral Peters’ anomaly in an infant with 22q11.2 deletion syndrome. Clin Dysmorphol. 2008;17:289–292. doi: 10.1097/MCD.0b013e3283079e7c. [DOI] [PubMed] [Google Scholar]
- 8.Guerra D, Sanchez O, Richieri-Costa A. van den Ende–Gupta syndrome of blepharophimosis, arachnodactyly, and congenital contractures. Am J Med Genet Part A. 2005;136A:377–380. doi: 10.1002/ajmg.a.30665. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Hall CM, Ransley YF, Murday VA. A new autosomal recessive syndrome of characteristic facies, joint contractures, skeletal abnormalities, and normal development: Second report with further clinical delineation. J Med Genet. 1995;32:809–812. doi: 10.1136/jmg.32.10.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamosh A, Sobreira N, Hoover-Fong J, Sutton VR, Boehm C, Schiettecatte F, Vall D. PhenoDB: A new web-based tool for the collection, storage, and analysis of phenotypic features. Hum Mutat. 2013;34:566–571. doi: 10.1002/humu.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii J, Adachi H, Aoki J, Koizumi H, Tomita S, Suzuki T, Tsujimoto M, Inoue K, Arai H. SREC-II, a new member of the scavenger receptor type F family, trans-interacts with SREC-I through its extracellular domain. J Biol Chem. 2002;277:39696–39702. doi: 10.1074/jbc.M206140200. [DOI] [PubMed] [Google Scholar]
- 12.Leal GF, Silva EO. Van den Ende–Gupta syndrome: Evidence for genetic heterogeneity. Am J Med Genet Part A. 2009;149A:1293–1295. doi: 10.1002/ajmg.a.32871. [DOI] [PubMed] [Google Scholar]
- 13.Mataftsi A, Sowden JC, Nischal KK. Atypical Peters plus syndrome with new associations. J AAPOS. 2010;14:560–561. doi: 10.1016/j.jaapos.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Mataftsi A, Islam L, Kelberman D, Sowden JC, Nischal KK. Chromosome abnormalities and the genetics of congenital corneal opacfication. Mol Vis. 2011;17:1624–1640. [PMC free article] [PubMed] [Google Scholar]
- 15.Patel N, Salih MA, Alshammari MJ, Abdulwahhab F, Adly N, Alzahrani F, Elgamal EA, El Khashab HY, Al-Qattan MM, Alkuraya FS. Letter to editor: Expanding the clinical spectrum and allelic heterogeneity in van den Ende–Gupta. Clin Genet. 2013 doi: 10.1111/cge.12205. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Phadke SR, Gulati R, Agarwal SS. Further delineation of a new (Van Den Ende–Gupta) syndrome of blepharophimosis, contractural arachnodactyly, and characteristic face. Am J Med Genet. 1998;77:16–18. doi: 10.1002/(sici)1096-8628(19980428)77:1<16::aid-ajmg4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Plüddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues MM, Calhoun J, Weinreb S. Sclerocornea with an unbalanced translocation (17p, 10q) Am J Ophthalmol. 1974;78:49–53. doi: 10.1016/0002-9394(74)90008-7. [DOI] [PubMed] [Google Scholar]
- 19.Schweitzer DN, Lachman RS, Pressman BD, Graham JM., Jr van den Ende–Gupta syndrome of blepharophimosis, arachnodactyly, and congenital contractures: Clinical delineation and recurrence in brothers. Am J Med Genet Part A. 2003;118A:267–273. doi: 10.1002/ajmg.a.10143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.