Abstract
Obesity, an established risk factor for breast cancer (BC), is associated with systemic inflammation. The breast contains adipose tissue (bAT), yet whether it plays a role in BC progression in obese females is being intensively studied. There is scarce knowledge on the lipid composition of bAT in health and disease. The purpose of this pilot study was: 1) to determine whether obesity and BC are associated with inflammatory changes in bAT 2) to analyze for the first time the lipid profile of bAT in obese and lean mammary tumor-bearing and normal mice. Syngeneic E0771 mammary tumor cells were implanted into the mammary fat pad of lean and diet-induced obese C57BL/6 mice. BATs were analyzed four weeks after tumor cell inoculation by immunohistochemistry and mass spectrometry. Phospholipids were identified and subjected to ratiometric quantification using a TSQ Quantum Access Max triple quadrupole mass spectrometer utilizing precursor ion scan or neutral ion loss scan employing appropriate class specific lipid standards in a two step quantification process. Four main classes of phospholipids were analyzed: phosphatidylcholines phosphatidylserines, phosphatidylethanolamines and phosphatidylinositols. Our results showed that bAT in obese (normal and tumor-bearing) mice contained hypertrophic adipocytes compared with their corresponding samples in lean mice; higher numbers of macrophages and crown-like structures were observed in obese tumor bearers compared to obese normal mice. BAT from normal obese mice revealed higher concentrations of phosphatidylethanolamines. Furthermore, bAT from tumor-bearing mice expressed higher phosphatidylcholines than that from non-tumor bearing mice, suggesting the presence of the tumor is associated with phosphatidylcholines. Conversion of phosphatidylethanolamines to phosphatidylcholines will be investigated in E0771 cells. Additional studies are projected to investigate macrophage activation by these specific classes of phospholipids. Occurrence of triglycerides and free fatty acids will be examined in bAT and similar lipidomic analyses will be carried out visceral adipose tissue, highly inflamed in obesity.
Keywords: Breast cancer, obesity, inflammation, macrophages, phospholipids, lipidomics
Introduction
Obesity, a world epidemic and a new disease in the United States, is an established risk factor for breast and other cancers [1,2]. Obesity is associated with systemic inflammation and increased visceral adipose tissue [3]. Macrophages play a central role in inflammation and also in its resolution, given that these innate immune cells can exhibit pro-inflammatory (M1) and also anti-inflammatory/immunosuppressive (M2) immune responses [4]. In obesity, visceral adipose tissue contains high numbers of pro-inflammatory M1 macrophages, crown like structures (CLS) and hypertrophic and hyperplastic adipocytes, which is why it is considered a setting with low level chronic inflammation [5,6]. Lean adipose tissue, on the other hand, contains fewer numbers of anti-inflammatory M2 macrophages. Macrophages participate in all stages of tumorigenesis; during the early phases of tumor development (initiation), macrophages exhibit more pro-inflammatory/pro-mutagenic M1 phenotypes while in more advanced tumors they are associated with an immunosuppressed M2 phenotype [7–9]. Interestingly, we have found mixtures of M1 and M2 macrophages in mouse mammary tumor models [10,11]. Mechanisms of tumorigenesis from excess adipose tissue involve low level chronic inflammation (including increased amounts of pro-inflammatory molecules and macrophages) and angiogenesis [12].
The mammary gland is comprised by a significant amount of adipose tissue (breast adipose tissue, bAT), subcutaneous in location, which is important for mammary gland development. We have been recently interested in investigating whether the bAT – which, together with macrophages is a major component of the mammary tumor microenvironment - plays a role in mammary tumorigenesis in obese females. In the present investigation we first sought to examine whether the bAT in obese female mice (mammary tumor-bearing and normal mice) displays inflammatory features, that is, contains higher numbers of macrophages, CLS and hypertrophic adipocytes than similar tissues in lean mice.
Importantly, activation of lipid metabolism is an early event in carcinogenesis [13–15]. Research in the lipidomics field has been proliferating with the advent of new technologies. Currently there is no information on the lipid composition of different fat depots in the body, especially in the context of obesity, and even less among obese tumor hosts. Capitalizing on our group’s experience in phospholipid analysis [16,17], we pursued the identification of the main phospholipids present in the bAT of obese and lean normal and mammary tumor-bearing mice. Our main aim here was to examine whether there is an association between bAT inflammation, obesity and mammary cancer with a particular phospholipid signature in the mammary gland.
New profiling methods employing shotgun lipidomics, a technique used in mass spectrometry via direct loading of crude lipid extracts into the mass spectrometer for intra-source separation and identification of numerous lipids, allows for extensive cellular lipid profiles of different tissues being accrued with relative ease [16–20]. Using the new technology of shotgun lipidomics allowed us to analyze bAT including the differences in profiles of phospholipids between lean control mice (non-tumor bearing), lean mammary tumor-bearing, obese control mice, and obese mammary tumor-bearing mice. Large-scale studies will be required for further characterization and confirmation of phospholipid quantities, but vast amounts of information can be preliminarily gathered with a small sample size like this one in the present pilot study. Profiling the differences in lipid composition will be the first step to understanding whether there is a particular or unique lipid composition in bAT in breast cancer, in obesity or in their intersection, for diagnostics and therapeutics purposes.
2. Experimental
2.1. Materials and Methods
2.1.1. Mice, diets, tumors
C57BL6 female mice of 10 weeks of age (NCI Frederick, MD) were used. Institutional animal care and use committee (IACUC) approved all the animal experiments. Diet-induced-obesity: To induce obesity, we used a high fat diet (HFD) containing 60%Kcal from fat (TD.06414, Harlan Laboratories, Inc., Madison, WI), whereas a control low fat diet (LFD) containing 10%Kcal/fat was employed to generate lean control mice (TD.94048, Harlan Laboratories, Inc., Madison, WI). Five mice were kept per cage for both types of diets and body weight was evaluated weekly. Obese status was assessed by body weight (BW) increase and was defined as ≥25% BW higher than normal BW group (lean). Tumor cell inoculation: Twelve weeks after the diets were introduced, E0771 mammary tumor cells, syngeneic to C57BL6 mice, were resuspended in PBS: Matrigel (BD Pharmingen, San Jose, CA) 1:1 and subcutaneously (s.c) injected in the fat pad of the fourth mammary gland in the lower abdomen at 2.5×105 cells/50 μl/mouse. Primary tumor growth was measured at weekly intervals using calipers, and mice were kept in their respective diets until they were euthanized 4 weeks post- tumor implantation by CO2.
2.1.2. Tissue procurement
Mammary (breast) adipose tissues (bAT) adjacent but not within the tumor microenvironment, all equivalent in wet weight, were excised from all the mice of the different experimental groups. Samples were either fixed in 4% paraformaldehyde and paraffin-embedded for histology/IHC analysis, or stored at −80°C until lipids were later extracted for lipidomics analysis. Experimental groups were: obese tumor-bearing (n=5), obese control (n=2), lean tumor bearing (n=4) and lean control mice (n=2).
2.1.3. Histology and Immunohistochemistry
Tissue sections were stained with Hematoxylin and Eosin (H&E) to reveal the histology of the adipose tissues. Immunohistochemistry (IHC) was performed to identify macrophages as previously reported [11] using the VECTASTAIN ABC Kit as described by the manufacturer (Vectors Lab, CA). The primary antibody used for macrophage IHC detection was a rat monoclonal anti-mouse F4/80 (Abcam, MA). Antigen retrieval was performed in Citrate Buffer (Antigen Unmasking Solution, Vector Laboratories). The first antibody was incubated overnight at 4°C.
2.1.4. Lipid extraction
Lipids were extracted using the modified Bligh-Dyer method [21–25]. The tissue was first alternated between −80°C and water bath 37°C for a total of 5 cycles to break the cellular membranes; tissues were then minced. A 1:1 mixture of Chloroform: Methanol was then added to the tissue and each sample was homogenized for 2 minutes. Chloroform was then added to the mixture and the tissue was homogenized for 30 seconds. A vortex was then used to mix the samples. The samples underwent centrifugation at 13,500 rpm for 15 minutes in a cold room. The lipid layer was then visualized and extracted. The lipid was divided into 7 aliquots and then dried using a Speed-Vac (Model 7810014; Labconco, Kansas City, MO). The lipids were flushed with Argon gas and stored in the −80°C right after being dried. The remaining aqueous phase, which consisted of proteins, was analyzed for protein concentration using Bradford’s method [26].
2.1.5. Mass spectrometer
A triple quadrupole mass spectrometer (TSQ Quantum Access Max; Thermo Fisher Scientific, Pittsburgh, PA) was used for the analysis. An aliquot of 100 uL for each sample was suspended in 200 uL mixture of Acetonitrile: Isopropanol (1:1) solution (Sigma Aldrich) and directly infused into the mass spectrometer. Lipid class settings were determined from previously published papers [18–20,22–25] and analysis was performed for each of the phospholipids. A nanospray was used (Advion, Ithaca, NY) for infusion. The sample was scanned for a total of two minutes. At least two scans were taken to insure reproducibility. The lipid standards used were the same as previous studies of phospholipids (all procured from Avanti Polar Lipids, Albaster, AL) namely 1,2-ditridecanoyl-sn-glycero-3-phosphocholine (molecular mass 649.89, catalog no. 850340) for PCs, 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (molecular mass 810.03, catalog no. 840035) for PSs, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (molecular mass 744.04, catalog no. 850725) for PEs and 1,2-dioleoyl-sn-glycero-3-phospho-(10-myo-inositol) (molecular mass 880.15, catalog no. 850149) for PIs [22,25]. Also, samples were analyzed without standards in order to ensure that the mass by charge (m/z) of the standard was not a normal constituent of the biological tissue.
2.1.6. Lipid analysis
MzMine 2.10 was then used to analyze the data. For the phosphatidylcholines (PC) any signal that was below 1e2 was considered noise. The data was also crop filtered for m/z that was 350–900. For the remaining classes given the low signal anything below 1e0 was considered noise. A centroid peak was used for determination of m/z and a database from LIPID MAPS was used for identification. Radiometric quantification was used with internal standard and the protein concentration of each sample was determined using the Bradford assay. The concentration of lipid in picomoles was then normalized using the protein concentration. For each of the four groups, the lipid concentrations of the different samples were averaged together that appeared in the same group. The number of carbon chains of each lipid identified was estimated and grouped together after averages were performed.
2.1.7. Statistical analysis
Prism software was used to statistically analyze our results. One or two-way ANOVAs were used when comparing more than two experimental groups; ANOVA was then followed by Tukey’s Multiple Comparisons Test (TMCT) to compare the means between pairs of groups among the larger group analyzed by ANOVA. Levels of significance were provided by Prism analysis for * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001 and **** P ≤ 0.0001, although actual p values are not provided by this software in the TMCT analysis. Error bars represent standard error of the mean (SEM) and alpha was set at 5%.
3. Results
3.1. Breast adipose tissue adjacent but not within the mammary tumor microenvironment is inflamed in obese mammary tumor bearers and in fewer amounts also in obese normal mice
To examine whether obesity, mammary cancer or their intersection may impact inflammation in the bAT that is not within the tumor microenvironment but is close to the tumor, we studied bAT samples of lean and obese normal (control) and mammary tumor-bearing mice. Our results (Figure 1) show that the bAT in obese (normal and tumor-bearing) mice contains larger, hypertrophic adipocytes compared with the corresponding samples in lean mice. Interestingly, higher numbers of F4/80+ macrophages and CLS are observed in obese tumor bearers than in obese normal (control) mice. Among the lean mice, where adipocytes are smaller and not hypertrophic compared with obese mice, bAT from tumor bearers exhibit more F4/80+ macrophages than normal (control) mice.
Figure 1.
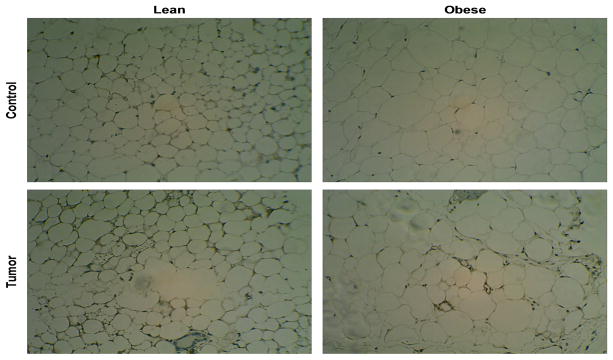
Immunohistochemistry analysis displays the inflammatory makeup of the four experimental groups pertaining to adipocyte morphology and F4/80+ macrophages colonization. Slides were all focused to a 20x magnification and were tagged with F4/80 marker to highlight macrophages recruited within the bAT (F4/80+ cells in brown).
3.2. Phosphatidylethanolamines and phosphatidylcholines predominate over phosphatidylserines and phosphatidylinositols in the bAT from all four experimental groups
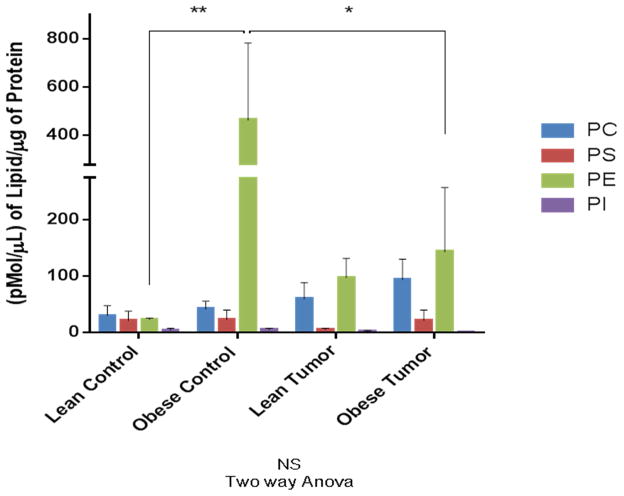
We analyzed the phospholipid composition of the bAT in all the mice comprising our four experimental groups (Figure 2). Our results show the mean concentration levels of the four main classes of phospholipids present in the bAT samples analyzed: phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI). Two-way ANOVA comparison of all the samples was not significant; however, Tuckey’s Multiple Comparison Test (TMCT) revealed a significant difference between PE levels in lean controls vs. obese controls (p ≤ 0.01), and a significant difference between PE levels in obese controls vs. obese tumor bearers (p ≤ 0.05). The largest total concentration observed was in PE in the obese control condition. The least amount of phospholipids per class was observed in the lean control mice. The group of obese control mice showed the highest concentration of PE, while the other phospholipid classes remained relatively low concentration, with obese tumor bearers showing higher PE concentration than lean tumor bearers. Lean tumor bearers had the second highest concentration of PCs and the lowest of PS, and obese tumor bearers had the highest concentration of PCs and second highest concentration of PEs (Figure 2). Interestingly, incremental concentrations of PCs were observed from lean controls to obese tumor bearers. In general, PIs were the phospholipids expressed at the lowest concentrations in bAT, and seemed not to be modulated by obesity or by tumor presence. They were followed in amount by PS.
Figure 2.
PE and PC prevail among the four phospholipid classes studied whenever obesity or tumor conditions are present. Bar graph shows comparison of all experimental groups being studied in terms of mean values expressed in pmol/μl of lipid by μg of protein.
3.3. Higher concentrations of phospholipids are associated with higher-numbered fatty acids side chains in the bAT of all the experimental groups
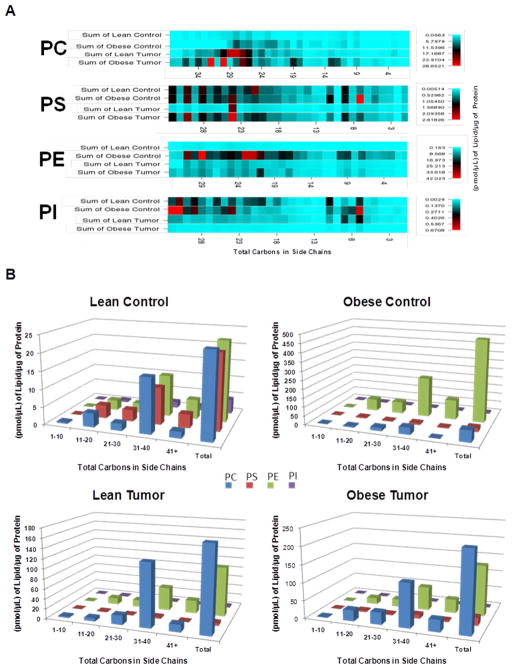
All phospholipids contain two molecules of fatty acids (side chains). The phospholipid compositions identified are represented by side chain distributions of the fatty acids and a heat map. This provides a greater understanding of the chemical composition of the four phospholipid classes analyzed. Fatty acids may vary in length according to the numbers of carbon atoms they contain. We added the total number of carbon atoms of the two chains of fatty acids comprising the different classes of phospholipids analyzed, and investigated whether there was a pattern of distribution among the four phospholipid classes, their concentrations in the bATs and the total number of carbon atoms among the different experimental groups. Our data, presented as a heat map (Figure 3A) demonstrate that the highest concentrations of phospholipids contain fatty acids with high total numbers of carbon atoms. Lean control mice had very few PC and PE phospholipids and lean tumor bearers group had a loss of PS. Figure 3B confirms that the side chain distribution still favors significantly more PCs and PEs, control obese group had higher numbers of PEs, whereas PCs prevail in the tumor condition. The lean control group seemed to have smaller chain PIs, but very little amounts compared with the other classes.
Figure 3.
Phospholipids are highly concentrated in higher-numbered total carbons of fatty acid side chains. A: Heat map diagrams of the four classes of phospholipids express the phospholipid concentration. Using the sum values for the lipids and the sum of the side chains, a table was constructed of the concentrations for each of the four experimental groups and arranged by total carbon atoms in side chains. A CIMminer-generated heat map was created (Genomics and Bioinformatic Group, Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute). The darker colors represent the higher concentrations. Carbon chain sizes were grouped together and estimated. B: Relative side chain distribution of each class based on the four experimental groups. The sum of the sample concentrations was used for each condition in the four phospholipid classes. The fatty acid side chains of each of the phospholipids identified were added and set together into specific ranges to demonstrate the concentration of phospholipids based on the total carbons in the side chains of the lipids.
3.4 Unique PC species are present in the bAT of obese and lean tumor bearers when compared to their obese and lean controls
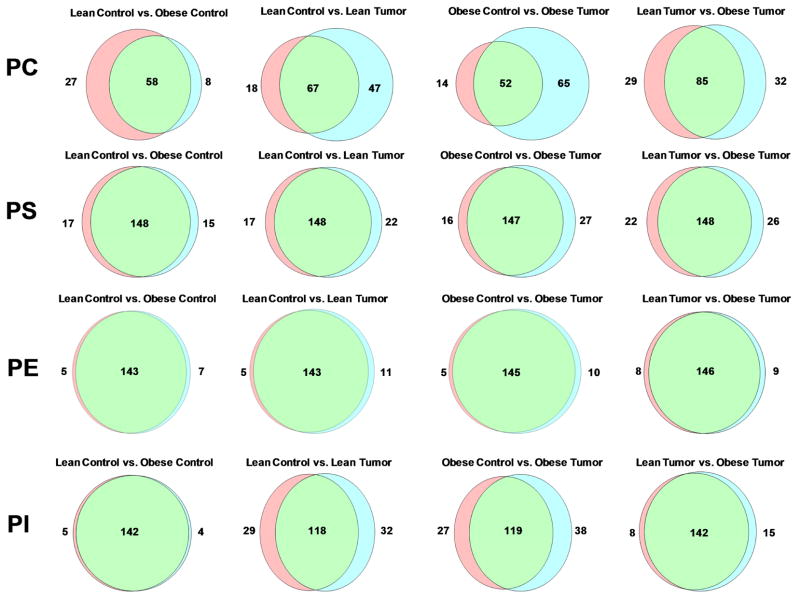
Combinations of fatty acids with different lengths and saturation levels within each phospholipid class account for the enormous variability within these lipids. We analyzed whether there was a particular pattern of fatty acid uniqueness among the four phospholipid classes expressed in the bAT among the different experimental groups. Our results using Venn diagrams revealed (Figure 4) that the greatest variability among the phospholipids of the bATs occur in PCs, particularly among obese and lean tumor bearers (lean tumor bearers vs lean controls; obese tumor bearers vs. obese controls and obese tumor bearers vs. obese controls). Our results indicate that the presence of the tumor in the mammary gland endows bAT – which is not in direct contact with the tumor - with unique specimens of PC both in obese and lean tumor bearers, with a higher number of unique specimens in the obese tumor bearers than in the lean tumor bearers. In contrast, no unique specimens of PE – the most prevalent phospholipid in bAT - could be found when comparing the four experimental groups among themselves.
Figure 4.
Venn diagrams represent the common and unique lipid across the phospholipid classes. Using a Venn Diagram Plotter program, a diagram is generated proportional to the numbers of common and unique lipids entered for the common and unique values. The green section represents the common lipids between the two compared conditions, and the red and blue sections highlight the unique lipids found in the respective conditions.
Discussion
Tumor stroma in addition to the tumor cells comprises the tumor microenvironment, which plays a critical role in carcinogenesis [27]. Among the major tumor microenvironment’s cellular players in tumor progression are the macrophages [28]. In addition to their participation in tumor progression, macrophages seem to be affected by tumors, and proximity to a tumor has been shown to play a significant role in the alteration of macrophages phenotypes and functions. When comparing tumor-associated macrophages with peritoneal macrophages in tumor-bearing mice, very distinct phenotypes where determined in the two different subpopulations [11].
We here demonstrated how the lipid profiles of bAT that is not part of the tumor microenvironment were affected based on the different cell composition of the mammary bAT from tumor-bearing obese or lean mice and obese normal mice when compared to lean normal mice. Even though the number of control mice was very small (n=2) for both lean and obese controls, in the case of the obese controls the values were consistently very different between themselves, resulting in a high SEM. Despite that, TMCT analysis demonstrated statistically significant differences (p ≤ 0.01 and p ≤ 0.05) when comparing lean control vs. obese control and obese control vs. obese tumor-bearers, respectively. We believe that the relevance of our pilot study is in the fact that trends in the expression of all these phospholipids among the different experimental groups have been revealed.
Other investigators have provided evidence for inflammation in the bAT of obese female mice and women [29,30]. Adipocytes, together with macrophages, are among the most important cell types in the mammary tumor microenvironment [31]. We have recently shown that these two cell types crosstalk with mammary tumor cells in the mammary tumor microenvironment, resulting in the production of pro-inflammatory chemokines, cytokines and growth factors that contribute to macrophage chemotaxis to the tumor microenvironment and to additional tumor-promoting functions [32]. Tumor-associated macrophages favor tumor progression and are signs of poor tumor prognosis [33]. We have also recently demonstrated that bAT within the mammary tumor microenvironment and also distal from it are inflamed in diet-induced obese (DIO) mice fed a 33% HFD, and revealed that the proximity to mammary tumor cells enhances bAT inflammation [32].
In the current investigation we analyzed DIO mice fed a different HFD containing 60%kcal/fat using the same E0771 mammary tumor model; we now focused on the bAT that is not within nor distal from the mammary tumor microenvironment, but adjacent to the tumor in the mammary gland. We showed that there were higher numbers of macrophages in this bAT from obese mice as compared to lean from both normal and tumor-bearing mice. We revealed that the presence of both obesity and tumor resulted in the highest bAT inflammation in this fat tissue that is adjacent but not in direct contact with the mammary tumor. This could probably be due to the local diffusion of molecules produced by the tumor cells and/or by various stromal cells of the tumor microenvironment, as well as the superimposed systemic inflammation in the obese mice, acting together to increase inflammation in the bAT. These results were not surprising since we have shown that bAT that is distal (not adjacent) to the tumor in DIO tumor bearers fed a HFD containing less Kcal/fat (33%) also exhibited increased inflammation [32].
Obesity could result in increased inflammation and increased macrophage colonization in the bAT through dyslipidemia. For example, in cardiovascular disease dyslipidemia plays a role in increased inflammation. We showed higher amounts of phospholipids in obese bAT as compared to lean as expected. Interestingly, bAT from obese mice without tumors had elevated amounts of phosphatidyletahnolamines (PE). Some studies have shown PE lipids to be tumor suppressors and to induce apoptosis in tumor cells [34]. Obesity is a known risk factor for breast cancer and the increased inflammation may induce greater amounts of PE. Tumor- bearing bAT had greater amount of PC than bAT from mice without tumors. Given the lower amounts of PE in bAT from tumor-bearing mice than in the obese control animals, we could speculate that the tumor cells in the mammary tumor microenvironment may be converting PE to PC in order to ensure their survival. Studies have also shown tumor cells to undergo lipogenesis in order to ensure survival [35]. It may also be debris from the cells.
Phosphatidylserine (PS) has been shown to inhibit the nitric oxide pathway involved in the cytotoxic effect of the macrophages [36]. Importantly, PS also induces anti-inflammatory/immunosuppressive responses in macrophages when expressed by apoptotic cells that need to be silently phagocytosed by macrophages without triggering inflammation [37]. Our results showed that under lean conditions there was less PS in the bAT. High-resolution mass spectrometry may reveal specific PS that may be involved in bAT from tumor bearers as opposed to control mice.
Further studies may help characterize the inflammatory effect of phospholipids on macrophages, particularly the ones that were detected in the bAT of obese and lean tumor-bearing and normal mice. We have shown the effects of the lipid changes in bAT from tumor-bearing versus control obese and lean mice. It will be interesting to characterize the different phospholipid composition in different adipose tissue sites, such as the visceral fat, highly inflamed in obesity, and to compare it with our results in bAT. Moreover, we will pursue characterizing other lipid molecules in these different fat depots such as triglycerides and free fatty acids, which are activators of toll-like receptor 4 (TLR4) in macrophages and other cells [38].
New studies may also help determine if tumor cells produce an enzyme that converts PE to PC such as phosphatidylethanolamine N-methyltransferase (PEMT). PEMT catalyzes the methylation from PE to PC [39]. Increased genetic activity of PEMT has been associated with augmented obesity. However, increased choline intake has been shown to be less of a risk factor for cancer. The genotype PEMT-GG has been shown to be a lower risk for cancer [40]. PEMT is influenced by estrogen and activity responds to estrogen [39]. So far it has not been shown in the literature despite certain studies showing specific PC being known to be prognostic indicators and influencing aggressiveness of tumor [15].
PEMT is a component in the liver and lack of the enzyme results in steatohepatitis. However, the appearance of the lipid profile appears to show that cancer cells may contain PEMT in order to convert PE to PC. It would be interesting to see whether breast cancer cells do contain this enzyme and if it varies genotypically in cancer cells as opposed to hepatic cells. This may provide a new target for breast cancer therapy.
Breast adipose tissue is inflamed in obese mice, containing hypertrophic adipocytes.
Breast fat contains high numbers of macrophages and crown-like structures in obesity.
Highest numbers of macrophages and crown-like structures occur in obese tumor bearers.
Breast fat from normal obese mice has higher concentrations of phosphatidylethanolamines.
Breast fat from tumor bearers expressed higher phosphatidylcholines than normal mice.
The presence of the tumor is associated with phosphatidylcholines.
Acknowledgments
This work was supported by the National Institute of Health (NIH) grant R21CA153172 (to MTK), the Institutional Research and Development Initiative grant (IRDI, University of Miami, to MTK, AJM and AN) and by NIH grants EY016112, EY016112S1, P30-EY14801, a Research to Prevent Blindness (RPB) unrestricted grant to University of Miami. The TSQ Quantum Access Max procurement was supported by Department of Defense Grant W81XWH-09-1-0674 (to SB).
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael Margolis, Email: m.margolis@med.miami.edu.
Osvaldo Perez, Jr., Email: op52@nova.edu.
Mitchel Martinez, Email: mmartinez@sanjuanbautista.edu.
Ana M. Santander, Email: AMsantander@med.miami.edu.
Armando J. Mendez, Email: amendez@med.miami.edu.
Mehrdad Nadji, Email: mnadji@med.miami.edu.
Ali Nayer, Email: anayer@med.miami.edu.
Sanjoy Bhattacharya, Email: sbhattacharya@med.miami.edu.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the united states, 2009–2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of u.S. Adults. The New England journal of medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Vona-Davis L. Biochemical and molecular mechanisms for the association between obesity, chronic inflammation, and breast cancer. Biofactors. 2014;40:1–12. doi: 10.1002/biof.1109. [DOI] [PubMed] [Google Scholar]
- 4.Torroella-Kouri M, Rodriguez D, Caso R. Alterations in macrophages and monocytes from tumor-bearing mice: Evidence of local and systemic immune impairment. Immunologic research. 2013;57:86–98. doi: 10.1007/s12026-013-8438-3. [DOI] [PubMed] [Google Scholar]
- 5.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. Journal of lipid research. 2011;52:480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature reviews Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 10.Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA, Iragavarapu-Charyulu V, Cardentey Y, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither m1 nor m2 and are less differentiated. Cancer research. 2009;69:4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez D, Silvera R, Carrio R, Nadji M, Caso R, Rodriguez G, Iragavarapu-Charyulu V, Torroella-Kouri M. Tumor microenvironment profoundly modifies functional status of macrophages: Peritoneal and tumor-associated macrophages are two very different subpopulations. Cellular immunology. 2013;283:51–60. doi: 10.1016/j.cellimm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Hernandez AI, Catalan V, Gomez-Ambrosi J, Rodriguez A, Fruhbeck G. Mechanisms linking excess adiposity and carcinogenesis promotion. Frontiers in endocrinology. 2014;5:65. doi: 10.3389/fendo.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World journal of biological chemistry. 2012;3:167–174. doi: 10.4331/wjbc.v3.i8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notarnicola M, Tutino V, Caruso MG. Tumor-induced alterations in lipid metabolism. Current medicinal chemistry. 2014;21:2729–2733. doi: 10.2174/0929867321666140303122426. [DOI] [PubMed] [Google Scholar]
- 15.Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer research. 2011;71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya SK. Recent advances in shotgun lipidomics and their implication for vision research and ophthalmology. Current eye research. 2013;38:417–427. doi: 10.3109/02713683.2012.760742. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez-Algeciras M, Bhattacharya SK. Lipidomic mass spectrometry and its application in neuroscience. World journal of biological chemistry. 2013;4:102–110. doi: 10.4331/wjbc.v4.i4.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Analytical chemistry. 2009;81:4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwudke D, Schuhmann K, Herzog R, Bornstein SR, Shevchenko A. Shotgun lipidomics on high resolution mass spectrometers. Cold Spring Harbor perspectives in biology. 2011;3:a004614. doi: 10.1101/cshperspect.a004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, Zhao Z, Gross RW, Han X. Identification and quantitation of unsaturated fatty acid isomers by electrospray ionization tandem mass spectrometry: A shotgun lipidomics approach. Analytical chemistry. 2011;83:4243–4250. doi: 10.1021/ac2006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Edwards G, Aribindi K, Guerra Y, Lee RK, Bhattacharya SK. Phospholipid profiles of control and glaucomatous human aqueous humor. Biochimie. 2014;101:232–247. doi: 10.1016/j.biochi.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerra Y, Aljohani AJ, Edwards G, Bhattacharya SK. A comparison of trabecular meshwork sphingolipids and ceramides of ocular normotensive and hypertensive states of dba/2j mice. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:283–290. doi: 10.1089/jop.2013.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aljohani AJ, Munguba GC, Guerra Y, Lee RK, Bhattacharya SK. Sphingolipids and ceramides in human aqueous humor. Molecular vision. 2013;19:1966–1984. [PMC free article] [PubMed] [Google Scholar]
- 25.Aribindi K, Guerra Y, Lee RK, Bhattacharya SK. Comparative phospholipid profiles of control and glaucomatous human trabecular meshwork. Investigative ophthalmology & visual science. 2013;54:3037–3044. doi: 10.1167/iovs.12-10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 27.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nature reviews Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Current opinion in immunology. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Subbaramaiah K, Hudis CA, Dannenberg AJ. The prostaglandin transporter regulates adipogenesis and aromatase transcription. Cancer Prev Res (Phila) 2011;4:194–206. doi: 10.1158/1940-6207.CAPR-10-0367. [DOI] [PubMed] [Google Scholar]
- 30.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 32.Santander AM, Lopez-Ocejo O, Casas O, Agostini T, Sanchez L, Lamas-Basulto E, Carrio R, Cleary MP, Gonzalez-Perez RR, Torroella-Kouri M. Paracrine interactions between adipocytes and tumor cells recruit and modify macrophages to the mammary tumor microenvironment: The role of obesity and inflammation in the breast adipose tissue. Cancer Open Access Submitted. 2014 doi: 10.3390/cancers7010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira AK, Meneguelo R, Pereira A, Filho OM, Chierice GO, Maria DA. Synthetic phosphoethanolamine induces cell cycle arrest and apoptosis in human breast cancer mcf-7 cells through the mitochondrial pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2013;67:481–487. doi: 10.1016/j.biopha.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer research. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 36.Calderon CL, Torroella-Kouri M, Dinapoli MR, Lopez DM. Involvement of protein kinase c and not of nf kappa b in the modulation of macrophage nitric oxide synthase by tumor-derived phosphatidyl serine. International journal of oncology. 2008;32:713–721. [PubMed] [Google Scholar]
- 37.Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J Immunol. 2003;171:4672–4679. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- 38.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. The Journal of biological chemistry. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vance DE. Physiological roles of phosphatidylethanolamine n-methyltransferase. Biochimica et biophysica acta. 2013;1831:626–632. doi: 10.1016/j.bbalip.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, Teitelbaum SL, Bradshaw PT, Neugut AI, Santella RM, Chen J. Choline metabolism and risk of breast cancer in a population-based study. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:2045–2052. doi: 10.1096/fj.07-101279. [DOI] [PMC free article] [PubMed] [Google Scholar]