Abstract
Despite the importance of enhancing the accuracy and reliability of non-human primate stereotaxy, a number of limitations exist using currently described techniques. To overcome these problems, we present a simple universally available approach that combines pre-operative magnetic resonance imaging and the non-surgical creation of reference points (teeth marking). We have found that this approach improves stereotaxic targeting reliability and permits accurate reproducible stereotaxic localization at time points distant from the pre-operative imaging.
Keywords: Accuracy, Magnetic resonance imaging, Primate, Stereotaxy, Surgery, Technique
1. Introduction
Specific understanding into the basic underlying pathophysiologic dysfunction of subcortical structures in various neurological disorders and advances that permit targeted treatment of these abnormal structures (Benabid et al., 1991; Bobo et al., 1994) is rapidly expanding the development of new neurosurgical therapies for a variety of neurologic diseases. To safely and effectively translate these discoveries into clinical therapies, stereotaxic targeting of regions of the brain in naïve and diseased non-human primate models is often critical. Subsequently, the need for accurate and reliable stereotaxic techniques for non-human primates studies is expanding and is fundamentally important to maximize animal safety, research efficiency and data validity, while minimizing animal use and research costs.
While high-resolution pre-operative magnetic resonance (MR)-imaging of non-human primates in a stereotaxic frame can enhance the accuracy and precision of stereotaxy (Saunders et al., 1990), it can be associated with significant inaccuracies if the imaging is performed at time points distant from the actual stereotaxic surgery. A major cause of this inaccuracy is due to misalignment of the animal in the stereotaxic frame (compared to the alignment of the animal in the frame at pre-operative imaging) at the time of the stereotaxic surgery. To overcome this limitation, we describe a simple and universally applicable method that allows reliable and accurate stereotaxic localization of small subcortical targets in non-human primates at time points distant from pre-operative MR-imaging.
2. Animals and methods
2.1. Animals
All animal procedures were performed in accordance with the regulations of the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health. Consecutive non-human primates (Macaca mulatta and Macaca fascicularis) that underwent stereotaxic targeting of deep brain structures for cannula or electrode placement using the below described technique in the Surgical Neurology Branch of the National Institutes of Health between June 2002 and June 2005 were included.
2.2. Accuracy of technique
Precise targets for placement of either an infusion cannula or a recording electrode within the striatum, brainstem, hippocampus or basal forebrain were chosen on pre-operative MR-imaging and recorded. Stereotaxic coordinates for these targets were then calculated from pre-operative images and recorded. Targeting accuracy was then determined by directly comparing the desired pre-operative MR-imaging target to the actual stereotaxic placement (of the cannula or electrode) on post-operative MR-imaging. Errors in stereotaxic placement between the desired pre-operative target (as determined from pre-operative MR-imaging) and the final cannula or electrode placement (as determined from post-operative MR-imaging) were measured directly from co-registered images. For inaccurately placed cannulas or electrodes, errors in each of the three planes (dorsoventral, anteroposterior, and/or mediolateral) were determined based on the comparison of the co-registered pre-operative and post-operative MR-images and recorded.
2.3. Stereotaxic technique
2.3.1. Pre-operative tooth reference marking and MR-imaging
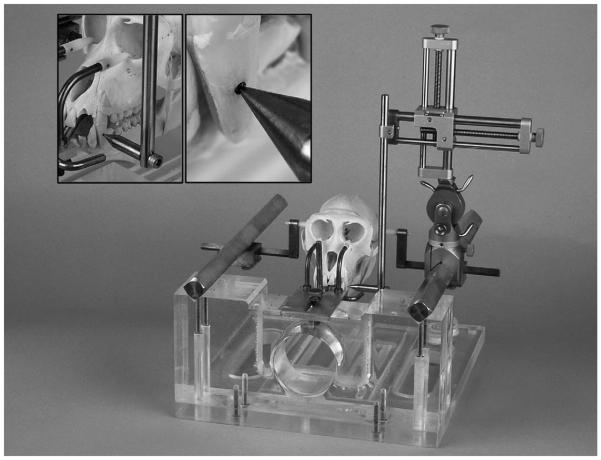
While under anesthesia, animals were secured in the center of a non-human primate stereotaxic head frame (Crist Instruments; Hagerstown, MD) using Vitamin E-filled ear bars. Once the animal’s head was secured in the frame, a small divot (1.0 mm in diameter) was drilled in the enamel of the incisors on each side of the upper mandible (Fig. 1) using a small round dental burr bit (1.0 mm in diameter). The reference teeth were cleaned with alcohol and the drilled divots were marked with a permanent black marker to assure easy visualization of the divot (Fig. 1). The tooth marker bar was attached to a standard electrode carrier (Model 1460; David Kopf, Tujunga, CA) and used as a stereotaxic localizer to obtain coordinates from the bilateral tooth markings in the anterioposterior, dorsoventral, and mediolateral dimensions (Fig. 1). The coordinates were recorded for the left- and right-sided tooth markings. The animal then underwent high-resolution (coronal plane; slice thickness 1–3 mm; no spacing) 1.5 or 3 T MR-imaging of the brain. Once the imaging was completed, the animal was awakened and taken to surgery at a distant time point from MR-imaging.
Fig. 1.
Photograph of the non-human primate stereotaxic frame with a non-human primate (Macaca mulatta) skull in place. (Left, inset) Demonstration of the stereotaxic localizer tip in the divot of the left incisor (dark area). (Right, inset) Close-up of the stereotaxic localizer tip in the divot of the left incisor (dark area). The divots drilled in the enamel of the bilateral incisors serve as permanent stereotaxic reference points.
2.3.2. Stereotaxic surgery
Based on the pre-operative MR-imaging, coordinates for the desired deep brain target were defined in all three planes before surgery. Using the Vitamin E-filled ear bars as bilateral reference points, target coordinates were calculated using standard MR-imaging software in all three planes. On the day of surgery (at a variable point in time after the pre-operative MR-scan was performed), the animal was anesthetized and placed in the stereotaxic frame. The divots were identified and darkened with a black marker to enhance visualization if necessary. The tooth marker was attached to the stereotaxic manipulator to measure the anterioposterior, dorsoventral, and mediolateral coordinates. The coordinates derived on the day of surgery were compared to the coordinates measured at the time of the pre-operative MR-imaging. If the coordinates for the reference tooth markings at the time of surgery did not match coordinates derived at the time of pre-operative MR-imaging, the animal was removed from the stereotaxic frame and then replaced/repositioned in the frame until the tooth markings correlated precisely. Once the tooth reference coordinates obtained on the day of surgery matched those taken at the time of the pre-operative scan, confirming the head is positioned exactly as it was at the time of pre-operative planning MR-scan, the animal underwent the stereotaxic surgery using the pre-operative image derive coordinates for the target. Anteroposterior coordinates were derived using the ear bars as the reference point, mediolateral coordinates were derived using the sagittal sinus as the reference point and dorsoventral coordinates were derived using the dura as the reference point.
3. Results
3.1. Animal and target characteristics
Between July 2002 and July 2004, 55 non-human primates underwent stereotaxic targeting of precise targets within deep brain structures for placement of either an infusion cannula (n = 45) or recording electrode (n = 10). The specific targets were located within the striatum (n = 5), brainstem (n = 30) (Fig. 2), hippocampus (n = 10), or basal forebrain (n = 10).
Fig. 2.

Post-operative magnetic resonance imaging demonstrating accurate, non-orthogonal, stereotaxic placement of an outer guide cannula (white arrowheads) and inner infusion cannula (black arrowheads) to a pre-operative imaging derived target (white cross) 6 weeks after pre-operative imaging was performed.
3.2. Accuracy and reliability of technique
Seventeen animals (31%) had to be repositioned in the stereotaxic frame (mean 1.8 times; range one to three times) so that tooth marking coordinates obtained at pre-operative MR-imaging accurately matched the tooth marking coordinates at the time of stereotaxic cannula or electrode placement. The cannula or recording electrode was placed precisely to target in 50 cases (91%) as determined by comparing the desired pre-operative MR-imaging target to actual location placement on post-operative MR-imaging (Fig. 2). In the remaining five animals (9%), the maximum error (in any single plane) that the cannula or electrode was placed from the desired target was less than or equal to 2 mm (Table 1). One of the 17 (6%) repositioned animals had an inaccurate cannula or electrode placement, while the other four cases of inaccurate placement occurred in animals that did not require repositioning. The time between obtaining the pre-operative MR-imaging and the stereotaxic surgery ranged from 1 week to 6 months. There was no decrease in accuracy with an increase in time between pre-operative imaging and stereotaxic surgery.
Table 1.
Plane and magnitude of error in the five animals with imprecise stereotaxic placement
| Animal number | Target | Anteroposterior error (mm) | Dorsoventral error (mm) | Mediolateral error (mm) |
|---|---|---|---|---|
| 1 | Left ventrolateral pons | 0 | 2.0 | 0 |
| 2 | Left ventrolateral pons | 0 | 2.0 | 0 |
| 3 | Right medioventral basal forebrain | 0 | 0 | 1.5 |
| 4 | Right globus pallidus externa | 0 | 1.0 | 1.5 |
| 5 | Right globus pallidus interna | 0 | 1.0 | 1.2 |
4. Discussion
4.1. Other stereotaxic techniques
Because of the variable size of individual non-human primate brains, targeting of deep brain structures using standardized stereotaxic atlases is fraught with inaccuracy (Aggleton and Passingham, 1981). To correct this inaccuracy and to enhance the reliability of non-human primate stereotaxy, a variety of techniques have been devised including intraoperative plain film roentenography, surgical placement of cranial fiducials, and frameless stereotaxy. Intraoperative plain film roentenography (Aggleton and Passingham, 1981; Ilinsky and Kultas-Ilinsky, 1982), while easily obtained and widely accessible, only enhances the accuracy of targeting structures close to known bony landmarks. Moreover, because this technique is based on coordinates derived from averaged measures, it has the same inherent problems as using a stereotaxic atlas. Surgical placement of cranial fiducials can provide increased accuracy of stereotaxic targeting (Alvarez-Royo et al., 1991; Dubach et al., 1985), but requires an additional operation and is associated with an increased risk of infection, particularly if the fiducials are implanted for prolonged periods of time before the actual stereotaxic surgery. Finally, frameless stereotaxy has been described for improving non-human primate stereotaxy (Frey et al., 2004), but requires specially designed equipment at an increased expense and necessitates surgical placement of skull fiducials for pre-operative imaging.
4.2. Current technique
To overcome the problems associated with previously described techniques for non-human primate stereotaxy, we employ an easy, readily available method that uses pre-operative MR-imaging and the non-surgical creation of lasting reference points (teeth marking) to obtain accurate and reproducible localization of small targets throughout the brain. The use of high resolution pre-operative MR-imaging for targeting deep brain structures for stereotaxis enhances accuracy and precision. This has been shown to avoid many of the factors associated with using standardized stereotaxic atlases or other radiographic techniques that can decrease stereotaxic accuracy including individual animal brain variation (due to age, size or state of health), image distortion and visibility of the target on imaging (Saunders et al., 1990; Subramanian et al., 2005)).
The use of tooth markings in non-human primate stereotaxis results in the reproducible and accurate replacement of the animal in the ear bars of the stereotaxic frame (a critical and frequent source of error in animal stereotaxy) and permits the precise targeting of pre-operative MR-imaging derived targets at distant time points from imaging. The creation of lasting reference points by drilling the bilateral incisors avoids potential problems associated with the use of “natural” tooth markings including changes in dentition over time (tooth chipping or wear) and the frequent lack of precise static points on teeth to reference. The enhanced accuracy of using this technique for stereotaxis can be inferred from the number of times (31% of the time) the animals had to be repositioned to match the tooth marks at the second stereotaxic procedure. All things constant, if these animals were not repositioned to match the original (pre-operative MR-imaging) tooth mark coordinates, the pre-operative MR-imaging derived targets would be missed by the same magnitude and direction as the tooth marking mismatch (Table 1).
5. Conclusion
We have used pre-operative MR-imaging combined with creation of tooth marking as reference points for accurate and reproducible stereotaxic localization of small targets throughout the brains of non-human primates. This technique gives considerable flexibility in regard to scheduling and preparing for stereotaxic surgeries that can be performed at distant time points from the pre-operative MR-imaging.
References
- Aggleton JP, Passingham RE. Stereotaxic surgery under X-ray guidance in the rhesus monkey, with special reference to the amygdala. Exp Brain Res. 1981;44(3):271–6. doi: 10.1007/BF00236564. [DOI] [PubMed] [Google Scholar]
- Alvarez-Royo P, Clower RP, Zola-Morgan S, Squire LR. Stereotaxic lesions of the hippocampus in monkeys: determination of surgical coordinates and analysis of lesions using magnetic resonance imaging. J Neurosci Meth. 1991;38(2/3):223–32. doi: 10.1016/0165-0270(91)90172-v. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337(8738):403–6. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach MF, Tongen VC, Bowden DM. Techniques for improving stereotaxic accuracy in Macaca fascicularis. J Neurosci Meth. 1985;13(2):163–9. doi: 10.1016/0165-0270(85)90029-9. [DOI] [PubMed] [Google Scholar]
- Frey S, Comeau R, Hynes B, Mackey S, Petrides M. Frameless stereotaxy in the nonhuman primate. NeuroImage. 2004;23:1226–34. doi: 10.1016/j.neuroimage.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ilinsky IA, Kultas-Ilinsky K. Stereotactic surgery in the rhesus monkey (Macaca mulatta) utilizing intracerebral landmarks. Appl Neurophysiol. 1982;45(6):563–72. doi: 10.1159/000101664. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Exp Brain Res. 1990;81(2):443–6. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Subramanian T, Deogaonkar M, Brummer M, Bakay R. MRI guidance improves accuracy of stereotaxic targeting for cell transplantation in parkinsonian monkeys. Exp Neurol. 2005;193(1):172–80. doi: 10.1016/j.expneurol.2004.11.032. [DOI] [PubMed] [Google Scholar]