Abstract
In some adult patients with cervical syringomyelia, MRI studies do not identify primary disease within the foramen magnum or spinal canal. To identify the etiology of this idiopathic type of syringomyelia, clinical features and posterior fossa (PF) measurements from 17 of these patients, 17 patients with Chiari I-type syringomyelia, and 32 control subjects were compared. Idiopathic syringomyelia and Chiari I-type syringomyelia manifested central cervical myelopathy and a small PF with narrow CSF spaces, suggesting that they develop by the same mechanism.
Syringomyelia is a polyetiologic disorder characterized by a fluid-filled cyst within the spinal cord. In this report, the term “syringomyelia” is used broadly to include hydromyelia, cystic enlargement of the spinal cord central canal with a preserved ependymal lining, and spinal cord cysts that may or may not connect with the central canal of the spinal cord and are lined primarily by glial fibers.1 Syringomyelia is associated with conditions that obstruct CSF flow at the foramen magnum or spinal levels, such as Chiari I malformation (CM1), basilar invagination, and arachnoiditis. MRI and pathologic studies indicate that lesions obstructing the subarachnoid space are present in most cases of syringomyelia and that syrinx fluid usually does not communicate with the fourth ventricle in adult patients.2 Recent clinical and animal research suggests that syringomyelia associated with CM1 results from spinal subarachnoid CSF entering the spinal cord through the perivascular spaces, being propelled by enlarged pulsatile CSF pressure waves that originate from the piston-like motion of the herniated cerebellar tonsils on the spinal subarachnoid space. Excess extracellular fluid accumulates and coalesces into a syrinx.3
Most cases of syringomyelia are associated with CM1.2 MRI diagnoses CM1 if the cerebellar tonsils extend 3 to 5 mm below the foramen magnum (FM),4 although associated features include compression of cerebellar cisterns, decreased posterior fossa (PF) volume, and dysplasia of the posterior aspect of the skull base.2 CM1 arises because an underdeveloped PF with decreased volume cannot accommodate the normally developed hindbrain.3 Development of syringomyelia in CM1 is more closely associated with narrowing of CSF pathways than with extent of tonsillar ectopia at the FM.5
Earlier we analyzed the disease course in patients who had syringomyelia with tonsillar herniation but did not identify the etiology of syringomyelia in patients without tonsillar herniation.6 The current study was designed to discover the cause of this “idiopathic” type of syringomyelia by comparing clinical and MRI data from these patients with data from patients with Chiari I-type syringomyelia and from control subjects. We excluded patients with other causes for syringomyelia. Our hypothesis was that “idiopathic” cervicothoracic syringomyelia is associated with a small PF.
Patients and methods
This study included 17 adult patients with idiopathic syringomyelia affecting the cervical spinal cord, 17 randomly chosen adult patients with cervical syringomyelia associated with CM1, and 32 adult control subjects (8 normal subjects and 24 persons evaluated for disorders not accompanied by spinal cord pathology [migraine headache, 10; benign paroxysmal positional vertigo, 4; trigeminal neuralgia, 4; tension headache, 5; and cluster headache, 1]). The idiopathic syringomyelia group consisted of 2 women and 15 men, age 33 to 64 years (mean 49 years), with cerebellar tonsils positioned above the FM and MRI-verified and clinically symptomatic cervicothoracic syringomyelia for 1 to 43 years (mean 23 years). Group CM1 consisted of 4 women and 13 men, age 21 to 63 (mean 49) years, with tonsillar ectopia of ≥5 mm below the FM and MRI-verified and clinically symptomatic syringomyelia for 2 to 47 (mean 22) years.
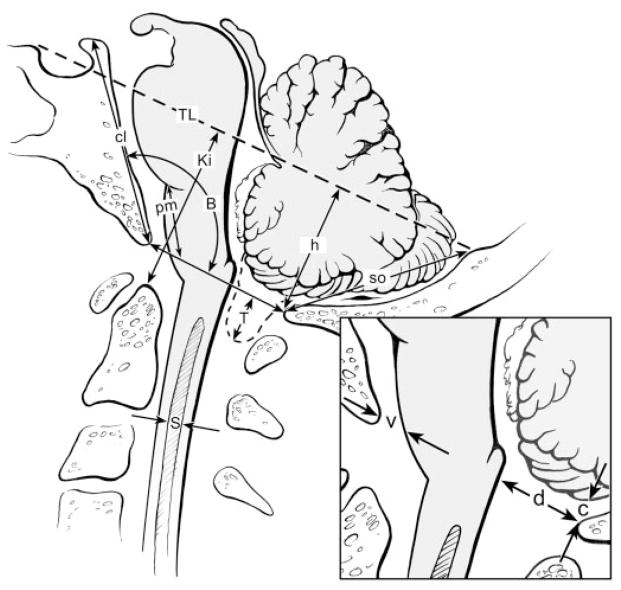
All 34 patients with syringomyelia and 32 control subjects underwent clinical examination and MRI scans of the brain and cervical and thoracic spine. In patients, other causes of syringomyelia such as tumor, arachnoiditis, or previous meningitis, subarachnoid hemorrhage, or trauma were excluded by history, clinical examination, and MRI study. Imaging was performed on the 1.0 T General Electric Signa Horizon (Milwaukee, WI) or 0.28 T Bruker with SGI workstation (Ettlingen, Germany). Standard T1- and T2-weighted images were taken in sagittal, coronal, and axial planes. RARE (rapid acquisition with relaxation enhancement) MR-hydrography imaged syrinx cavities in three planes.7 Measurements of CSF spaces, bony structures, and angles at the craniovertebral junction and PF were performed on sagittal slice MR images (figure 1).3,8 Measurements included 1) cerebellar tonsillar herniation below FM (T); 2) Twining line (TL; tuberculum sellae to internal occipital protuberance) to opisthion (h; PF height); 3) Klaus index (Ki; tip of dens to Twining line); 4) pontomedullary junction to FM (pm)9; 5) supraocciput (so; internal occipital protuberance to opisthion); 6) clivus (cl; apex of dorsum sellae to basion; 7) Boogaard angle formed by the clivus, basion, and opisthion (B); 8) vertical distance between cerebellum and opisthion (c); 9) anteroposterior diameter of ventral FM CSF pathway (v); 10) anteroposterior diameter of dorsal FM CSF pathway (d); and 11) widest anteroposterior diameter of syrinx (S) (figure 2).
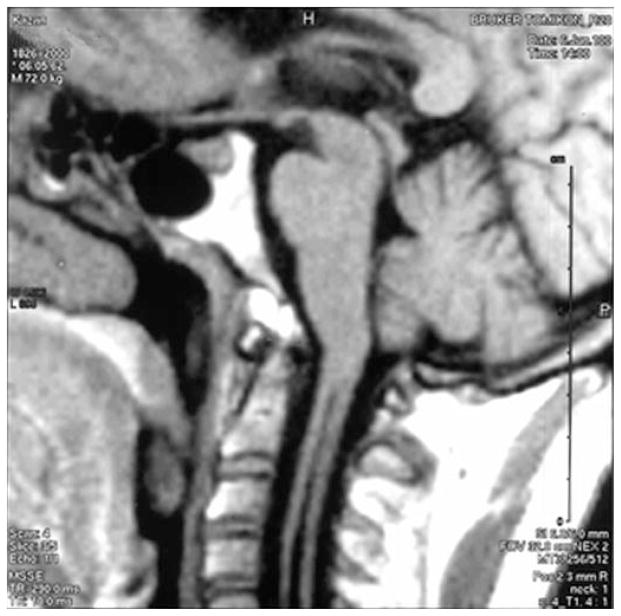
Figure 1.
Syringomyelia in the absence of herniation of the cerebellar tonsils is evident on a sagittal slice T1-weighted MR image. Note the narrowing of the CSF pathways at the superior margin of the foramen magnum.
Figure 2.
Measurements were taken from sagittal slice T1-weighted MR images as indicated in this drawing and included tonsillar herniation (T); posterior fossa height (h); Klaus index (Ki); pontomedullary height (pm); supraocciput (so); clivus (cl); Boogaard angle (B); cerebellum to opisthion (c); and ventral subarachnoid space (v); dorsal subarachnoid space (d); syrinx diameter (s); and Twining line (TL; tuberculum sellae to internal occipital protuberance).
Clinical features in patients are listed in the table. Data were analyzed statistically using the Student t-test and nonparametric Mann–Whitney U test; p values of <0.05 were considered significant.
Table.
Radiographic evaluation and clinical semiology of syringomyelia
| Factor | Idiopathic | Chiari I | Control |
|---|---|---|---|
| No. of patients | 17 | 17 | 32 |
| Age, y | 49.4 ± 10.9 | 48.7 ± 11.6 | 50.2 ± 9.2 |
| Age at onset, y | 26.2 ± 10.9 | 27.4 ± 11.5 | |
| MRI features | |||
| Tonsillar herniation, mm | 0 ± 0 | 7.6 ± 1.5 | |
| PF height, mm | 24.5 ± 3.4* | 25.5 ± 4.3* | 32.0 ± 3.0 |
| Klaus index, mm | 33.4 ± 3.5* | 34.5 ± 6.5 | 38.0 ± 5.0 |
| Distance pm, mm | 16.5 ± 2.4* | 13.6 ± 3.0† | 19.0 ± 3.0 |
| Length of so, mm | 36.8 ± 4.5* | 36.5 ± 4.6* | 41.0 ± 5.0 |
| Length of cl, mm | 40.0 ± 3.5* | 39.3 ± 4.2* | 43.4 ± 4.4 |
| Boogaard angle, ° | 145.3 ± 6.7* | 144.3 ± 10.7* | 133.8 ± 6.5* |
| Distance c, mm | 3.8 ± 2.6* | 1.4 ± 1.5*† | 8.8 ± 2.5 |
| Ventral CSF space, mm | 5.7 ± 1.9* | 6.1 ± 2.9* | 12 ± 2.3 |
| Dorsal CSF space, mm | 11.5 ± 4.7* | 0.8 ± 1.3*† | 19 ± 2.3 |
| Syrinx sagittal diameter, mm | 2.7 ± 1.9 | 5.5 ± 4.7† | N/A |
| Small cerebellar cisterns, % | 17 (100) | 17 (100) | |
| Cervicomedullary kinking, % | 2 (12) | 9 (53)† | |
| 4th ventricle to syrinx communication, % | 0 (0) | 0 (0) | |
| Clinical features, % | |||
| Segmental sensory loss | 16 (94) | 15 (88) | |
| Muscle atrophy | 10 (59) | 11 (65) | |
| Pyramidal signs | 14 (82) | 12 (71) | |
| Impaired position sense | 6 (35) | 5 (29) | |
| Suboccipital headache | 4 (24) | 4 (24) | |
| Ataxia | 8 (47) | 5 (29) | |
| Scoliosis/kyphosis | 9 (53) | 10 (59) | |
Values are means ± SD or no. (%).
Significant, p < 0.05, compared with control.
Significant, p < 0.05, compared with idiopathic syringomyelia.
PF = posterior fossa; pm = pontomedullary junction to foramen magnum; so = supraocciput; cl = clivus (apex of dorsum sellae to basion).
Results
There were no significant differences in clinical symptoms and signs of myelopathy between idiopathic and Chiari type-I syringomyelia (see the table). Patients in the idiopathic and Chiari type-I syringomyelia groups, compared with control, had significantly shortened PF bones (so, cl), reduced height of the posterior fossa (h, Ki), and increased B angle, indicating underdevelopment of osseous PF structures and skull base flattening. Caudal brainstem displacement (decreased pm) was present in idiopathic and Chiari type-I syringomyelia but was greater and associated with cervicomedullary kinking in CM1. Width of the ventral FM CSF pathway (v) was significantly reduced in idiopathic and Chiari I-type syringomyelia. Width (d) and height (c) of the dorsal CSF pathway at the FM were reduced in idiopathic and Chiari I-type syringomyelia, but significantly more in CM1. Syrinx diameter (S) was greater in CM1-type than idiopathic syringomyelia.
Discussion
All patients were rural tenants of the Republic of Tartarstan in the Russian Federation who were evaluated using modern MRI techniques and were found to have syringomyelia that was not associated with spinal lesions.
Recent clinical investigations suggest that craniocervical decompression, a treatment for Chiari I-type syringomyelia, is also effective for idiopathic syringomyelia. For example, syringomyelia resolved after PF decompression in five children who had syringomyelia without tonsillar herniation. In these patients, significant intradural adhesions, osseous abnormalities, and caudal descent of the brainstem compressed or distorted the PF contents and disrupted normal CSF flow, similar to that seen in patients with tonsillar ectopia and syringomyelia.9 The authors of this report denote this condition as “Chiari zero malformation” (CM0).9 In a report of 364 symptomatic patients with broadly defined CM1 examined by MRI, cerebellar tonsils herniated significantly below the FM in most (91%), but retrocerebellar CSF spaces were obliterated in all (100%) patients.2 In this study, 14 of 22 patients (64%) with insignificant (<5-mm) tonsillar herniation had syringomyelia compared with 224 of 342 (65%) patients with significant tonsillar herniation (CM1).2 Other investigators described “tight cisterna magna syringomyelia” in four adult patients with syringomyelia but without tonsillar herniation.10 In our study, patients with CM1-type syringomyelia and idiopathic syringomyelia had identical clinical manifestations of syringomyelia (see the table). Both groups of patients had similar morphometric abnormalities of the PF and craniocervical junction, namely, shortened bones and narrow CSF pathways. Shortening of the bones of the skull base lowered the torcular and tentorium and compromised the volume of the PF. Caudal brainstem displacement (pm) was greater in patients with tonsillar herniation (CM1) than in idiopathic syringomyelia.9
The syrinx diameter was larger in CM1-type syringomyelia than idiopathic syringomyelia, which is presumably related to greater constriction of CSF pathways in that type compared with idiopathic syringomyelia. Despite the difference in syrinx diameter, myelopathy was of equal severity in the patient groups. One explanation is that in idiopathic syringomyelia, the syringes were larger early in the disease, but by the time of the MRI scanning, the syringes had collapsed because the CSF pathways enlarged at the craniocervical junction or the syringes drained spontaneously (5/17). In syringomyelia, the pace of neurologic deterioration is rapid initially and is related to syrinx size, but later, after neurologic signs become well established, the pace of deterioration slows and the syrinx becomes smaller and no longer distends the spinal cord.6
Idiopathic syringomyelia and Chiari type-I syringomyelia are associated with a small PF with narrow CSF spaces. The etiology of CSF flow disturbances and syringomyelia in idiopathic and Chiari I-type syringomyelia appears to be PF underdevelopment and neural displacement. A PF with decreased compliance and narrow CSF spaces promotes the development of accentuated pulsatile CSF subarachnoid pressure waves that promote the development of syringomyelia.3
Syringomyelia that appears to be idiopathic may be associated with morphometric abnormalities of the PF fossa and constriction of the subarachnoid CSF pathways, the so-called “CM0.” Recent studies suggest that surgical decompression of the PF and craniocervical junction expands narrowed subarachnoid CSF pathways and resolves this type of syringomyelia.9,10
References
- 1.Vinters HV. Neuropathology of syringomyelia. In: Batzdorf U, editor. Syringomyelia: current concepts in diagnosis and treatment. Baltimore: Williams & Wilkins; 1991. pp. 35–58. [Google Scholar]
- 2.Milhorat TH, Chou MW, Trinidad EM, et al. Chiari 1 malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005–1017. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 3.Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91:553–562. doi: 10.3171/jns.1999.91.4.0553. [DOI] [PubMed] [Google Scholar]
- 4.Aboulezz AO, Sartor K, Geyer CA, Gado MH. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: a quantitative approach with MR imaging. J Comput Assist Tomogr. 1985;9:1033–1036. doi: 10.1097/00004728-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Masur H, Oberwittler C, Reuther G, Heyen P. Cerebellar herniation in syringomyelia: relation between tonsillar herniation and the dimensions of the syrinx and the remaining spinal cord. Eur Neurol. 1995;35:162–167. doi: 10.1159/000117114. [DOI] [PubMed] [Google Scholar]
- 6.Bogdanov EI, Mendelevich EG. Syrinx size and duration of symptoms predict the pace of progressive myelopathy: retrospective analysis of 103 unoperated cases with craniocervical junction malformations and syringomyelia. Clin Neurol Neurosurg. 2002;104:90–97. doi: 10.1016/s0303-8467(01)00189-5. [DOI] [PubMed] [Google Scholar]
- 7.Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Res Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt H, Sartor K, Heckl RW. Bone malformations of the craniocervical region. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 32. Amsterdam: Elsevier/North-Holland Biomedical Press; 1978. pp. 1–98. [Google Scholar]
- 9.Tubbs RS, Elton S, Grabb P, Dockery SE, Bartolucci AA, Oakes WJ. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery. 2001;48:1050–1054. doi: 10.1097/00006123-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Kyoshima K, Kuroyanagi T, Oya F, Kamijo Y, El-Noamany H, Kobayashi S. Syringomyelia without hindbrain herniation: tight cisterna magna. J Neurosurg (Spine 2) 2002;96:239–249. doi: 10.3171/spi.2002.96.2.0239. [DOI] [PubMed] [Google Scholar]