Abstract
Background
Human herpesvirus-6 (HHV-6), a ubiquitous β-herpesvirus, is the causative agent of roseola infantum and has been associated with a number of neurologic disorders including seizures, encephalitis/meningitis, and multiple sclerosis. Although the role of HHV-6 in human CNS disease remains to be fully defined, a number of studies have suggested that the CNS can be a site for persistent HHV-6 infection.
Objective
To characterize the extent and distribution of HHV-6 in human glial cells from surgical brain resections of patients with mesial temporal lobe epilepsy (MTLE).
Method
Brain samples from eight patients with MTLE and seven patients with neocortical epilepsy (NE) undergoing surgical resection were quantitatively analyzed for the presence of HHV-6 DNA using a virus-specific real-time PCR assay. HHV-6 expression was also characterized by western blot analysis and in situ immunohistochemistry (IHC). In addition, HHV-6-reactive cells were analyzed for expression of glial fibrillary acidic protein (GFAP) by double immunofluorescence.
Results
DNA obtained from four of eight patients with MTLE had significantly elevated levels of HHV-6 as quantified by real-time PCR. HHV-6 was not amplified in any of the seven patients with NE undergoing surgery. The highest levels of HHV-6 were demonstrated in hippocampal sections (up to 23,079 copies/106 cells) and subtyped as HHV-6B. Expression of HHV-6 was confirmed by western blot analysis and IHC. HHV-6 was co-localized to GFAP-positive cells that morphologically appeared to be astrocytes.
Conclusions
HHV-6B is present in brain specimens from a subset of patients with MTLE and localized to astrocytes in the absence of inflammation. The amplification of HHV-6 from hippocampal and temporal lobe astrocytes of MTLE warrants further investigation into the possible role of HHV-6 in the development of MTLE.
The human herpesvirus-6 (HHV-6) is a ubiquitous β-herpesvirus,1,2 which infects a wide spectrum of cell types including glial cells.3–5 The virus is acquired early in childhood and is the causative agent of roseola infantum (exanthem subitum), a benign and otherwise self-limiting disease.6 Primary infection may result in seizures and severe neurologic complications such as meningitis or meningoenceph-alitis.7 As with all herpesviruses, HHV-6 can establish lifelong latent infection,8 and reactivation can occur in immunosuppressed patients such as bone marrow transplant recipients.9 Two HHV-6 variants have been characterized with respect to their antigenic and genomic composition.10 HHV-6B is primarily associated with most symptomatic infections during infancy,11 whereas HHV-6A has been suggested to be more neurotropic and is associated with viral persistence and reactivation in the CNS.12 HHV-6 variant A has also been detected more frequently in multiple sclerosis patient samples compared with variant B.13 However, HHV-6B has also been shown to have neurotropic potential4,14 and has recently been associated with limbic encephalitis.15
Although the role of HHV-6 in human CNS disease remains to be fully defined, a number of studies have suggested that the CNS can be a site for persistent HHV-6 infection.16,17 Autopsy material from a wide variety of normal18 and diseased19,20 brains has documented HHV-6 infection in the CNS, although its role as a causative agent is unclear. Cell types reported to be infected include oligodendrocytes, astrocytes, and possibly neurons.21,22 In limbic encephalitis, HHV-6 DNA was detected in the CSF, and astrocytes were shown to be the most represented cell type harboring HHV-6B in the hippocampus.15 This localization is consistent with the report of HHV-6 hippocampal encephalitis after bone marrow transplantation23 and of hippocampal injury in patients with prolonged focal febrile seizures,24,25 a frequent complication of a primary HHV-6 infection.
In an attempt to characterize further the extent and distribution of HHV-6 in human glial cells, brain tissues from surgical specimens were processed to establish cultures of primary astrocytes and oligodendrocytes to be used for in vitro virologic examinations. Brain resections are a therapy for pharmacologically untreatable seizures,26 and specimens from patients with mesial temporal lobe epilepsy (MTLE) and neocortical epilepsy (NE) were collected for analysis.
Methods
Patients
Fifteen patients with epilepsy intractable to medical management were enrolled in the clinical research protocol “Research Study of Specimens Obtained During Epilepsy Surgery” (National Institute of Neurological Disorders and Stroke 02-N-0014) after being evaluated at the NIH and at the Children’s National Medical Center, Washington, DC, for surgical treatment. The Institutional Review Board of the National Institute of Neurological Disorders and Stroke approved the research protocol. Evaluation before surgery included ictal video EEG monitoring, MRI, and PET. Informed consent was obtained from the 10 adult patients who had surgery at NIH and from the parents of the five children who had surgery at the Children’s National Medical Center (table). No patient had any history or clinical features suggestive of an inflammatory disorder. For 12 of these patients, a paired blood sample taken at the time of surgery was available for analysis. Peripheral blood mononuclear cells (PBMC) from 23 age-and sex-matched normal blood donors were used as controls.
Table.
Clinical characteristics of patients studied
| Patient no. | Age, y/sex | Previous febrile seizures | Age at onset, y | MRI/pathology | Epileptic focus | Samples |
|---|---|---|---|---|---|---|
| MTLE patients | ||||||
| 1 | 41/M | No | 26 | R MTS | RT | Lateral temporal lobe, hippocampus, parahippocampus |
| 2 | 31/M | Yes | 17 | R MTS | RT | Lateral temporal lobe, hippocampus, PBMC |
| 3 | 21/F | No | 5–6 | R MTS | RT | Lateral temporal lobe, hippocampus, PBMC |
| 4 | 13/F | Yes | 4 | L MTS | LT | Lateral temporal lobe, hippocampus, PBMC |
| 6 | 51/M | No | 26 | L MTS | LT | Lateral temporal lobe, hippocampus |
| 7 | 19/F | No | 11 | R MTS | RT | Lateral temporal lobe, hippocampus, PBMC |
| 11 | 32/F | Yes | <1 | L MTS | LT | Lateral temporal lobe, hippocampus, PBMC |
| 15 | 40/M | No | 9 | R MTS | RT | Lateral temporal lobe, hippocampus, PBMC |
| NE patients | ||||||
| 5 | 6/F | No | 5 mo | Cortical dysplasia | RF | Lateral frontal lobe, medial frontal lobe, PBMC |
| 8 | 38/M | No | 37 | R temporal angioma | RT | Lateral temporal lobe, hippocampus, PBMC |
| 9 | 14/M | No | 8 | Normal, rare abnormal neurons | RF | Frontal lobe, PBMC |
| 10 | 40/M | No | 39 | Astrocytoma | RF | Lateral temporal lobe, hippocampus |
| 12 | 13/M | No | <1 | Porencephalic cyst | RT | Lateral temporal lobe, PBMC |
| 13 | 55/F | No | 53 | R frontal astrocytoma | RF | Tumor frontal lobe, normal frontal lobe, PBMC |
| 14 | 51/M | No | 40 | L temporal astrocytoma | LT | Lateral temporal lobe, PBMC |
MTLE =mesial temporal lobe epilepsy; NE =neocortical epilepsy; MTS =mesial temporal sclerosis; RT =right temporal lobe; PBMC =peripheral blood mononuclear cells; LT =left temporal lobe; RF =right frontal lobe.
Pathologic evaluation of epilepsy brain tissue
Numerous samples from hippocampus and lateral temporal lobe tissue from MTLE patients were fixed in formalin and processed for neuropathology evaluation. All patients with MTLE showed variable degrees of neuronal loss and gliosis preferentially affecting the CA1 and CA3 areas, but also markedly affecting CA4, CA2, and the dentate gyrus in more severe cases. There was no evidence of inflammation or neuronal inclusions in any case of MTLE.
DNA extraction from blood and brain tissue samples
Whole blood from patients and normal donors was collected in acid citrate dextrose (solution A) tubes (BD Vacutainer, Franklin Lakes, NJ), and PBMC were isolated on Ficoll (BioWhittaker, Walkersville, MD) gradients. Brain tissue samples obtained during surgery were collected in sterile containers for pathology examinations. Selected tissue samples were placed in Hybernate A medium (BrainBits, Springfield, IL) containing B27 supplement (Gibco Invitrogen Corp., Grand Island, NY) and penicillin–streptomycin (BioWhittaker) on ice for immediate processing. Samples were first washed three times in Hybernate A medium. Meninges and blood vessels were removed, and the tissue was finely minced into cubes of <2 mm3 with a scalpel. Cellular DNA was extracted using the QIAamp DNAeasy blood kit for PBMC and DNAeasy tissue kit for brain samples (Qiagen, Valencia, CA) according to the manufacturer’s instructions, with an overnight incubation with proteinase K in the case of brain samples. All DNA extractions and the subsequent real-time PCR were performed in separate hoods using separate sets of pipettes.
Quantitative real-time PCR
DNA from triplicate samples and controls was tested in parallel for gene sequences homologous to the immediate early (IE) regions of HHV-6A and HHV-6B as described.27 Controls were DNA samples extracted from cultured SupT-1 cells uninfected or infected with HHV-7 and SupT-1 cells infected with HHV-6A (strains GS or U1102) or HHV-6B (strain Z29).28 The DNA concentration for each sample was adjusted to 10 ng/μL in diethylpyrocarbonate-treated water, and 100 ng, corresponding to 10 μL of each diluted sample or control, was added in triplicate to a 96-well TaqMan plate (ABI, Weiterstadt, Germany).
Results were plotted and sorted using the Sequence Detector System program (PerkinElmer, Wellesley, MA). With use of a known concentration of variant-specific HHV-6 plasmids, a standard curve was generated, from which a lower level of sensitivity was determined to be 10 viral DNA copies/100 ng of DNA. To normalize results, a calibration curve using human genomic β-actin was incorporated in all assays.29 Absolute viral and β-actin DNA copy number was assessed by averaging triplicate results. All DNA extracts were tested in separate experiments at least twice. Final viral DNA load/106 cells was calculated by the following formula: HHV-6 DNA copy number/(β-actin DNA copy number/2) × 106 =copy number of HHV-6 DNA/106 cells. To ensure the reliability of the HHV-6 DNA results from each TaqMan reaction, a cutoff threshold of 100 viral DNA copies/106 cells was determined to be the lower limit of sensitivity of this assay.
Protein extraction and sodium dodecyl sulfate–polyacrylamide gel electrophoresis/western blot analysis
Protein extracts from 20 mm3 of frozen brain tissue samples were resuspended in 100 μL of 2× Tris–glycine sodium dodecyl sulfate sample buffer (KD Medical, Columbia, MD) supplemented with 5% β-mercaptoethanol and 0.002% bromophenol blue, followed by five freeze–thaw cycles and sonication. Samples were boiled for at least 5 minutes and applied to a 10% Tris–glycine gel (Gibco Invitrogen Corp.) and electrophoresed for 50 minutes at 200 V. The gel was then transferred to a Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Little Chalfont, UK). Western blot analysis was performed using a hybridoma supernatant containing the HHV-6-specific monoclonal antibody anti-p41 (kindly provided by Dr. M. Handy, Advanced Biotechnologies, Columbia, MD)30 at a dilution of 1:200. Visualization was achieved by incubation for 1 hour with a horseradish peroxidase-conjugated anti-mouse IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:50,000 dilution and subsequent enhanced chemiluminescence detection (Pierce Biotechnology, Rockford, IL).
In situ immunohistochemistry
In situ immunohistochemistry (IHC) was performed on formalin-fixed paraffin-embedded brain sections following standard methods.19 In brief, after hydration and rinsing, slides were blocked in a 10% horse serum (Gibco Invitrogen Corp.)/phosphate-buffered saline (PBS) solution and then incubated overnight at 4 °C with a 2% horse serum/PBS solution containing anti-HHV-6 gp116/54/64 mouse monoclonal antibody that reacts with both HHV-6A and B variants (1:50) (Advanced Biotechnologies).31 IHC reactivity was detected using a DAKO LSAB 2 System (DAKO Corp., Carpinteria, CA) following the manufacturer’s instructions with a 10-minute counterstain in Mayer’s hematoxylin (Fluka Chemie, Steinheim, Germany). Slides were observed under a microscope (Carl Zeiss Microimaging, Thornwood, NY) at 40× or 32×.
Double-immunofluorescence assay for glial fibrillary acidic protein and HHV-6 gp116/54/64
After rehydrating and blocking as above, formalin-fixed paraffin-embedded brain tissue slides were incubated overnight at 4 °C with a 2% horse serum/PBS solution containing rabbit anti-glial fibrillary acidic protein (anti-GFAP) (DAKO Corp.; 1:100) and mouse anti-HHV-6 gp116/54/64 monoclonal antibodies (1:50). After washing three times with PBS, a solution containing the secondary antibodies was added. The secondary antibodies were fluorescein isothiocyanate (FITC)–conjugated anti-rabbit IgG (DAKO Corp.; 1:100) and Alexa fluor–conjugated anti-mouse IgG2b (Molecular Probes, Eugene, OR; 1:200) for the detection of HHV-6 gp116/54/64 and GFAP-reactive astrocytes. Slides were incubated for an additional hour at room temperature and rinsed three times with PBS. After mounting with 4,6-diamidino-2-phenylindole (DAPI) mounting medium (Vector Laboratories, Burlingame, CA), samples were examined with a fluorescent microscope (Carl Zeiss Microimaging) at 20× and 40× with exposures of 100 milliseconds for the DAPI filter, 400 milliseconds for the FITC filter, and 500 milliseconds for the rhodamine filter. Slides with HHV-6-infected and -uninfected SupT-1 cell lines and astrocytic line U251 were used as controls. Single staining with each of the two primary antibodies was also applied on slides as a control.
Results
Quantitative detection of HHV-6 DNA in PBMC and fresh brain tissue samples
Initially, DNA from PBMC of 23 normal donors was examined for the amount of HHV-6B IE gene sequences. As shown in figure 1, levels of HHV-6 in PBMC were below the level of sensitivity of this assay (100 copies/106 cells) and consistent with reports demonstrating low frequency of HHV-6 DNA in PBMC, often only detectable with nested PCR amplification.32 By contrast, brain tissue from four of eight patients with MTLE (see the table) had elevated levels of HHV-6 (see figure 1). Higher levels of HHV-6 were detected in hippocampal samples relative to lateral temporal lobe material obtained from the same patient with values as high as 23,079 copies/106 cells in the hippocampus of MTLE Patient 2. None of the brain specimens from seven patients with NE (see the table) had amplifiable HHV-6 IE sequences. Sufficient brain material was available from five MTLE patients and four NE patients from which specificity analysis demonstrated no amplification with the HHV-6A primer sets. The difference in frequency of HHV-6B DNA detection between MTLE and NE patients was statistically significant (χ2, p =0.0289). When available, PBMC from patients with and without MTLE were tested for HHV-6 DNA and, similar to healthy normal controls, were below the limits of detection of this assay (see figure 1).
Figure 1.
Quantitative detection of human herpesvirus-6 variant B (HHV-6B) DNA in samples from normal donors and from brain surgery patients. PBMC ND = peripheral blood mononuclear cells (PBMC) from normal donors; PBMC Epilepsy patients = available PBMC from all brain surgery patients examined (mesial temporal lobe epilepsy [MTLE] and neocortical epilepsy [NE] patients); MTLE Brain resections = fresh brain tissue samples from surgical resections of MTLE patients; NE Brain resections = fresh brain tissue samples from surgical resections of NE patients; circles = PBMC; squares = hippocampus samples; triangles = lateral temporal lobe samples; rectangles = frontal lobe samples. Samples above the cutoff threshold (dotted line) were considered positive. ■ ▲ = Patient 2;

 = Patient 3; ▨
= Patient 3; ▨
 = Patient 6;
= Patient 6;

 = Patient 15.
= Patient 15.
Western blot analysis for HHV-6 protein in MTLE brain tissue extracts
To characterize further the presence of HHV-6B antigen in brain samples from patients with MTLE, sufficient frozen material was available from both hippocampus and lateral temporal lobe of Patient 2 to analyze by western blot using a monoclonal antibody specific for the HHV-6 p41 antigen.30 As illustrated in figure 2, protein extracts of the MTLE hippocampal sample specifically reacted with the anti-HHV-6 p41 antibody in a dose-dependent manner. The reactive band from hippocampal samples was slightly higher than expected, having a molecular mass between 45 and 50 kd. Possible explanations for the higher molecular mass observed include glycosylation or posttranslational modifications of the protein within CNS. No reactivity was observed in protein extracts from the lateral temporal lobe. Protein extracts from the lateral temporal lobe of Patient 3, with no detectable HHV-6 DNA (see figure 1), were also negative for anti-HHV-6 p41 reactivity by western blot (data not shown). These results are consistent with the detection of significantly higher HHV-6B viral DNA loads in the hippocampus of MTLE Patient 2 (see figure 1).
Figure 2.
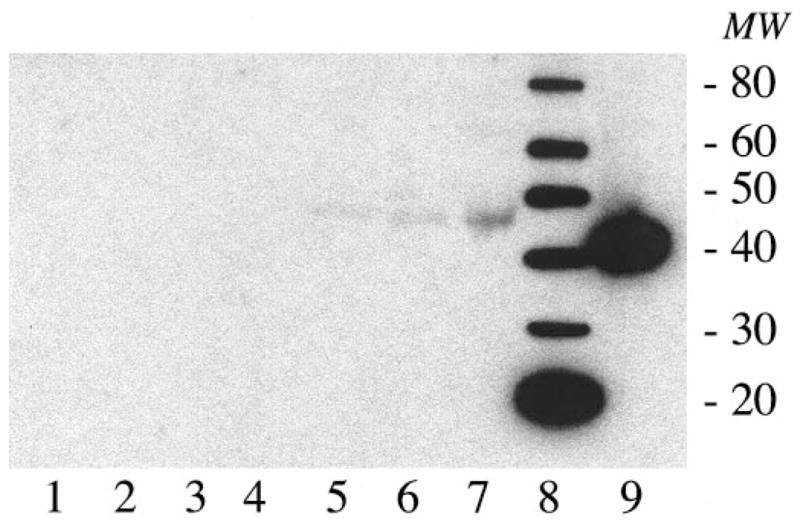
Immunoreactivity to human herpesvirus-6 (HHV-6) p41 antigen protein extracts from frozen samples from Patient 2. Lane 1 = uninfected SupT-1 cells; lanes 2 to 4 = lateral temporal lobe, 5, 10, and 20 μL; lanes 5 to 7 = hippocampus, 5, 10, and 20 μL; lane 8 = protein ladder; lane 9 = Z29-infected SupT-1 cells. Respective band molecular weights are shown on the right.
Immunohistochemical detection of HHV-6 gp116/54/64 antigen from formalin-fixed paraffin-embedded MTLE brain tissue
To support the observations of increased HHV-6B DNA viral loads and detection of HHV-6 viral antigens by western blot from frozen MTLE brain material, formalin-fixed paraffin-embedded tissue was analyzed for the expression and localization of HHV-6 by immunohistochemistry with an HHV-6-specific monoclonal antibody to the gp116/54/64 protein, a late viral protein present in both HHV-6 variants.31 Samples from MTLE Patient 2 with the highest levels of HHV-6 DNA (see figure 1), MTLE Patient 3 with the lowest detectable level, and MTLE Patient 1 with undetectable levels of HHV-6 were processed for immunohistochemistry. As shown in figure 3, significant staining for the HHV-6 gp116/54/64 antigen was observed in hippocampal and lateral temporal lobe regions of MTLE Patient 2 (see figure 3, A and B) and the hippocampus of MTLE Patient 3 (see figure 3C). Fresh surgical material from these same regions was positive for HHV-6 DNA sequences by PCR (see figure 1). Consistent with the diagnosis of MTLE, no evidence of inflammation was observed in any of the samples examined. Intense brown staining was seen in cells that morphologically resemble astrocytes (see figure 3, A through C, filled arrows), whereas a significant number of cells remained negative (open arrows), increasing confidence in the observed specificity of HHV-6 staining in MTLE PCR-positive brain samples. No HHV-6-specific staining was observed in lateral temporal lobe samples of MTLE Patient 3 or from any region of MTLE Patient 1 (see figure 3, D through F). PCR detection of HHV-6 from fresh brain material from these areas failed to amplify HHV-6 DNA sequences (see figure 1). Similarly, there was no HHV-6-positive staining from formalin-fixed brain material from other disease controls including oligodendroglioma, ganglion cell tumor, cavernous angioma, and glioblastoma multiforme (data not shown).
Figure 3.

In situ immunohistochemistry staining and localization of human herpesvirus-6 (HHV-6) gp116/54/64 in formalin-fixed paraffin-embedded tissue from patients with mesial temporal lobe epilepsy and control subjects. Samples are from hippocampus (A) (32×) and lateral temporal lobe (B) (40×) from Patient 2; hippocampus (C) (40×) and lateral temporal lobe (D) (32×) from Patient 3; and hippocampus (E) (32×) and lateral temporal lobe (F) (32×) from Patient 1. Positively stained cells of tissue samples from Patients 2 and 3 morphologically appear to be astrocytes (filled arrows), whereas oligodendrocytes and a proportion of astrocyte-appearing cells (open arrows) are negatively stained.
Co-localization of anti-HHV-6 gp116/54/64 and anti-GFAP reactivity in formalin-fixed paraffin-embedded MTLE brain tissue
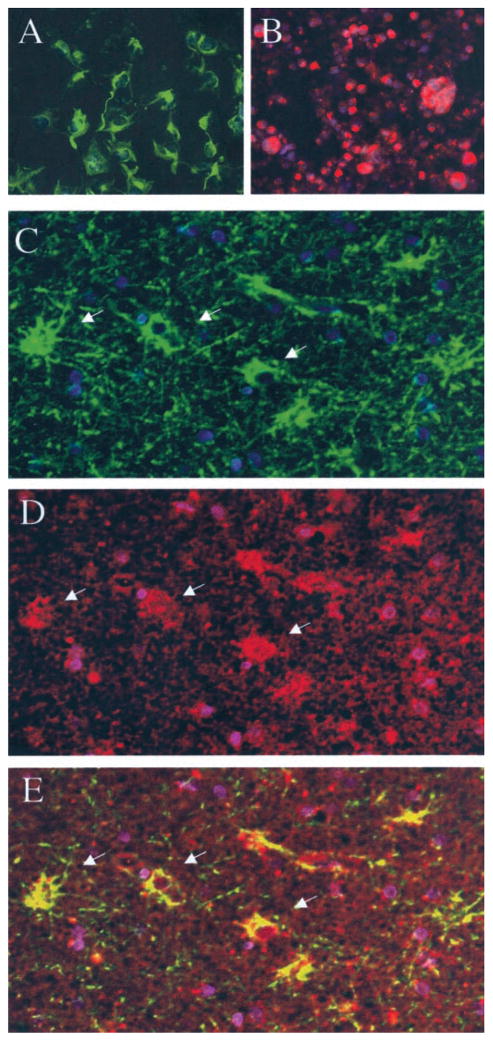
To characterize the cell type harboring HHV-6 in the brain tissue of HHV-6-positive MTLE patients, formalin-fixed paraffin-embedded tissue sections were simultaneously stained for GFAP, an intracellular marker of astrocytes, and the HHV-6 gp116/54/64. The results are illustrated in figure 4. As controls, the anti-GFAP monoclonal antibody specifically stained an astrocytic cell line U251 that was unreactive with the anti-HHV-6 monoclonal antibody (see figure 4, A, green). Conversely, a HHV-6-infected T-cell line specifically reacted with the anti-HHV-6 antibody but not with anti-GFAP (see figure 4, B, red). No co-localization of these two reagents could be demonstrated after these images were overlaid (failure to detect yellow). By contrast, analysis of lateral temporal lobe tissue from Patient 2 demonstrated some cells reactive for GFAP, with a typical star-shaped appearance highly suggestive of astrocytes (see figure 4, C, green) and for HHV-6 gp116/54/64 (see figure 4, D, red). The juxtaposition of the two color images obtained from the same tissue showed that HHV-6 gp116/54/64 reactivity co-localized with GFAP (see figure 4, E, yellow). Tissue sections from MTLE Patient 1 hippocampus that was negative for HHV-6 DNA (see figure 1) had GFAP-positive cells that did not stain for HHV-6 gp116/54/64 (data not shown).
Figure 4.
Co-localization of anti-human herpesvirus 6 (anti-HHV-6) gp116/54/64 reactivity in glial fibrillary acidic protein (GFAP)–reactive cells using double-immunofluorescence assay on formalin-fixed paraffin-embedded brain tissue. Positive staining for GFAP (green) but not for HHV-6 gp116/54/64 (red) of astrocytic cell line U251 was visualized with both fluorescein isothiocyanate and rhodamine filters (A). Positive staining for HHV-6 gp116/54/64 but not for GFAP of HHV-6B (strain Z29)–infected SupT-1 cell line (B) was seen after simultaneous incubation with both sets of antibodies. Lateral temporal lobe of Patient 2 shows presence of GFAP-reactive cells (green) (C, arrows) and of HHV-6 gp116/54/64-positive cells (red; D, arrows) incubated with both anti-GFAP and anti-gp116/54/64 primary antibodies. Juxtaposition of the combined images shows co-localization of GFAP and HHV-6 gp116 reactivity (yellow) (E, arrows) (20× magnification). All fields were counterstained with 4,6-diamidino-2-phenylindole (blue) to visualize nuclei.
Discussion
DNA obtained from a subset of patients with MTLE had significantly elevated levels of HHV-6 as quantified by real-time PCR compared with other brain material from patients with NE undergoing surgery. The highest levels of HHV-6 were demonstrated in hippocampal sections and subtyped as HHV-6B. Expression of HHV-6 was confirmed by western blot analysis and IHC. HHV-6-specific antigen was co-localized to cells that were positive for GFAP and morphologically appeared to be astrocytes. Collectively, these results demonstrate that HHV-6B is present in brain specimens from some patients with MTLE and localized to astrocytes in the absence of inflammation. Additional studies of tissue from normal controls and patients without seizures will be necessary to establish the specificity of our finding.
We used a highly reliable and quantitative PCR method specific for the two variants of HHV-6.27 Of the 15 patients analyzed in this study, HHV-6 variant B was detected in a subset of patients with MTLE and mesial temporal sclerosis (four of eight patients). In contrast, DNA from surgical material from patients with NE did not have HHV-6 viral sequences in the range of detection of this assay. Most striking was the magnitude of HHV-6 viral DNA in the MTLE samples. This is in marked contrast to the amount of HHV-6 in PBMC from all patients and control subjects that was below assay detection limits.
The significance of PCR detection of viral DNA sequences in brain tissue alone is uncertain.33 Therefore, the unexpected finding of such high HHV-6 viral loads in MTLE patient brain resections prompted a further evaluation of this material for specific HHV-6 protein expression. Western blot analysis from the hippocampus of MTLE Patient 2 demonstrated specific anti-HHV-6 p41 reactivity in frozen brain tissue extracts taken from the same brain regions that had the highest copies of HHV-6 viral DNA. Protein extracts of the patient’s lateral temporal lobe were negative. As the p41 protein of HHV-6 is an early protein of this β-herpesvirus and is associated with active virus replication,30 the detection of HHV-6 protein in brain regions with high HHV-6 viral loads supports the observations that active HHV-6 virus replication may be present specifically in the hippocampus of this MTLE patient. A high proportion of patients with MTLE may have a history of complex or prolonged febrile seizures,34 and recurrent seizures themselves may lead to hippocampal injury.35–37 It has been suggested that an early acute neurologic injury to the hippocampus from childhood infection, ischemia, trauma, or febrile convulsions may predispose the hippocampus to progressive deterioration, permanent damage, and eventual sclerosis.38–40 Despite its wide host cell range and ubiquity, in MTLE, HHV-6 may be tropic for the hippocampus. This observation is supported by the absence of viral DNA in the two hippocampal samples from NE patients. Although the number of NE specimens studied in the current investigation was limited, the results suggest an association of this virus with MTLE. In addition, these results are consistent with the recent qualitative demonstration of HHV-6 DNA in the brain specimens of 6 of 17 Japanese patients with temporal lobe epilepsy.41
Formalin-fixed MTLE brain material demonstrated HHV-6 expression in astrocyte-resembling cells that were reactive for GFAP, an astrocytic marker. Again, these results are supported by the observations of HHV-6-infected astrocytes in patients with limbic encephalitis,15 suggesting that astrocytes may be preferentially infected with HHV-6B. This finding in MTLE patients is of interest as astrocytes are known to closely interact with neurons and are critical in modulating synaptic transmission.42,43 It has been suggested that the astrogliosis observed in MTLE lesions might have a crucial role in the disease by releasing epileptogenic factors eventually affecting neuronal activity and survival. 44,45
Collectively, the findings reported here suggest an association of HHV-6 in a subset of patients with MTLE. The possibility that a reactivation of HHV-6 in infected astrocytes might have a role in the development of MTLE epilepsy warrants future investigation.
Acknowledgments
The authors thank Dr. Wolfgang Siegert for kindly providing the variant-specific HHV-6 plasmids, primers, and probes; Rachel Barreto, Cynthia Soderblom, Elena Martinelli, Hamid Dean Refai, and René Smith for their assistance in this study; and Dr. Samantha Soldan for her helpful comments.
References
- 1.Salahuddin SZ, Ablashi DV, Markham PD, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 2.Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001;33:829–833. doi: 10.1086/322691. [DOI] [PubMed] [Google Scholar]
- 3.Lusso P, Markham PD, Tschachler E, et al. In vitro cellular tropism of human B-lymphotropic virus (human herpesvirus-6) J Exp Med. 1988;167:1659–1670. doi: 10.1084/jem.167.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albright AV, Lavi E, Black JB, Goldberg S, O’Connor MJ, Gonzalez–Scarano F. The effect of human herpesvirus-6 (HHV-6) on cultured human neural cells: oligodendrocytes and microglia. J Neurovirol. 1998;4:486–494. doi: 10.3109/13550289809113493. [DOI] [PubMed] [Google Scholar]
- 5.He J, McCarthy M, Zhou Y, Chandran B, Wood C. Infection of primary human fetal astrocytes by human herpesvirus 6. J Virol. 1996;70:1296–1300. doi: 10.1128/jvi.70.2.1296-1300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanishi K, Okuno T, Shiraki K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Asano Y. Central nervous system complications in human herpesvirus-6 infection. Brain Dev. 2000;22:307–314. doi: 10.1016/s0387-7604(00)00113-3. [DOI] [PubMed] [Google Scholar]
- 8.Luppi M, Marasca R, Barozzi P, et al. Three cases of human herpesvirus-6 latent infection: integration of viral genome in peripheral blood mononuclear cell DNA. J Med Virol. 1993;40:44–52. doi: 10.1002/jmv.1890400110. [DOI] [PubMed] [Google Scholar]
- 9.Singh N, Paterson DL. Encephalitis caused by human herpesvirus-6 in transplant recipients: relevance of a novel neurotropic virus. Transplantation. 2000;69:2474–2479. doi: 10.1097/00007890-200006270-00002. [DOI] [PubMed] [Google Scholar]
- 10.Ablashi DV, Balachandran N, Josephs SF, et al. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991;184:545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- 11.Dewhurst S, McIntyre K, Schnabel K, Hall CB. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. J Clin Microbiol. 1993;31:416–418. doi: 10.1128/jcm.31.2.416-418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall CB, Caserta MT, Schnabel KC, et al. Persistence of human herpesvirus 6 according to site and variant: possible greater neurotropism of variant A. Clin Infect Dis. 1998;26:132–137. doi: 10.1086/516280. [DOI] [PubMed] [Google Scholar]
- 13.Akhyani N, Berti R, Brennan MB, et al. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis. 2000;182:1321–1325. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- 14.McCullers JA, Lakeman FD, Whitley RJ. Human herpesvirus 6 is associated with focal encephalitis. Clin Infect Dis. 1995;21:571–576. doi: 10.1093/clinids/21.3.571. [DOI] [PubMed] [Google Scholar]
- 15.Wainwright MS, Martin PL, Morse RP, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50:612–619. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 16.Caserta MT, Hall CB, Schnabel K, et al. Neuroinvasion and persistence of human herpesvirus 6 in children. J Infect Dis. 1994;170:1586–1589. doi: 10.1093/infdis/170.6.1586. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa T, Asano Y, Akimoto S, et al. Latent infection of human herpesvirus 6 in astrocytoma cell line and alteration of cytokine synthesis. J Med Virol. 2002;66:497–505. [PubMed] [Google Scholar]
- 18.Chan PK, Ng HK, Hui M, Ip M, Cheung JL, Cheng AF. Presence of human herpesviruses 6, 7, and 8 DNA sequences in normal brain tissue. J Med Virol. 1999;59:491–495. doi: 10.1002/(sici)1096-9071(199912)59:4<491::aid-jmv11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Cuomo L, Trivedi P, Cardillo MR, et al. Human herpesvirus 6 infection in neoplastic and normal brain tissue. J Med Virol. 2001;63:45–51. [PubMed] [Google Scholar]
- 20.Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer’s disease. J Pathol. 2002;197:395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 21.Knox KK, Brewer JH, Henry JM, Harrington DJ, Carrigan DR. Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease. Clin Infect Dis. 2000;31:894–903. doi: 10.1086/318141. [DOI] [PubMed] [Google Scholar]
- 22.Challoner PB, Smith KT, Parker JD, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:7440–7444. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujimura H, Iseki T, Date Y, et al. Human herpesvirus-6 encephalitis after bone marrow transplantation: magnetic resonance imaging could identify the involved sites of encephalitis. Eur J Haematol. 1998;61:284–285. doi: 10.1111/j.1600-0609.1998.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 24.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43:413–426. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 25.Theodore WH, Bhatia S, Hatta J, et al. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999;52:132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 26.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 27.Nitsche A, Muller CW, Radonic A, et al. Human herpesvirus 6A DNA is detected frequently in plasma but rarely in peripheral blood leukocytes of patients after bone marrow transplantation. J Infect Dis. 2001;183:130–133. doi: 10.1086/317651. [DOI] [PubMed] [Google Scholar]
- 28.Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol. 2000;47:306–313. [PubMed] [Google Scholar]
- 29.Yamano Y, Nagai M, Brennan M, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- 30.Chang CK, Balachandran N. Identification, characterization, and sequence analysis of a cDNA encoding a phosphoprotein of human herpesvirus 6. J Virol. 1991;65:2884–2894. doi: 10.1128/jvi.65.6.2884-2894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balachandran N, Amelse RE, Zhou WW, Chang CK. Identification of proteins specific for human herpesvirus 6-infected human T cells. J Virol. 1989;63:2835–2840. doi: 10.1128/jvi.63.6.2835-2840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Luca D, Dolcetti R, Mirandola P, et al. Human herpesvirus 6: a survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J Infect Dis. 1994;170:211–215. doi: 10.1093/infdis/170.1.211. [DOI] [PubMed] [Google Scholar]
- 33.DeBiasi RL, Kleinschmidt–DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5–S11. doi: 10.1016/s1386-6532(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 34.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 35.Kalviainen R, Salmenpera T. Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog Brain Res. 2002;135:279–295. doi: 10.1016/S0079-6123(02)35026-X. [DOI] [PubMed] [Google Scholar]
- 36.Theodore WH, Gaillard WD. Neuroimaging and the progression of epilepsy. Prog Brain Res. 2002;135:305–313. doi: 10.1016/S0079-6123(02)35028-3. [DOI] [PubMed] [Google Scholar]
- 37.Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol. 1999;45:568–576. doi: 10.1002/1531-8249(199905)45:5<568::aid-ana4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648–655. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grunewald R. Childhood seizures and their consequences for the hippocampus. Brain. 2002;125:1935–1936. doi: 10.1093/brain/awf195. [DOI] [PubMed] [Google Scholar]
- 40.Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–251. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- 41.Uesugi H, Shimizu H, Maehara T, Arai N, Nakayama H. Presence of human herpesvirus 6 and herpes simplex virus detected by polymerase chain reaction in surgical tissue from temporal lobe epileptic patients. Psychiatry Clin Neurosci. 2000;54:589–593. doi: 10.1046/j.1440-1819.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- 42.Keyser DO, Pellmar TC. Synaptic transmission in the hippocampus: critical role for glial cells. Glia. 1994;10:237–243. doi: 10.1002/glia.440100402. [DOI] [PubMed] [Google Scholar]
- 43.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 44.Represa A, Niquet J, Pollard H, Ben–Ari Y. Cell death, gliosis, and synaptic remodeling in the hippocampus of epileptic rats. J Neurobiol. 1995;26:413–425. doi: 10.1002/neu.480260313. [DOI] [PubMed] [Google Scholar]
- 45.Hinterkeuser S, Schroder W, Hager G, et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]