Abstract
Object
Accurate real-time imaging of coinfused surrogate tracers can be used to determine the convective distribution of therapeutic agents. To assess the effect that a concentration of a Gd-based surrogate tracer has on the accuracy of determining the convective distribution, the authors infused different concentrations of Gd-diethylenetriamine pentaacetic acid (DTPA) in primates during MR imaging.
Methods
Five nonhuman primates underwent convective infusion (1 or 5 mM, 21–65 μl) of Gd-DTPA alone, Gd-DTPA and 14C-sucrose, or Gd-DTPA and 14C-dextran into the bilateral striata. Animals underwent real-time MR imaging during infusion (5 animals) and autoradiographic analysis (2 animals).
Results
Gadolinium-DTPA could be seen filling the striata at either concentration (1 or 5 mM) on real-time MR imaging. While the volume of distribution (Vd) increased linearly with the volume of infusion (Vi) for both concentrations of tracer (1 mM: R2 = 0.83; 5 mM: R2 = 0.96), the Vd/Vi ratio was significantly (p < 0.0001) less for the 1-mM (2.3 ± 1.0) as compared with the 5-mM (7.4 ± 1.9) concentration. Autoradiographic and MR volumetric analysis revealed that the 5-mM concentration most accurately estimated the Vd for both small (sucrose [359 D], 12% difference between imaging and autoradiographic distribution) and large (dextran [70 kD], 0.2% difference) molecules compared with the 1-mM concentration (sucrose, 65% difference; dextran, 68% difference).
Conclusions
The concentration of infused Gd-DTPA plays a critical role in accurately assessing the distribution of molecules delivered by CED. A 5-mM concentration of Gd-DTPA most accurately estimated the Vd over a wide range of molecular sizes.
Keywords: convection-enhanced delivery, gadolinium, oncology, magnetic resonance imaging, surrogate tracer, volume of distribution
Convection-enhanced delivery is being used in translational and clinical studies to distribute putative therapeutic molecules to the CNS.1,2,5,7,13 Because CED relies on bulk flow to distribute infusate in the interstitial spaces of the parenchyma, it can be used to homogeneously distribute small- and large-molecular-weight compounds over targeted clinically relevant volumes across the blood-nervous system barrier. 3 Paradigms to monitor the distribution of CED infusate using coinfused MR imaging surrogate tracers, including Gd-based agents, are being developed.18,22 The utilization of coinfused surrogate tracers allows real-time monitoring of CED to ensure adequate distribution of therapeutics, determine the efficacy of drugs, enhance safety, and provide insights into the properties of CED under various conditions.
The optimal use of surrogate imaging tracers in convective delivery paradigms will depend on the accuracy of these agents in tracking the distribution of coinfused therapeutic compounds. While data indicate that Gd-DTPA can be used to track putative therapeutic agents delivered by convection,9,13,17,21 little is known about the impact that specific features, particularly concentration, will have on its MR imaging properties and tracking accuracy. To determine the effect of Gd-DTPA concentration on MR imaging visualization and tracking accuracy during convective delivery of an infusate, we perfused the striata of primates with different concentrations of Gd-DTPA and radiolabeled compounds and analyzed the effect on imaging and tracking accuracy.
Methods
Preparation of Gd-DTPA
Clinical grade Gd-DTPA (Magnevist, Bayer Health-Care Pharmaceuticals, Inc.) was used for all in vitro and in vivo studies. Gadolinium-DTPA was diluted in sterile 1× phosphate-buffered saline (KD Medical) to the specified concentrations for in vitro (see below) and in vivo (Table 1) experiments.
TABLE 1.
Infusate and observation characteristics in study primates*
| Animal No. | Striatal Side | Vi (μl) | Infusate | Final Vd/Vi Ratio on Imaging† | Final Vd/Vi Ratio on QAR‡ |
|---|---|---|---|---|---|
| 1 | lt | 63.2 | 5 mM Gd-DTPA | 7.94 | NA |
| rt | 65.2 | 1 mM Gd-DTPA | 3.37 | NA | |
| 2 | lt | 38.4 | 5 mM Gd-DTPA | 6.44 | NA |
| rt | 36.3 | 1 mM Gd-DTPA | 2.25 | NA | |
| 3 | lt | 23.3 | 5 mM Gd-DTPA | 6.89 | NA |
| rt | 21.4 | 1 mM Gd-DTPA | 1.33 | NA | |
| 4 | lt | 46.5 | 5 mM Gd-DTPA & 14C-sucrose | 6.44 | 5.76 |
| rt | 46.7 | 1 mM Gd-DTPA & 14C-sucrose | 2.23 | 6.46 | |
| 5 | lt | 51.3 | 5 mM Gd-DTPA & 14C-dextran | 6.31 | 6.33 |
| rt | 54.6 | 1 mM Gd-DTPA & 14C-dextran | 1.73 | 5.42 |
NA = not applicable.
Vd determined by 3D MR imaging volumetric analysis.
Vd determined by quantitative autoradiography.
In Vitro Imaging of Gd-DTPA
Gadolinium-DTPA was diluted in phosphate-buffered saline to concentrations of 0.1, 1, 5, 25, 50, 125, 250, and 500 mM and placed in 1.5-ml plastic vials (Eppendorf AG). Then T1-weighted MR images (slice thickness 1 mm, spacing 0 mm) were obtained through the Gd-DTPA solutions using a 3-T MR scanner (Phillips).
Preparation of 14C-Sucrose and 14C-Dextran for In Vivo Imaging With Gd-DTPA
14C-sucrose and 14C-dextran (Sigma-Aldrich) were coinfused with Gd-DTPA (Table 1). 14C-sucrose (specific activity 50 μCi) and 14C-dextran (specific activity 50 μCi) were added to 1- and 5-mM concentrations of Gd-DTPA before infusion.
Convective Infusion of Gd-DTPA and Radiolabeled Compounds
All animal investigations and procedures were performed in accordance with National Institutes of Health guidelines on animal research and under an approved National Institute for Neurological Disorders and Stroke Animal Care and Use Committee animal study protocol. Five adult nonhuman primates (Macaca mulatta) underwent convective infusion of Gd-DTPA alone (3 animals), Gd-DTPA with 14C-sucrose (1 animal), or Gd-DTPA with 14C-dextran (1 animal) to the bilateral striata (Table 1). Briefly, animals were sedated, intubated, and placed under general anesthesia.8 Temperature and cardiopulmonary function were monitored during anesthesia. Animals were secured in a stereotactic frame (Crist Instrument Company). The vertex of the skull was exposed through a midline scalp incision. Using a high-speed drill, two 8-mm bur holes were made in the skull overlying bilateral striata. Outer guide cannulae (outer diameter 0.027 in, inner diameter 0.02 in) were stereotactically placed through the dural openings 5 mm along the target trajectory and secured with methylmethacrylate. Inner cannulae (outer diameter 0.014 in, inner diameter 0.006 in) were then placed through outer guide cannulae to the striatal targets.
Once inner cannulae were on target, infusate was delivered by convection (rate of 1 μl/minute) using a previously described system.14 Briefly, a PHD 2000 Harvard syringe pump (Harvard Apparatus, Inc.) was used to generate continuous hydrostatic pressure that was transmitted directly to an infusate-filled, gas-tight glass Hamilton syringe (250 μl total volume, Hamilton Company) connected to thick-walled polyethylene tubing (outer diameter 0.05 in, inner diameter 0.023 in, Plastics One). The tubing was in turn connected to the inner infusion cannulae. On completing the infusion, the cannulae were removed.
All 5 animals were killed by intravenous barbiturate overdose (pentobarbital 90 mg/kg) immediately after the infusion was complete. On euthanizing the animals, brains used for QAR (2 animals) were removed and cut into whole coronal brain blocks. Each block was coated with optimal cutting temperature embedding matrix (Tissue-tek OCT, Sakura Finetek) and snap frozen in an isopentane dry ice bath. Slabs were subsequently placed into a −80°C freezer for storage until sectioning.
Imaging of Gd-DTPA Distribution
After placement of the infusion cannulae, coronal T1-weighted MR images were obtained to determine the precise location of the cannulae within the bilateral striata in all animals. Once cannula placement was confirmed, T1-weighted MR images were obtained in 3 planes (sagittal, axial, and coronal slice thickness 1 mm, spacing 0 mm) using a 3-T MR unit. Images were obtained at approximately 10- to 30-minute intervals until the infusions were complete. Two of the animals infused with Gd-DTPA alone (1 and 5 mM) underwent MR imaging at 10, 20, 30, and 80 minutes after the infusion using the same MR imaging parameters described above.
Evaluation of Gd-DTPA as a Surrogate MR Imaging Tracer
Quantitative Autoradiography
Frozen 3/8-in brain slabs were coronally cut into 20-μm-thick serial sections, with autoradiograms prepared from every fifth section. The tissue sections and 14C standards were exposed on BAS-MS9000 imaging plates and developed using an FLA-5000G bioimaging analyzer (Fuji Medical Systems). The area of distribution on each slide was measured using the Image Gauge software program (version 4.23, Fuji Medical Systems). A segmentation threshold of 10% of the value obtained from the region of interest containing the maximum optical density was used to determine the area of distribution.18 The volume of distribution (Vd) was calculated by summing the areas and multiplying by 0.1 mm.11,12,16,18
Magnetic Resonance Imaging Analysis
The MR images were evaluated using an image analysis and visualization software package (MEDx version 3.44, Medical Numerics). Three-dimensional Vds of the region infused with contrast agent were calculated using a segmentation threshold as the MR signal intensity value at least 10% above the mean MR signal from the surrounding non-infused anatomical region.18 The Vd of the MR contrast agent was then compared with the autoradiographic Vd of 14C-sucrose and 14C-dextran to assess the ability of Gd-DTPA at different concentrations to track drug delivery.
Homogeneity Analysis
Autoradiograms were used to determine the concentration profile across the region of infusion. Specific activity (concentration) measurements from 14C-sucrose and 14C-dextran across the infused region were determined based on optical density measurements. These values were plotted on a graph to derive a concentration-distance profile. Differences in specific activity (concentration) were analyzed across the plateau region of perfusion.
Statistical Analysis
Statistical analysis was performed using commercially available software (PASW Statistics version 18.0, SPSS, Inc.), and specific statistical tests were used as defined in the text.
Results
In Vitro Imaging of Gd-DTPA
To assess the concentration range of Gd-DTPA that would be visible on MR imaging during infusions in vivo, we performed MR imaging of serial dilutions of Gd-DTPA (0.1, 1, 5, 25, 50, 125, 250, and 500 mM) in vitro. These T1-weighted MR images of serial dilution samples revealed that Gd-DTPA was visible between 0.1- and 50-mM concentrations but was outside the apparent visible range above the 50-mM concentrations. The maximal intensity in vitro on T1-weighted MR imaging was at a concentration of 5 mM (Fig. 1).
Fig. 1.
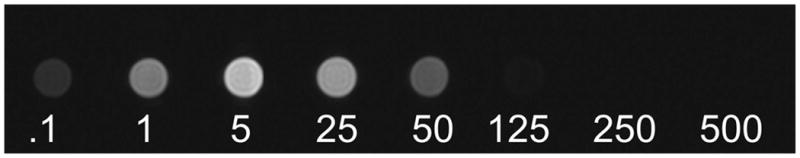
Magnetic resonance images of serial dilutions of Gd-DTPA in vitro (mM concentration).
Convective Delivery of Gd-DTPA
Imaging Characteristics
To assess the feasibility of infusing variable concentrations of Gd-DTPA in the brain, we perfused the bilateral striata of 5 primates (Table 1) either with Gd-DTPA alone (1 or 5 mM) or with radiolabeled molecules (14C-sucrose and 14C-dextran) coinfused with Gd-DTPA (1 or 5 mM). Real-time T1-weighted MR imaging during the infusion of Gd-DTPA revealed that both concentrations (1 and 5 mM) could be seen progressively filling the bilateral striata with an increasing volume of infusion (Vi). While the regions of Gd-DTPA infusion could be clearly distinguished from the surrounding striatal gray matter at either concentration, the image intensity and Vd of the 1-mM concentration was qualitatively less than the region perfused with the 5-mM concentration at a similar Vi (Fig. 2).
Fig. 2.

Axial real-time T1-weighted MR image of bilateral striata with 1 mM (arrowhead) and 5 mM (arrow) of Gd-DTPA revealing reduced
Ratio of Vd/Vi for Varying Gd Concentrations
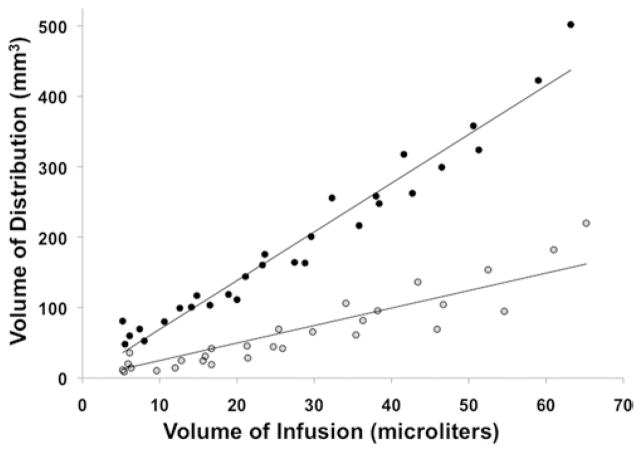
To determine the Vd/Vi ratio for both concentrations of Gd-DTPA, we performed 3D volumetric analysis of MR imaging of the Vd at each time point during infusion. Because the region of increased T1 hyperintensity could be clearly distinguished from the surrounding parenchymal region, the precise Vd for each concentration was obtained. Whereas the Vd increased linearly with both the 1-mM (R2 = 0.83) and 5-mM (R2 = 0.96) concentrations, the mean Vd/Vi ratio was significantly (p < 0.0001, paired t-test) less for the 1-mM Gd-DTPA infusion as compared with the 5-mM Gd-DTPA infusion. Based on MR imaging volumetric analysis, the mean Vd/Vi ratio at 1 mM was 2.3 ± 1.0 and the ratio at 5 mM was 7.4 ± 0.9 (Fig. 3).
Fig. 3.
Graph demonstrating the relationship between Vd and Vi. There was a highly linear relationship between Vd (determined by MR imaging) and Vi of Gd-DTPA at the 1-mM (R2 = 0.83, gray circles) and 5-mM concentration (R2 = 0.96, black circles). The mean Vd/Vi ratio (7.4 ± 0.9) for the 5-mM concentration was significantly (p < 0.05) higher than that for the 1-mM concentration (2.3 ± 1.0).
Tracking Accuracy Based on Surrogate Concentration
To assess the accuracy of either concentration of Gd-DTPA in determining the actual distribution of a small- and large-molecular-weight compound, we compared the Vd from MR imaging volumetric analysis with the Vd obtained from QAR for 14C-sucrose and 14C-dextran. Imaging and QAR comparative analysis revealed that the 5-mM concentration of Gd-DTPA most accurately tracked both sucrose and dextran distribution. Whereas the difference between 5-mM imaging and autoradiographic Vd was 12% for sucrose and 0.2% for dextran, the difference between 1-mM imaging and autoradiographic Vd was 65% for sucrose and 68% for dextran.
Infusion Homogeneity
To determine the homogeneity of 14C-sucrose and 14C-dextran infusions, we first calculated the concentration of both molecules across the infused region by using specific activity measurements directly from autoradiograms. Quantification of the specific activity across the perfused region revealed that the pattern of distribution of both molecules was square shaped. The profiles were associated with a steep drop off in concentration at the edges of perfusion and a flat concentration profile across the region of infusion (Fig. 4). Analysis of concentration across the plateau region revealed a difference in concentration of 12.5% for 14C-sucrose and 8.6% for 14C-dextran.
Fig. 4.
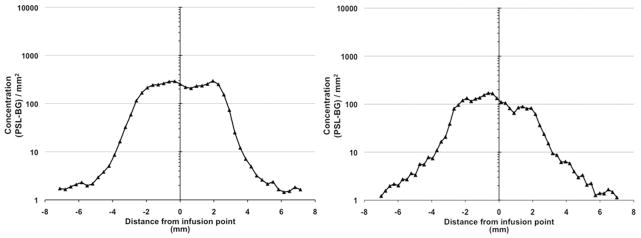
Quantitative analysis of the specific activity (concentration) of 14C-sucrose (left) and 14C-dextran (right) across perfused regions of bilateral striata revealing a square-shaped distribution of substrate of both molecules. PSL-BG = photostimulated luminescence given per area (concentration of radiation).
Postinfusion MR Imaging
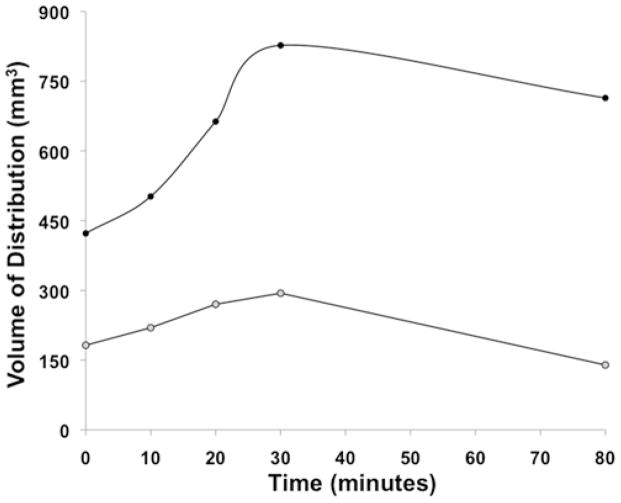
To determine the imaging features of different concentrations of Gd-DTPA after infusion, we performed delayed MR imaging once the infusions were stopped (at 10, 20, 30, and 80 minutes postinfusion) in 2 animals infused with Gd-DTPA alone. Delayed imaging revealed maximum expansion of MR-visible Vds of 96% and 61% at 30 minutes after stopping infusions for the 1- and 5-mM concentrations, respectively (Fig. 5). Following this expansion of the Vd shell after ceasing the infusion, there was a decline in the imaged Vd that coincided with an overall decreasing MR imaging Gd-DTPA hyperintensity.
Fig. 5.
Graph showing Gd-DTPA postinfusion Vd. The Vd increased (maximal increase 30 minutes after infusion) significantly (p < 0.05) more for the 5-mM concentration (96% increase over Vd at infusion cessation, black circles) than for the 1-mM concentration (61% increase, gray circles).
Discussion
Previous Studies
Previous translational and early clinical studies have investigated the use of Gd-DTPA (1 or 5 mM) as a surrogate marker to predict convective distribution by preinfusion modeling or as a real-time MR imaging surrogate tracking agent for compounds delivered via CED.8,9,13,17,22 Consistent with the mean Vd/Vi ratios (1 mM, 2.3; 5 mM, 7.4) demonstrated in the current study, past investigations documented a mean Vd/Vi ratio of 3:1 using 1 mM Gd-DTPA22 and mean ratios of 5:1 to 8:1 using 5 mM Gd-DTPA.8,17 While these investigations used Gd-DTPA concentrations similar to those described in the present study, they did not examine the effect of concentration on drug tracking accuracy. Here, we analyzed the effect of concentration on MR imaging characteristics, including imaged Vd and tracking accuracy, by infusing 1 and 5 mM Gd-DTPA simultaneously in bilateral striata of primates and by determining Vd by QAR.
Implications for Imaging Convective Delivery
Gadolinium-DTPA
Gadolinium-DTPA (MW 469 D) is a US FDA-approved intravenous MR imaging contrast agent. It consists of a metal ion (Gd3+) bound to the DTPA chelator. Gadolinium-DTPA was studied as a potential convective surrogate imaging tracer because of its accessibility and well-established reliability as an MR imaging agent. Further, Gd-DTPA has a long record of safety for visualizing pathology of the nervous system. Similar to other compounds best suited for CED, Gd-DTPA has a low octanol-water coefficient (log P = −2.8),23 which makes it impermeable to the intact blood-brain barrier and also allows it be an excellent surrogate imaging agent.
In Vitro Imaging of Gd-DTPA
Magnetic resonance imaging of serial concentrations of Gd-DTPA (Fig. 1) revealed that the surrogate tracer was visible in vitro at concentrations between 0.1 and 50 mM. The highest T1-weighted intensity was seen with the 5-mM concentration. One- and 5-mM concentrations were selected for the in vivo infusion because they have been used in vivo to track CED of therapeutic molecules and represent the highest T1-weighted intensities on MR imaging in vitro, minimizing tissue dilutional effects. Because convective delivery distributes molecules in the interstitial spaces over a Vd that is greater than the Vi (Vd/Vi ratio), a tissue dilutional effect may need to be taken into consideration when selecting the infused concentration of imaging agents.
Effect of Gd-DTPA Concentration on Tracking Accuracy
An important feature of any surrogate imaging agent is its ability to accurately demonstrate anatomical and volumetric distribution of the coinfused drug. Our findings indicated that the concentration of Gd-DTPA has a central role in tracking accuracy. Data revealed that a 5-mM concentration of Gd-DTPA is optimal for tracking coinfused therapeutic compounds over a broad range of molecular weights that is inclusive of most drugs. These data also indicated that using lower concentrations of Gd-DTPA (1 mM) to track molecules across this size range will result in a gross underestimation of the region perfused by drug. Alternatively, it is possible that lower concentrations of Gd-DTPA (1 mM) may more accurately track very large compounds with lower Vd/Vi ratios (2:1 to 4:1), including nanoparticles and viral capsids.4,10,19,20,22
Homogeneity
Consistent with the bulk flow properties of convection and similar to previous studies analyzing the distribution of molecules by CED,3,6,12,13,16,18 the distribution of 14C-sucrose and 14C-dextran was homogeneous across the perfused region. While it is not currently possible to determine the concentration of infusate directly from MR imaging of a surrogate tracer, the homogeneous distribution across a perfused region should permit extrapolation of the drug concentration based on simple volumetric calculations. Provided that surrogate tracer correctly represents drug Vd and that drug distribution is homogeneous across the perfused region, as shown in this and other studies,12,13,16 an accurate estimation of tissue concentration can be calculated by dividing the infusate concentration by the MR imaging Vd/Vi ratio.
Postinfusion MR Imaging
There was a significant increase in Gd-DTPA Vd that was maximal at 30 minutes after infusion was complete. While postinfusion increases in Vd can be the result of continued convective flow, our findings indicate that diffusion may play a particularly potent role in the postinfusion Vd expansion associated with higher concentrations of infusate and smaller molecules.6,13,14 The maximum postinfusion volumetric expansion was significantly larger with the 5-mM (95% increase in Vd) as compared with the 1-mM (61% increase in Vd) concentration. Previous studies examining diffusion after convection of a large-molecular-weight compound (Gd-albumin, MW 72 kD) revealed a smaller maximal expansion (40% increase) in the Vd that occurred over significantly longer periods of time (days).14
When bulk flow plays the dominant role in drug distribution, such as occurs during CED, small and large molecules with comparable properties (blood-barrier permeability, degradation times, and binding properties) will distribute similarly. At times when diffusion plays a larger role in Gd-DTPA distribution, tracking inaccuracies can develop between small and large molecules. Specifically, differences between imaged Gd-DTPA distribution and the actual distribution of coinfused therapeutic compounds with different diffusion characteristics can develop at the edge of an infusion front during prolonged perfusion or after the completion of an infusion when convective forces are reduced. Subsequently, large-molecular-weight compounds that are perfused over large volumes and long periods of time may require large-molecular-weight surrogate tracers for accurate image tracking.
Previously, Croteau and colleagues6 modeled and studied the tracking accuracy of a small-molecular-weight CT surrogate tracer (iopamidol, MW 777 D) and a large-molecular-weight coinfused compound (dextran, MW 70 kD). Consistent with known properties of bulk flow distribution, modeling revealed the ability of a large-molecular-weight compound (dextran) to accurately track a low-molecular-weight tracer (iopamidol), which was directly related to infusion rate (or convective flow). Specifically, these authors found that the Vi of iopamidol that could be infused with a 20% error in the Vd demonstrated on imaging and the actual dextran distribution was described by the equation Vi (μl) = 600 × infusion rate (μl/minute).
Safety
Previous animal and clinical investigations9,15,17 have shown the infusion of Gd-DTPA at 1- and 5-mM concentrations to be safe. In those trials, no evidence of clinical deterioration occurred after infusion. Further, histological analysis in the animals did not demonstrate evidence of toxicity at either concentration. Histopathological evaluation of tissues was conducted after convective infusion and revealed that there was a limited region of gliosis (25-μmol radius from cannula) surrounding the infusion cannula tract with either concentration. 6,14
Imaging of CED
Real-time imaging of convective delivery can provide delivery information not previously available and will be critical for future translational and clinical studies. The opportunity to accurately determine drug distribution during infusion has a number of advantages. Real-time imaging can confirm adequate and accurate delivery of putative therapeutic agents to the desired region. Similarly, it will enhance the ability to determine drug efficacy by ensuring drug was delivered to the desired region and volume. Real-time imaging will enhance the safety of convective delivery of a drug by permitting infusion cessation after the desired region has been perfused. Finally, imaging will provide essential insights into the convective properties that exist under varying human pathological conditions and optimal conditions/parameters for CED.
Conclusions
Concentration of infused Gd-DTPA plays a critical role in accurately assessing the distribution of molecules delivered by CED. A 5-mM concentration of Gd-DTPA most accurately estimated the Vd over a wide range of molecular sizes.
Acknowledgments
This work was supported by the intramural research program at the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Abbreviations used in this paper
- CED
convection-enhanced delivery
- DTPA
diethylenetriamine pentaacetic acid
- MW
molecular weight
- QAR
quantitative autoradiography
- Vd
volume of distribution
- Vi
volume of infusion
Footnotes
Disclosure
Author contributions to the study and manuscript preparation include the following. Conception and design: Asthagiri, Lonser. Acquisition of data: Asthagiri, Walbridge, Heiss. Analysis and interpretation of data: Asthagiri, Heiss, Lonser. Drafting the article: Asthagiri, Lonser. Critically revising the article: Asthagiri, Walbridge, Lonser. Approved the final version of the paper on behalf of all authors: Asthagiri. Statistical analysis: Asthagiri. Administrative/technical/material support: Lonser. Study supervision: Asthagiri, Lonser.
References
- 1.Androutsellis-Theotokis A, Rueger MA, Park DM, Mkhikian H, Korb E, Poser SW, et al. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci U S A. 2009;106:13570–13575. doi: 10.1073/pnas.0905125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 3.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen MY, Hoffer A, Morrison PF, Hamilton JF, Hughes J, Schlageter KS, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J Neurosurg. 2005;103:311–319. doi: 10.3171/jns.2005.103.2.0311. [DOI] [PubMed] [Google Scholar]
- 5.Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croteau D, Walbridge S, Morrison PF, Butman JA, Vortmeyer AO, Johnson D, et al. Real-time in vivo imaging of the convective distribution of a low-molecular-weight tracer. J Neurosurg. 2005;102:90–97. doi: 10.3171/jns.2005.102.1.0090. [DOI] [PubMed] [Google Scholar]
- 7.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 8.Heiss JD, Walbridge S, Asthagiri AR, Lonser RR. Image-guided convection-enhanced delivery of muscimol to the primate brain. Laboratory investigation. J Neurosurg. 2010;112:790– 795. doi: 10.3171/2009.7.JNS09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagannathan J, Walbridge S, Butman JA, Oldfield EH, Lonser RR. Effect of ependymal and pial surfaces on convection-enhanced delivery. Laboratory investigation. J Neurosurg. 2008;109:547–552. doi: 10.3171/JNS/2008/109/9/0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krauze MT, Mcknight TR, Yamashita Y, Bringas J, Noble CO, Saito R, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005;16:20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 12.Lonser RR, Gogate N, Morrison PF, Wood JD, Oldfield EH. Direct convective delivery of macromolecules to the spinal cord. J Neurosurg. 1998;89:616–622. doi: 10.3171/jns.1998.89.4.0616. [DOI] [PubMed] [Google Scholar]
- 13.Lonser RR, Schiffman R, Robison RA, Butman JA, Quezado Z, Walker ML, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 14.Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg. 2002;97:905–913. doi: 10.3171/jns.2002.97.4.0905. [DOI] [PubMed] [Google Scholar]
- 15.Lonser RR, Warren KE, Butman JA, Quezado Z, Robison RA, Walbridge S, et al. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J Neurosurg. 2007;107:190–197. doi: 10.3171/JNS-07/07/0190. [DOI] [PubMed] [Google Scholar]
- 16.Lonser RR, Weil RJ, Morrison PF, Governale LS, Oldfield EH. Direct convective delivery of macromolecules to peripheral nerves. J Neurosurg. 1998;89:610–615. doi: 10.3171/jns.1998.89.4.0610. [DOI] [PubMed] [Google Scholar]
- 17.Murad GJ, Walbridge S, Morrison PF, Szerlip N, Butman JA, Oldfield EH, et al. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg. 2007;106:351– 356. doi: 10.3171/jns.2007.106.2.351. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TT, Pannu YS, Sung C, Dedrick RL, Walbridge S, Brechbiel MW, et al. Convective distribution of macromolecules in the primate brain demonstrated using computerized tomography and magnetic resonance imaging. J Neurosurg. 2003;98:584–590. doi: 10.3171/jns.2003.98.3.0584. [DOI] [PubMed] [Google Scholar]
- 19.Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Szerlip NJ, Walbridge S, Yang L, Morrison PF, Degen JW, Jarrell ST, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg. 2007;107:560–567. doi: 10.3171/JNS-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 21.Varenika V, Dickinson P, Bringas J, LeCouteur R, Higgins R, Park J, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. Laboratory investigation. J Neurosurg. 2008;109:874–880. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 23.Wiegers CB, Welch MJ, Sharp TL, Brown JJ, Perman WH, Sun Y, et al. Evaluation of two new gadolinium chelates as contrast agents for MRI. Magn Reson Imaging. 1992;10:903–911. doi: 10.1016/0730-725x(92)90444-5. [DOI] [PubMed] [Google Scholar]