Abstract
Individuals in areas of Plasmodium falciparum endemicity develop immunity to malaria after repeated exposure. Knowledge of the acquisition and nature of protective immune responses to P. falciparum is presently limited, particularly for young children. We examined antibodies (IgM, IgG, and IgG subclasses) to merozoite antigens and their relationship to the prospective risk of malaria in children 1 to 4 years of age in a region of malaria endemicity in Papua New Guinea. IgG, IgG1, and IgG3 responses generally increased with age, were higher in children with active infection, and reflected geographic heterogeneity in malaria transmission. Antigenic properties, rather than host factors, appeared to be the main determinant of the type of IgG subclass produced. High antibody levels were not associated with protection from malaria; in contrast, they were typically associated with an increased risk of malaria. Adjustment for malaria exposure, using a novel molecular measure of the force of infection by P. falciparum, accounted for much of the increased risk, suggesting that the antibodies were markers of higher exposure to P. falciparum. Comparisons between antibodies in this cohort of young children and in a longitudinal cohort of older children suggested that the lack of protective association was explained by lower antibody levels among young children and that there is a threshold level of antibodies required for protection from malaria. Our results suggest that in populations with low immunity, such as young children, antibodies to merozoite antigens may act as biomarkers of malaria exposure and that, with increasing exposure and responses of higher magnitude, antibodies may act as biomarkers of protective immunity.
INTRODUCTION
In areas of malaria endemicity, immunity that protects from high (H)-density parasitemia and symptomatic disease develops over a number of years (1). Knowledge of the precise nature of the protective immune responses to Plasmodium falciparum, in terms of the immune mechanisms, the specific target antigens, the nature of responses, and the rate of acquisition of immunity, has been sought, and while advances have been made, our current understanding is still limited (2, 3). Past experiments involving the passive transfer of immunoglobulin from immune adults into P. falciparum-infected individuals provided strong evidence that antibodies (Abs) play an important role in mediating immunity and target the blood stages of infection (4–6). Targets of antibodies include antigens expressed by the merozoite stage of the parasite, and these antibodies function by inhibiting merozoite invasion of red blood cells and by opsonizing merozoites for uptake by phagocytes and antibody-dependent cellular inhibition (7–14).
An important approach for identifying antigens as targets of protective immunity in humans is to assess the acquisition of antibodies and the association between antigen-specific responses and protection from symptomatic malaria in malaria-exposed populations (3), particularly in longitudinal cohort studies that prospectively examine the relationship between antibody responses and different malaria-based outcomes over time (15). Studies examining the protective associations for antibodies to merozoite antigens have reported various results (15–24). Some have provided evidence supporting a role of specific antibodies in protection, whereas others have found little evidence of a protective role or even an increased risk of symptomatic malaria (15). These differences may be explained by study design with respect to the age of participants, malaria transmission intensity, and the level of immunity in the populations (15, 25, 26).
A further aspect of the complexity of efforts aimed at such identification is the presence of significant heterogeneity in P. falciparum transmission intensity (26–31), even within small geographical areas. This leads to different levels of P. falciparum exposure within populations and, therefore, impacts acquisition of immunity and risk of malaria. Understanding how these factors influence functional immunity is important for defining key targets of immunity; however, addressing these issues is challenging and new approaches and insights are needed. A recent study used a novel molecular method to define the number of new P. falciparum clones acquired over time (the molecular force of infection [molFOI]) (32) and demonstrated a strong relationship between this parameter and factors that influence heterogeneity in exposure within a population (e.g., seasonality, location, and the use of bed nets). Furthermore, molFOI, as a marker of an individual's exposure to malaria, was the major predictor of clinical disease in a cohort of young children still actively acquiring immunity to P. falciparum (32).
In populations where antibody levels have not yet reached thresholds that are predictive of clinical immunity, their close association with recent exposure may also make them good biomarkers of malaria risk as they may identify individuals with the highest level of exposure to Plasmodium infection and therefore those most at risk of developing symptomatic malaria. There is increasing interest in using antibodies specific for merozoite antigens as serological biomarkers of Plasmodium exposure or as biomarkers of immunity to help monitor changes in malaria transmission over time, to evaluate the impact of malaria control interventions, and to identify populations at high risk of developing symptomatic malaria to inform malaria control programs (33–37). However, to achieve this, a greater knowledge of antibody responses to malaria antigens and how they are acquired relative to exposure, age, and immunity is needed (38). Relatively little is known about the early acquisition and role of parasite-specific antibodies in young children, particularly in populations outside Africa, how such responses compare to responses in older children with higher levels of immunity, or the extent to which immunity may depend on the magnitude of antibody responses or the quality or nature of these responses (including antibody isotypes and IgG subclasses). Differences in levels and patterns of transmission of malaria, host and parasite genetics, and other population factors could potentially influence acquisition of humoral immunity and contribute to differences in antibody associations observed in different populations. The majority of studies examining the acquisition and role of malaria-specific antibodies in young children have been conducted in Africa. It is therefore desirable to examine this in additional cohort studies in geographically distinct populations to determine the generalizability of observations. A clear understanding of the human immune responses to malaria will facilitate rational vaccine design and evaluation in clinical trials. Defining the role of specific antibodies and whether they act to prevent symptomatic malaria or are indicators of an individual's previous exposure to Plasmodium infection will provide insight into how vaccines based on specific antigens may work. It may also enable the identification of potential endpoints for measuring the efficacy of vaccines in clinical trials.
In the present study, we aimed to determine the acquisition of antibodies, including IgM and IgG subclasses, to several merozoite antigens in a longitudinal cohort of young children aged 1 to 4 years resident in Papua New Guinea (PNG) who were actively acquiring immunity to P. falciparum malaria (39). We examined the role of these antibodies in relation to the prospective risk of developing malaria to determine whether they may play a role in protection in this age group and evaluated the influences of age, active P. falciparum infection, and spatial heterogeneity in exposure levels to better understand the relationship between antibodies and malaria risk. Additionally, molFOI (32) was measured by sensitive molecular methods, enabling us to derive new insights into the acquisition of immunity, to better understand the relationship between antibodies, heterogeneity in P. falciparum exposure, and malaria risk, and to evaluate molFOI as a valuable tool for use in studies of human immunity. We have previously shown that antibodies to merozoite antigens are associated with a reduced risk of developing high-density parasitemia and symptomatic P. falciparum episodes in a cohort of older children (5 to 14 years of age) in PNG (23, 24). Therefore, we also compared responses in two cohorts of young and older children to determine whether a threshold level of antibody may be required for protective immunity and whether the differences in the nature of antibody isotypes and IgG subclass responses may be related to protective immunity.
MATERIALS AND METHODS
Study population.
A cohort study was undertaken in the Ilaita area of Maprik District, East Sepik Province, Papua New Guinea. Details of the study are described elsewhere (39). Briefly, 264 children aged 1 to 4 years (median, 1.70 years; range, 0.9 to 3.2 years) were enrolled in the study and venous blood was collected. Of these, 190 were enrolled at the start of the study and 74 over the 6 months that followed. Antibody assays were performed using samples from 183 of the 190 children enrolled at the commencement of the study. All data presented for the current analysis relate to these 183 children only. After enrollment, children were followed for a total of 16 months. Active morbidity surveillance was carried out every 2 weeks and involved clinical examination for signs and symptoms of malaria. Additionally, children were also actively checked for malaria infection every 8 weeks, with visits scheduled over 2 consecutive days (with 2 samples collected 24 h apart) to improve detection of low (L)-level infections. This sampling schedule was implemented to maximize detection of parasite clones while ensuring continued participant compliance. Passive case detection for reinfection and symptomatic illness was maintained at local health facilities. A clinical episode of P. falciparum malaria was defined as the presence of fever (axillary temperature of >37.5°C) and parasitemia at >2,500 parasites/μl. All children with parasitologically confirmed malaria were treated with artemether-lumefantrine (Coartem), and children with moderate to severe anemia (hemoglobin [Hb] level of <7.5 g/dl) received artemether-lumefantrine and 4 weeks of iron and folate supplementation according to national treatment guidelines. At enrollment, 30 children were treated for symptomatic parasitemia. Parasite positivity was determined by semiquantitative post-PCR and ligase detection reaction-fluorescent microsphere assay (LDR-FMA) (40), and densities were determined by light microscopy. Plasma samples from anonymous Melbourne residents with no known previous exposure to malaria were included as negative controls in all assays.
Samples and data were also used from a second longitudinal study of 206 children aged 5 to 14 years in the Madang Province, PNG (24, 41). At enrollment, all children received 7 days of artesunate orally to clear any parasitemia (treatment efficacy, 95%). The cohort was actively reviewed every 2 weeks for a 6-month period for symptomatic illness and parasitemia by PCR and microscopy. New infections were distinguished from treatment failures by merozoite surface protein 2 (MSP2) genotyping. A clinical episode of P. falciparum malaria was defined as fever and a P. falciparum parasite load of >5,000 parasites/μl. Plasma samples collected at baseline were used in this study for comparisons with the cohort of younger children.
Informed consent was obtained from all participants in the studies, and ethics approval was obtained from the Medical Research Advisory Council, PNG, and the Human Research Ethics Committees of The Walter and Eliza Hall Institute of Medical Research and Alfred Health (for the Burnet Institute).
ELISA.
Samples collected from the enrollment bleed were used in the enzyme-linked immunosorbent assay (ELISA). Recombinant MSP1-19 (3D7 sequence), full-length MSP2 (FC27 and 3D7), and AMA-1 (full ectodomain; 3D7) were generated as 6His-tagged proteins in Escherichia coli using established methods (42, 43); the correct conformation of AMA-1 was confirmed by binding with monoclonal antibodies (MAbs) 1F9 and 2C5, which recognize conformationally dependent epitopes (44, 45). Erythrocyte binding antigens (EBA) EBA175, EBA140, and EBA181 were expressed as glutathione S-transferase (GST) fusion proteins in E. coli (23). A subset of samples was also tested for IgG responses to AMA-1 (W2mef). As the correlation between the IgG responses to the AMA-1 3D7 and W2mef alleles was very high (r = 0.857), the analysis presented here was performed using data for AMA-1 3D7 only. Recombinant MSP2 and AMA-1 proteins were kindly provided by Robin Anders, La Trobe University, Australia.
ELISAs were performed using established methods (12, 23, 24). Briefly, 96-well plates (Immunolon 4 plates [Thermo Labsystems, Waltham, MA, USA] or Maxisorp plates [Nunc, Roskilde, Denmark]) were coated with 0.5 μg/ml of recombinant antigen–phosphate-buffered saline (PBS) and incubated overnight at 4°C. Skim milk–PBS–0.05% Tween was used for blocking and diluting plasma and antibodies. Plasma was added in duplicate at previously determined dilutions. For measuring total IgG, horseradish peroxidase (HRP)-conjugated sheep anti-human IgG (Chemicon, Melbourne, Australia) was used at a 1:2,500 concentration. For measuring IgM, HRP-conjugated sheep anti-human IgM (Chemicon, Melbourne, Australia) was used at a 1:5,000 concentration. For IgG subclasses, secondary antibodies were added at a dilution of 1:1,000 using mouse anti-human IgG subclass antibodies (IgG1 clone HP6069 and IgG3 clone HP6047 [Zymed/Invitrogen Corporation, Carlsbad, CA]). The tertiary antibody for the subclass assays was a sheep anti-mouse antibody (Chemicon International, Temecula, CA, USA), used at 1:2,500. OPD (o-phenylenediamine dihydrochloride; Sigma, Castle Hill, Australia) was used as the substrate, and the reaction was stopped with 3 M hydrochloric acid. Optical density (OD) was measured at 492 nm. All samples were tested in duplicate, and samples were retested if there was a discrepancy of greater than 25% between duplicates. Standardization of the assays was achieved using positive-control plasma pools on each plate. Background values (determined by examination of wells with no plasma) were subtracted from the mean of the values determined for each sample, and a cutoff threshold for positivity was determined as the mean plus 3 standard deviations of the results determined for the nine negative-control plasma samples (Australian residents) included in each assay.
Determining the molFOI.
The molecular force of infection (molFOI) was used to define the number of new P. falciparum clones acquired during the study follow-up period. The molFOI was determined using previously described methods (32). Briefly, DNA samples were genotyped for merozoite surface protein 2 (MSP2) using capillary electrophoresis for fragment sizing as described in reference 46 with minor modifications (47). High polymorphism of this marker was observed in this study population, with 52 different MSP2 genotypes identified (47). A new infection was defined as any MSP2 genotype detected in a given 8-week interval that was not detected during the previous interval, and the number of infections with new genotypes was calculated for each individual (32). Genotyping was undertaken on blood samples collected during both the morbidity surveillance and the 8 periods of weekly active surveillance.
Statistical analysis.
Antibody levels were not normally distributed, so nonparametric tests were used for analyses. At baseline, the differences in median optical densities (ODs) corresponding to age and P. falciparum infection status (defined by LDR-FMA) were compared using Kruskal-Wallis tests or Mann-Whitney U tests where appropriate, and correlations between ODs of different antibody responses were determined using Spearman's rank correlation. The association between the proportions of seropositive children according to age and P. falciparum infection status (defined by LDR-FMA) was assessed using chi-square tests. For determining the association between antibody levels and the presence of P. falciparum clinical malaria, children were stratified into three equal groups (tertiles), reflecting those exhibiting high (H)-level, medium (M)-level, and low (L)-level responses (i.e., high, medium, and low responders) according to the OD values for each antigen.
Children were followed up for a maximum of 8 periods during the study, each spanning 8 to 9 weeks and consisting of 3 fortnightly morbidity surveillance visits and concluding with the collection of 2 blood samples 24 h apart for active detection of malaria infection. Clinical malaria episodes were defined as a fever (i.e., axillary temperature >37.5°C) or history of fever during the previous 48 h in the presence of parasitemia at a density of >2,500 parasites/μl detectable by the use of a light microscope (48). Febrile episodes with only LDR-FMA-detectable parasite positivity were not considered to represent clinical episodes. For each 8- to 9-week follow-up interval, children were considered at risk from the first day after the second or only blood sample collected for active malaria follow-up was taken. Cross-sectional bleed days were therefore considered part of the preceding 8- to 9-week interval, and clinical episodes detected during these cross-sectional bleeds (2 samples were taken 24 h apart) were included in that interval. Children were considered not at risk at 2 weeks after treatment with artemether-lumefantrine and 4 weeks after treatment with amodiaquine (AQ) plus sulfadoxine-pyrimethamine (SP).
As previous analyses showed significant overdispersion in the number of episodes per child, a negative binomial model with generalized estimating equations (GEE) (based on an XTNBREG procedure) with an exchangeable correlation structure and a semirobust variance estimator was used for the analyses of incidence rates (39). Prior analyses assessed a range of factors to determine their association with a risk of malaria in this cohort (39). Based on these factors, the current analyses of antibody variables (tertiles to reflect low, medium, and high responses) were done using univariate analysis and adjusted for the predefined variables of village (11 categories), month (6 categories), year (continuous variable), age (continuous), P. falciparum infection status at the start of the interval (0 and 1, where “0” represents uninfected and “1” represents infected), prior drug use (2 categories), and average insecticide-treated net (ITN) use (continuous) (39). The inclusion of P. vivax as a confounder had no impact on the estimates of the association between P. falciparum antibodies and the subsequent risk of clinical disease. Therefore, only P. falciparum was included as a variable in the analyses presented, to enable evaluation of species-specific infection with respect to species-specific exposures and outcomes. An interaction term between each antibody variable and follow-up interval was also examined to assess any changes of an effect over time. No significant evidence of an interaction was found (P > 0.1), so the interaction term between each antibody variable and follow-up interval was not included in the final model. The association of combinations of antibody responses was also assessed by analyzing all pairwise combinations, by fitting a model with two main effects and an interaction term. All analyses were done using the STATA 9.2 statistical software package (StataCorp, College Station, TX, USA).
Individual differences in exposure were calculated using molFOI, defined as the number of genetically distinct P. falciparum clones a child acquired during 2-month intervals, expressed as the number of new infections per unit time. Samples from scheduled bleeds as well as morbidity surveillance were used. The force of infection for each child and interval was defined as the number of new clones acquired per year at risk (i.e., the molecular force of infection) and was cube-root transformed (32).
RESULTS
Acquisition of antibodies to merozoite antigens in young children: influence of age, infection, and spatial heterogeneity.
Antibody responses to blood-stage antigens MSP1-19, MSP2, and AMA-1 were determined at enrollment in 183 children, aged 1 to 4 years. Overall, the prevalences of IgM (31.2% to 63.4%) and IgG (43.7% to 61.2%) responses to various merozoite antigens in this age group were moderate and were lower than we had found previously for older children (aged 5 to 14 years) (see Table S1 in the supplemental material). For a given antigen, the prevalences of IgG and IgM were similar, with the exception of AMA-1 (IgM, 31.2%; IgG, 57.9%). Similarly, the prevalences of IgG subclass responses (33.9% to 63.9%) differed among the different antigens. In previous studies in PNG (23, 24, 49), we found that the dominant IgG subclass responses among PNG children are comprised of IgG1 and IgG3, with very little IgG2 or IgG4, consistent with reports from African studies (18, 50–52). Therefore, only IgG1 and IgG3 responses were examined in detail in the current study. For AMA-1 and MSP1-19, IgG1 levels were significantly higher than IgG3 levels (P = 0.016 for MSP1-19; P < 0.001 for AMA-1). For MSP2, IgG3 was the dominant subclass for both alleles (P < 0.001 for both 3D7 and FC27) (data not shown).
IgG and IgM levels in response to all antigens increased significantly with age (for IgG, P ≤ 0.005; for IgM, P ≤ 0.001) (Fig. 1 and data not shown). IgG1 and IgG3 subclass levels also increased significantly with age (for IgG1, P ≤ 0.0009; for IgG3, P ≤ 0.0045), with the exception of MSP1-19 IgG1 (P = 0.247) and IgG3 (P = 0.617) (data not shown).
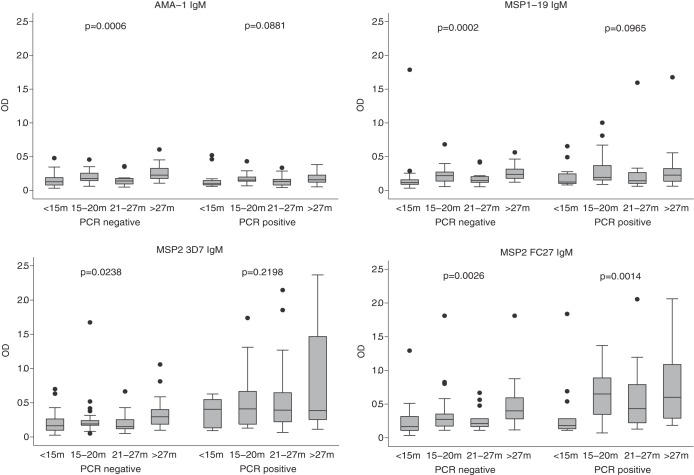
FIG 1.
IgM responses to merozoite antigens in relation to age and P. falciparum infection status. Children (n = 183) were divided into 4 age groups to examine associations with age. As indicated, the presence of P. falciparum was determined by PCR. Data are plotted as box-and-whisker plots (boxes show medians and interquartile ranges; error bars show 95% confidence intervals). m, months. Filled circles represent outlier values.
At enrollment, 93 (50.8%) children were positive for P. falciparum (by LDR-FMA). Levels of IgM for the two MSP2 alleles were significantly higher in those children with detectable P. falciparum infection compared to parasite-negative individuals (P = 0.0001; Fig. 1). Evidence for higher levels of IgM with P. falciparum infection at baseline was weaker for AMA-1 (P = 0.089) and was not significant for MSP1-19 (P = 0.425). IgG levels were significantly higher for all antigens in those children who were P. falciparum positive than in those who were parasite negative (P = 0.0001) (data not shown). IgG1 and IgG3 levels were significantly higher in those children who had concurrent parasitemia than in those who were parasite negative (P ≤ 0.0013), with the exception of IgG1 for MSP1-19, although the trend was still seen (P = 0.062).
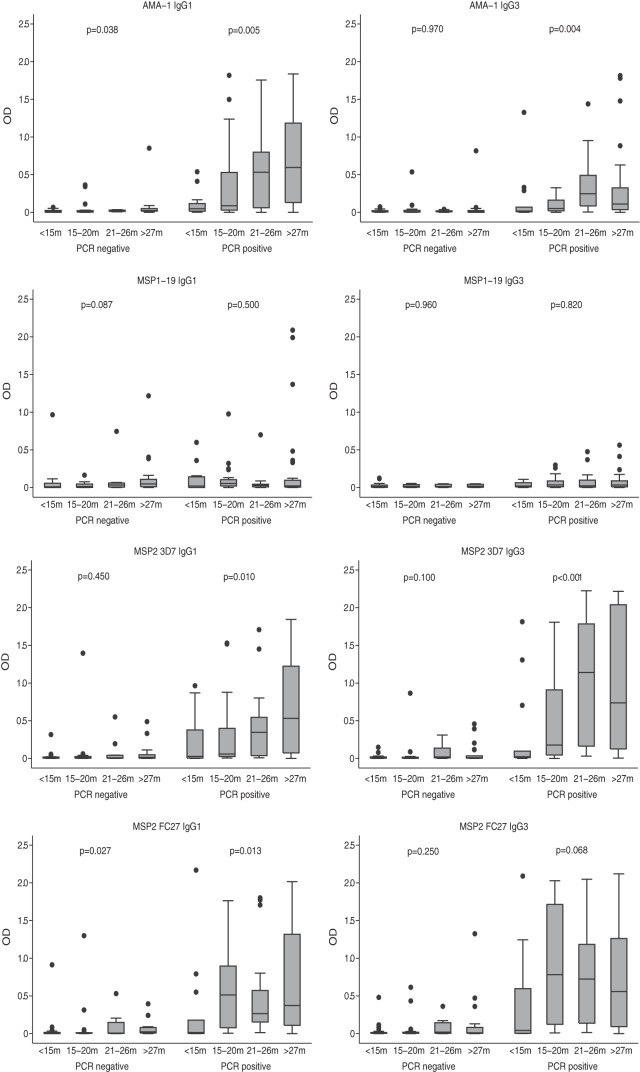
To better understand the acquisition of antibodies, we analyzed antibody levels stratified by age and the presence or absence of infection (Fig. 2). In parasite-positive individuals, significant age associations, and higher-level responses, were generally seen. There was a significant increase in IgG1 and IgG3 levels with age for AMA-3D7 and the 2 MSP2 alleles (P ≤ 0.013, except MSP2 FC27 IgG3, P = 0.068). In parasite-negative individuals, there was evidence of a significant increase of antibody responses with age only for AMA-1 IgG1 (P = 0.038) and MSP2 FC27 IgG1 (P = 0.027). In contrast, IgG subclass responses to MSP1-19 did not significantly increase with age in parasite-positive or -negative individuals, suggesting that the presence of these antibodies is not strongly reflective of recent exposure. IgG levels were significantly associated with age in those that were parasite negative and parasite positive (P ≤ 0.02), with the exception of MSP1-19 (P = 0.24 in P. falciparum-positive children) (data not shown).
FIG 2.
IgG subclass responses in relation to age and P. falciparum infection status. Children (n = 183) were divided into 4 age groups to examine associations with age. As indicated, the presence of P. falciparum was determined by PCR. Data are presented as box-and-whisker plots (boxes show medians and interquartile ranges; error bars show 95% confidence intervals).
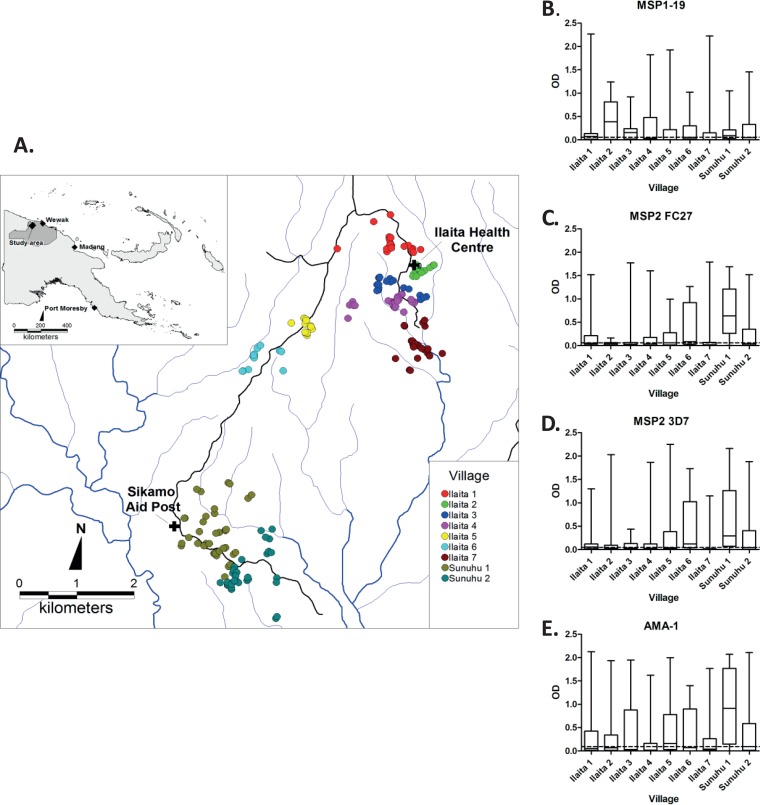
Given the heterogeneity of malaria transmission levels in the study population (39), and the interest in using antibodies as biomarkers of exposure in populations (38), we then examined antibody responses relative to spatial heterogeneity in malaria transmission. Overall, antigen-specific antibody levels differed significantly (P < 0.001) when individuals were grouped by village of residence (Fig. 3). Examining the relatedness of median antibody responses by village, MSP2-3D7 responses were significantly correlated with MSP2 FC27 (r = 0.85, P < 0.01) and AMA-1 responses (r = 0.70, P < 0.05), and MSP2-FC27 responses were correlated with AMA-1 (r = 0.78, P < 0.05). MSP1-19 responses were not correlated with any other median antibody responses (r = −0.03 to 0.27, P > 0.48). This suggests that antibodies to MSP2 and AMA-1 may be better markers of heterogeneity of exposure than antibodies to MSP1-19.
FIG 3.
IgG responses to merozoite antigens by village. (A) Schematic map of study area, including houses of participants (dots) and health centers (crosses) (modified from reference 39 under the Creative Commons Attribution License). (B to E) IgG responses to MSP1-19, MSP2 FC27, MSP2 3D7, and AMA-1 categorized by village. The dashed line in each panel indicates the overall median response for a given antigen. Data are shown as box-and-whisker plots (boxes show medians and interquartile ranges; error bars show minimums and maximums). Antibody levels were significantly different between villages for all antigens (P = <0.001).
Coacquisition of different antibodies to merozoite antigens.
To further understand the acquisition of immunity, we examined the coacquisition of multiple antibody types and specificities. IgG responses were significantly positively correlated between the different antigens, indicating coacquisition of IgG responses with different specificities, although the strength of correlations varied (r = 0.51 to 0.73, P < 0.001) (see Table S2 in the supplemental material). Significant positive correlations between the IgM responses were also observed for the different antigens (r = 0.39 to 0.76, P < 0.001) (see Table S2). Interestingly, IgM responses to MSP2 were significantly correlated with IgG responses to MSP2 and IgG responses to MSP1-19 and AMA-1 (r = 0.38 to 0.48, P < 0.001). In contrast, IgM responses to MSP1-19 or AMA-1 were only weakly or were not significantly correlated with IgG responses (r = 0.17 and P < 0.05 or r = −0.5 and P > 0.10, respectively; see Table S2). These results suggest that the antibody responses are dominated by IgG for AMA-1 and MSP1-19; in contrast, there appears to have been greater coacquisition of IgM together with IgG for MSP2 than was seen for the other antigens. The IgG1 and IgG3 subclass responses were highly significantly correlated for a given antigen (r = 0.58 to 0.87, P < 0.001) (Table 1). IgG1 and IgG3 responses were highly correlated between the two MSP2 alleles (r = 0.71 to 0.79, P < 0.001) and between the MSP2 alleles and AMA-1 (r = 0.56 to 0.73, P < 0.001). Weaker correlations were observed between the subclass responses to MSP1-19 and the other antigens (r = 0.21 to 0.48, P < 0.001).
TABLE 1.
Correlation between IgG1 and IgG3 responses to merozoite antigens of P. falciparum
| Antigen | Antibody | Correlation coefficienta |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MSP1-19 |
MSP2 3D7 |
MSP2 FC27 |
AMA-1 |
||||||
| IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | ||
| MSP1-19 | IgG1 | ||||||||
| IgG3 | 0.58 | ||||||||
| MSP2 3D7 | IgG1 | 0.38 | 0.38 | ||||||
| IgG3 | 0.36 | 0.44 | 0.78 | ||||||
| MSP2 FC27 | IgG1 | 0.37 | 0.39 | 0.75 | 0.74 | ||||
| IgG3 | 0.34 | 0.42 | 0.71 | 0.79 | 0.87 | ||||
| AMA-1 | IgG1 | 0.37 | 0.48 | 0.71 | 0.70 | 0.69 | 0.73 | ||
| IgG3 | 0.21 | 0.45 | 0.56 | 0.68 | 0.58 | 0.66 | 0.65 | ||
Correlation coefficients were determined by Spearman's method. All correlations are significant at a P value of <0.001.
We investigated whether antigen properties or host factors were the major determinants of IgG subclass responses to different merozoite antigens, by examining the relationship between IgG subclass responses between individuals for different antigens (24). Individuals who were identified as high responders (defined as >50th percentile; n = 92) for IgG3 to AMA-1 were no more likely to be high responders for IgG3 to MSP1-19 than for IgG1 to MSP1-19 (65% were high responders for IgG3 versus 55% for IgG1). Similarly, high responders for IgG1 to AMA-1 were no more likely to be high responders for IgG1 to MSP1-19 than for IgG3 to MSP1-19 (64% were high responders for IgG1 versus 69% for IgG3). Additionally, correlations between IgG3 responses to different antigens, or IgG1 responses to different antigens, were very similar to correlations for IgG1 versus IgG3 for different antigens (see Table S2 in the supplemental material), although there were some differences for AMA-1 correlations. These results suggest that antigen properties rather than host factors were the major determinants of IgG subclass responses to the different merozoite antigens.
Association between antibody responses and risk of symptomatic malaria.
Individuals were allocated into three equally sized groups based on antibody levels measured at enrollment (high [H], medium [M], and low [L]; based on tertiles) and were related to the prospective incidence of symptomatic P. falciparum malaria during the study follow-up period. In these analyses, high (H) or medium (M) responders were compared to low (L) responders.
Univariate analyses showed that children with high levels of AMA-1 IgG, IgG1, and IgG3 all had a significantly increased prospective risk of P. falciparum malaria during follow-up compared to children with low levels of antibodies (incidence rate ratio [IRR], 1.72 to 2.26, P < 0.001; Table 2). A similar increased risk was observed in children with high total IgG, IgG1, and IgG3 levels against both MSP2 allelic types (for 3D7, IRR of 1.66 to 1.91, P < 0.002; for FC27, IRR of 1.99 to 2.18, P < 0.001). Interestingly, high levels of antibodies specific for MSP1-19 were not consistently associated with an increased or decreased risk of P. falciparum malaria.
TABLE 2.
Antibody responses to P. falciparum merozoite antigens and prospective risk of symptomatic malaria with a density of >2,500/μla
| Antigen, antibody (responder level) | Value(s) for P. falciparum (density > 2,500/μl) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IRRb | 95% CI | P | aIRRc | 95% CI | P | aIRR-FOId | 95% CI | P | |
| MSP1-19 | |||||||||
| IgG (M) | 1.27 | 0.93, 1.73 | 0.129 | 1.04 | 0.75, 1.45 | 0.818 | 0.95 | 0.74, 1.22 | 0.669 |
| IgG (H) | 1.11 | 0.81, 1.52 | 0.517 | 0.99 | 0.74, 1.33 | 0.955 | 0.91 | 0.72, 1.16 | 0.465 |
| IgG1 (M) | 1.21 | 0.90, 1.63 | 0.21 | 1.15 | 0.86, 1.55 | 0.35 | 1.00 | 0.79, 1.27 | 0.988 |
| IgG1 (H) | 0.83 | 0.59, 1.15 | 0.259 | 0.84 | 0.63, 1.12 | 0.229 | 0.81 | 0.61, 1.06 | 0.127 |
| IgG3 (M) | 1.13 | 0.83, 1.55 | 0.428 | 1.16 | 0.87, 1.56 | 0.319 | 1.02 | 0.79, 1.30 | 0.903 |
| IgG3 (H) | 1.09 | 0.80, 1.47 | 0.596 | 1.12 | 0.80, 1.57 | 0.506 | 0.87 | 0.67, 1.13 | 0.293 |
| IgM (M) | 0.97 | 0.73, 1.31 | 0.858 | 0.95 | 0.72, 1.25 | 0.724 | 1.04 | 0.81, 1.35 | 0.750 |
| IgM (H) | 0.88 | 0.65, 1.20 | 0.43 | 0.90 | 0.65, 1.24 | 0.518 | 0.93 | 0.73, 1.18 | 0.541 |
| MSP2 3D7 | |||||||||
| IgG(M) | 1.34 | 0.95, 1.89 | 0.092 | 1.12 | 0.79, 1.58 | 0.528 | 1.21 | 0.92 1.58 | 0.178 |
| IgG (H) | 1.72 | 1.28, 2.33 | <0.001 | 1.19 | 0.80, 1.77 | 0.399 | 1.13 | 0.86, 1.48 | 0.381 |
| IgG1(M) | 1.10 | 0.77, 1.58 | 0.594 | 0.96 | 0.65, 1.42 | 0.836 | 0.97 | 0.73, 1.28 | 0.829 |
| IgG1(H) | 1.66 | 1.21, 2.27 | 0.002 | 1.18 | 0.77, 1.81 | 0.458 | 1.07 | 0.81, 1.40 | 0.633 |
| IgG3 (M) | 1.51 | 1.07, 2.13 | 0.018 | 1.52 | 1.07, 2.16 | 0.019 | 1.17 | 0.89, 1.55 | 0.253 |
| IgG3 (H) | 1.91 | 1.40, 2.61 | <0.001 | 1.35 | 0.89, 2.04 | 0.161 | 1.11 | 0.85, 1.47 | 0.436 |
| IgM (M) | 0.85 | 0.61, 1.19 | 0.352 | 0.80 | 0.58, 1.10 | 0.177 | 0.92 | 0.70, 1.22 | 0.583 |
| IgM (H) | 1.29 | 0.96, 1.73 | 0.092 | 1.10 | 0.80, 1.50 | 0.559 | 1.10 | 0.85, 1.42 | 0.480 |
| MSP2 FC27 | |||||||||
| IgG (M) | 1.52 | 1.07, 2.17 | 0.019 | 1.36 | 0.96, 1.93 | 0.08 | 1.26 | 0.95, 1.67 | 0.111 |
| IgG (H) | 1.99 | 1.44, 2.74 | <0.001 | 1.48 | 0.98, 2.24 | 0.06 | 1.24 | 0.92, 1.67 | 0.162 |
| IgG1 (M) | 1.54 | 1.09, 2.16 | 0.014 | 1.31 | 0.90, 1.89 | 0.161 | 1.19 | 0.90, 1.57 | 0.222 |
| IgG1 (H) | 2.18 | 1.62, 2.93 | <0.001 | 1.74 | 1.22, 2.49 | 0.002 | 1.32 | 1.00, 1.75 | 0.050 |
| IgG3 (M) | 1.52 | 1.08, 2.15 | 0.017 | 1.48 | 1.04, 2.10 | 0.03 | 1.29 | 0.99, 1.69 | 0.062 |
| IgG3 (H) | 2.17 | 1.58, 2.98 | <0.001 | 1.62 | 1.08, 2.42 | 0.02 | 1.33 | 0.99, 1.80 | 0.060 |
| IgM (M) | 0.91 | 0.66, 1.25 | 0.561 | 0.89 | 0.65, 1.24 | 0.5 | 0.92 | 0.72, 1.17 | 0.506 |
| IgM (H) | 1.29 | 0.96, 1.73 | 0.086 | 1.12 | 0.79, 1.57 | 0.528 | 0.97 | 0.75, 1.25 | 0.806 |
| AMA-1-3D7 | |||||||||
| IgG (M) | 1.49 | 1.08, 2.07 | 0.015 | 1.36 | 0.99, 1.87 | 0.058 | 1.28 | 0.97, 1.69 | 0.076 |
| IgG (H) | 2.15 | 1.56, 2.96 | <0.001 | 1.66 | 1.07, 2.58 | 0.023 | 1.29 | 0.97, 1.72 | 0.084 |
| IgG1 (M) | 1.13 | 0.82, 1.55 | 0.445 | 1.04 | 0.75, 1.44 | 0.809 | 1.01 | 0.77, 1.33 | 0.922 |
| IgG1 (H) | 1.72 | 1.29, 2.28 | <0.001 | 1.25 | 0.88, 1.78 | 0.214 | 1.11 | 0.86, 1.43 | 0.409 |
| IgG3 (M) | 1.55 | 1.11, 2.16 | 0.01 | 1.58 | 1.14, 2.19 | 0.006 | 1.43 | 1.09, 1.88 | 0.010 |
| IgG3 (H) | 2.26 | 1.67, 3.05 | <0.001 | 1.94 | 1.31, 2.87 | 0.001 | 1.44 | 1.09, 1.90 | 0.009 |
| IgM (M) | 0.90 | 0.68, 1.19 | 0.459 | 0.99 | 0.73, 1.33 | 0.932 | 0.91 | 0.71, 1.16 | 0.438 |
| IgM (H) | 0.77 | 0.56, 1.06 | 0.11 | 0.84 | 0.63, 1.14 | 0.265 | 0.86 | 0.66, 1.12 | 0.266 |
| All (H)e | 1.45 | 0.99, 2.13 | 0.058 | 1.23 | 0.82, 1.85 | 0.316 | 1.21 | 0.84, 1.74 | 0.317 |
Antibody levels were grouped into tertiles and related to the prospective incidence of symptomatic P. falciparum malaria. High-level antibody responders (designated H) or medium-level antibody responders (designated M) were compared to low-level responders for each antigen. CI, confidence interval. Boldface data represent statistically significant associations (P < 0.05).
Values represent incidence rate ratio (IRR).
Adjustments were performed for the variables of village (11 categories), month (6 categories), year (continuous variable), age (continuous), P. falciparum infection status at the start of the interval (0 and 1), prior drug use (2 categories), and average ITN use (continuous) as defined previously (aIRR) (39).
Adjustments were performed to account only for differences in exposure as defined previously (aIRR-FOI) (32).
The prospective risk of malaria during the study follow-up period was examined in 16 individuals who were high-level responders for all antigens and was compared with the results determined for the rest of the cohort.
Individuals with high IgM responses to MSP2-FC27 and MSP2-3D7 had an increased prospective risk of P. falciparum malaria episodes during the study follow-up period compared to low responders (Table 2), but these were of borderline statistical significance (IRR of 1.29 and P = 0.092 compared to IRR of 1.29 and P = 0.086, respectively). No significant associations were observed for IgM responses to the other antigens. We also evaluated associations between antibodies and risk of symptomatic P. falciparum infection of any parasite density during the follow-up period; the associations were very similar to those for symptomatic malaria using a parasitemia density cutoff value of 2,500 parasites/μl (see Table S3 in the supplemental material).
Previous analyses have identified several demographic, spatial, and temporal variables and use of bed nets as associated with risk of P. falciparum malaria in this cohort (39). When adjustments were made using these variables as potential confounding factors, the strength and significance of associations between antibodies and the prospective risk of symptomatic malaria during the follow-up period were reduced for most antibody responses, suggesting that in this cohort, antibodies are markers for an increased malaria risk rather than being causally linked with malaria risk; this suggests that levels of antibodies are higher in children who have greater exposure to malaria. However, children with high IgG1 and IgG3 responses to MSP2-FC27 (adjusted incidence rate ratio [aIRR], 1.62 to 1.74, P < 0.02) and high IgG and IgG3 responses to AMA-1 (aIRR, 1.66 to 1.94, P < 0.02) still had a significantly increased risk of developing malaria after adjusting for potential confounding factors (Table 2). These data suggest that IgG subclass-specific antibodies to MSP2-FC27 and AMA-1 may be useful biomarkers of the risk of symptomatic malaria in this population by identifying groups of children with higher exposure to malaria or who reside in pockets of higher malaria transmission.
The association of antibody responses with incidence of P. falciparum infection was examined in a subset of individuals (n = 16) who were high responders for all antigens. Following univariate analyses of data from these individuals, there was an increased risk for P. falciparum at a density of >2,500/μl during the follow-up period (IRR of 1.45, P = 0.058) (Table 2) compared to other children in the cohort. Significance was lost following adjustment for potential confounders.
Association between antibodies, force of infection, and malaria risk.
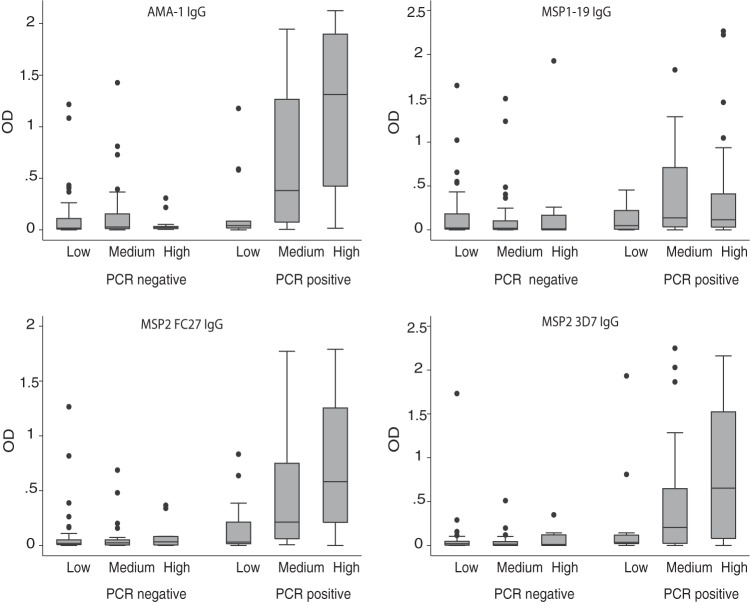
Heterogeneity in P. falciparum transmission intensity exists, even within small geographical areas, and this may lead to differences in malaria exposure (32). In this study, we used a novel molecular marker of exposure in the cohort, molFOI, and examined its relationship to antibody levels and associations with malaria risk. We examined IgG responses to merozoite antigens in relation to molFOI. Overall, higher levels of IgG corresponding to merozoite antigens were observed with increasing molFOI (Fig. 4). This was seen primarily among children with active parasitemia at baseline, as this group had higher levels of antibodies and incidence of malaria, and this age association was significant for MSP2 and AMA-1 antibodies (P < 0.01) but not for MSP1-19. This suggests that antibodies are strongly linked with the individual exposure level in this cohort.
FIG 4.
IgG responses to merozoite antigens in relation to molFOI and P. falciparum infection status. Children were divided into 3 groups (those with low, medium, and high molFOI levels) to examine associations between antibody levels (OD) at baseline and molFOI as a marker of exposure. As indicated, the presence of P. falciparum at baseline was determined by PCR. Data are presented as box-and-whisker plots (boxes show medians and interquartile ranges; error bars show 95% confidence intervals). Differences categorized by level of molFOI are significant for AMA-1 and MSP2 responses among the PCR-positive children (P < 0.01).
Heterogeneity in Plasmodium exposure can confound associations between measures of the immune response and risk of developing malaria (26–31). Previous analyses showed that molFOI could explain the majority of malaria risk in this cohort (32). Therefore, analyses examining associations between antibody responses and prospective risk of malaria were adjusted for molFOI, an approach that has not been reported previously. Adjustment for molFOI greatly reduced or eliminated the associations between antibodies and prospective risk of symptomatic P. falciparum infection at a density of >2,500/μl and P. falciparum infection of any density during the follow-up period (Table 2; see also Table S3 in the supplemental material); this further indicates that levels of antibodies are highest in those with the greatest exposure to infection and risk of malaria rather than being causally related to the risk of malaria. Many antibody responses that were significantly associated with an increased risk of P. falciparum infection before adjustment became nonsignificantly associated with an increased risk of P. falciparum infection after adjusting for molFOI. However, some weak associations remained after adjustment for AMA-1 IgG3 (aIRR-FOI, 1.44, P = 0.009) and MSP2-FC27 IgG1 (aIRR-FOI, 1.32, P = 0.050).
Contrasting malaria risk and IgG levels in two different cohorts.
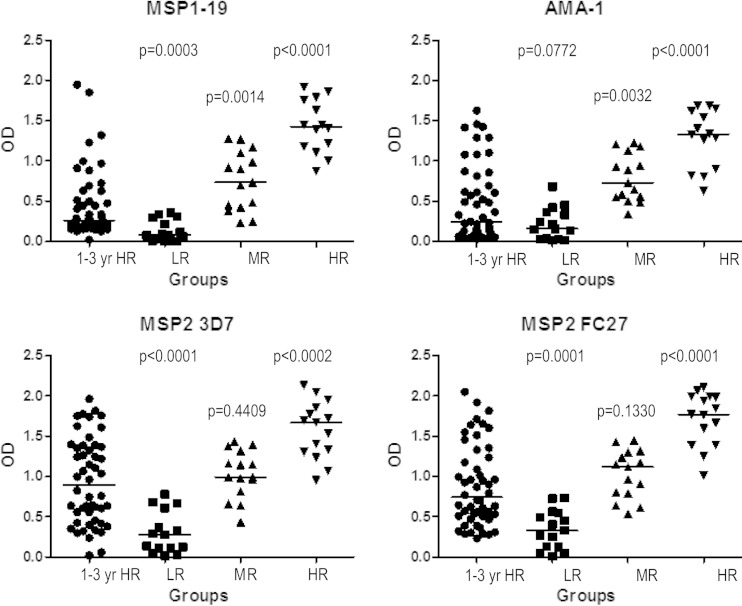
To understand how antibody levels might relate to protective associations, we compared levels of antibodies to MSP1-19, MSP2, and AMA-1 between children of this cohort and a previous cohort of older PNG children (5 to 14 years), in whom high antibody responses were associated with protection from symptomatic malaria (24). Plasma samples from the top 25% of 1- to 4-year-old responders for each antigen in the current study were tested for antibody reactivity directly alongside a random selection of 5- to 14-year-old low, medium, and high responders from the previous study (Fig. 5). This allowed a direct assessment to determine whether insufficient antibody levels were a possible explanation for why antibodies in these younger children were not associated with protection from symptomatic malaria.
FIG 5.
Comparison of IgG levels to merozoite antigens of P. falciparum in two cohorts of children of different ages. Total IgG levels specific for MSP1-19, AMA-1, MSP2 3D7, and MSP2 FC27 in the top quartile of responders for each antigen in the group of children 1 to 4 years of age (1-3 yr HR group; age at enrollment) were compared with those of low, medium, and high responders in the group of children 5 to 14 years of age. Horizontal bars indicate median antibody levels, and P values refer to comparisons between the top quartile of responders from the group of 1- to 4-year-old children and each of the low, medium, and high responder groups of the 5- to 14-year-old children. LR, low responders; MR, medium responders; HR, high responders.
Children in the top quartile of responders from the 1- to 4-year-old cohort had substantially lower levels of antibodies compared with the older high responders, who were largely protected from malaria. The top quartile of responders among the 1- to 4-year-old children had (i) higher levels of MSP1-19 IgG than the low responders among the 5- to 14-year-old children (P = 0.0003), but the levels were significantly less than those seen with the medium- or high-responder groups (P = 0.0014 or P < 0.0001, respectively) (Fig. 5); (ii) AMA-1 IgG levels similar to those seen with the 5-to-14-year-old low responders (P = 0.0772) and significantly lower levels than in the medium and high responders (P = 0.0032 and P < 0.0001, respectively); and (iii) levels of MSP2 that were similar to those seen with the 5- to 14-year-old medium responders but were significantly lower than those seen with the high responders (for MSP2 3D7, P < 0.0002; for MSP2 FC27, P < 0.0001). These results demonstrate that the median IgG levels in even the group of highest responders among the 1- to 4-year-old children were below those in the children who had protective immunity in the older cohort of 5- to 14-year-old children. This suggests that children in the younger cohort had antibody levels below the threshold required for protective immunity. The patterns of IgG subclass responses to different antigens were very similar between the two cohorts, suggesting that this is not an explanation for the different antibody associations.
We further investigated differences in antibody associations between the two cohorts by comparing IgG responses to the EBAs, which are merozoite invasion ligands and emerging as important targets of acquired immunity (12, 23, 37, 53, 54). Prior studies observed that IgG antibodies against the EBAs were among the responses most strongly associated with protective immunity in the cohort of older children, even after adjusting for potential confounders (23, 44). In the cohort of 1- to 4-year-old children, we measured total IgG responses to EBA175, EBA140, and EBA181 and found that high levels of IgG were associated with an increased prospective risk of malaria episodes (IRR, 1.58 to 1.99, P ≤ 0.005; Table 3), as seen for other merozoite antigens; this remained after adjustment for potential confounders (aIRR = 1.48 and P = 0.033 and aIRR = 1.58 and P = 0.029, respectively). In contrast, in the cohort of 5- to 14-year-old children, high levels of antibodies were significantly associated with protection from risk of malaria episodes (hazard ratio [HR], 0.2 to 0.32, P ≤ 0.02). Similar trends of protective associations in the 5- to 14-year-old children were observed for MSP2 and AMA-1 antibodies, contrasting with the association with increased risk of malaria for antibodies in the 1- to 4-year-old cohort (Table 3). These results suggest that while antibodies to merozoite antigens, including the EBAs, are associated with increased risk of malaria in younger children, these responses evolve to become biomarkers of protective immunity in older children.
TABLE 3.
Comparison of antibody responses to P. falciparum merozoite antigens and risk of symptomatic malaria in two cohorts of different age rangesa
| Anitigen | Cohort 1 (aged 1–4 years) |
Cohort 2 (aged 5–14 years) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRRb | 95% CI | P | aIRRc | 95% CI | P | HRd | 95% CI | P | aHRe | 95% CI | P | |
| EBA-181 | 1.99 | 1.46, 2.73 | <0.0001 | 1.48 | 1.03, 2.11 | 0.033 | 0.32 | 0.13, 0.82 | 0.02 | 0.4 | 0.16, 1.04 | 0.06 |
| EBA-140 | 2.04 | 1.45, 2.87 | <0.0001 | 1.58 | 1.05, 2.38 | 0.029 | 0.2 | 0.07, 0.58 | <0.01 | 0.25 | 0.09, 0.76 | 0.01 |
| EBA-175 | 1.58 | 1.15, 2.18 | 0.005 | 1.23 | 0.84, 1.79 | 0.28 | 0.21 | 0.07, 0.61 | <0.01 | 0.27 | 0.09, 0.81 | 0.02 |
| MSP1-19 | 1.11 | 0.81, 1.52 | 0.517 | 0.99 | 0.74, 1.33 | 0.955 | 0.42 | 0.17, 1.03 | 0.06 | 0.57 | 0.22, 1.44 | 0.24 |
| MSP2 | 1.72 | 1.28, 2.33 | <0.001 | 1.19 | 0.80, 1.77 | 0.399 | 0.39 | 0.14, 1.02 | 0.06 | 0.52 | 0.19, 1.38 | 0.19 |
| AMA-1 | 2.15 | 1.56, 2.96 | <0.001 | 1.66 | 1.07, 2.58 | 0.023 | 0.29 | 0.11, 0.80 | 0.02 | 0.34 | 0.12, 0.94 | 0.04 |
IgG antibody levels were measured by ELISA, individuals were grouped into tertiles based on IgG reactivity, and tertile groups were related to the prospective incidence of symptomatic P. falciparum malaria. Only results of comparisons between high-level and low-level responders are shown. Boldface data represent statistically significant associations (P < 0.05).
Values represent incidence rate ratio (IRR) (see Materials and Methods).
Adjustments were performed using the variables of village (11 categories), month (6 categories), year (continuous), age (continuous), P. falciparum infection status at the start of the interval (0 and 1), prior drug use (2 categories), and average ITN use (continuous) as defined previously (aIRR) (39).
Values represent hazard ratios and were calculated using the Cox proportional hazards model as described previously (23).
aHR, adjusted hazard ratio. Adjustments were performed for variables as defined previously (23).
DISCUSSION
Findings presented here provide important insights into understanding the acquisition of immunity in young children. Our key findings are that (i) acquisition of IgG subclass-specific responses to merozoites are associated with increasing age and are boosted by active infection, and levels are reflective of spatial heterogeneity in exposure; (ii) there is early coacquisition of multiple antibodies, dominated by IgG1 and IgG3, but MSP2 responses are noted by significant coacquisition of IgM; (iii) antigen properties, rather than host factors, appear to be the main determinant of the type of IgG subclass response among individuals; (iv) antibodies reflect exposure and are predictive of malaria risk among young children in the early stages of acquiring immunity and evolve to become biomarkers of protective immunity among older children with greater exposure; (v) results suggest that antibodies may need to reach a threshold level before they are associated with immunity or are involved in mediating protection from malaria; and (vi) the novel molecular marker molFOI closely reflects heterogeneity in malaria exposure and antibody acquisition and is a valuable tool for analyses of antibody associations with malaria risk.
In young children acquiring immunity, higher levels of antibodies to merozoite antigens of P. falciparum were not protective but were instead broadly predictive of an increased prospective risk of developing malaria. Our results suggest that high antibody levels identified children with greater malaria exposure and higher risk of P. falciparum exposure. Total IgG and IgG subclass responses to MSP2, AMA-1, EBA175, EBA140, and EBA181 were significantly associated with increased risk of malaria. Interestingly, responses to MSP1-19 were not significantly associated with risk. When associations were adjusted for markers of P. falciparum exposure (age, location, seasonality, concurrent P. falciparum infection, prior use of antimalarials, and ITN use [39]), associations with increased malaria risk were reduced overall but remained significant for some responses. On the other hand, adjusting associations with molFOI mostly eliminated these associations with risk. These findings support the conclusion that antibodies are good biomarkers of malaria risk in this young cohort, rather than mediating significant protective immunity, and that molFOI is better able to capture heterogeneity in malaria exposure in the cohort and account for the association between antibodies and this increased risk of malaria than other parameters.
A previous study found that molFOI was a major predictor of clinical disease in this cohort (32). This molecular method estimates the number of distinct P. falciparum clones acquired during the study follow-up period, providing a more accurate estimation of individual malaria exposure than techniques such as light microscopy and standard PCR or demographic parameters (32). Our analyses indicate that molFOI, as a single parameter, is able to explain most of the increased risk associated with high antibody responses in this cohort. This is an important finding, and we believe that our study is the first to apply a sensitive measure of FOI in the evaluation of antibody-mediated immunity to P. falciparum. Previously, studies have not been able to define exposure to P. falciparum at an individual level, which has limited the interpretation of the relationship between antibodies and prospective malaria risk, particularly where extensive heterogeneity in exposure is present. It is possible that strain-specific immunity might protect against infection with specific clones, which may influence the estimation of molFOI. However, this is likely to have minimal effect since acquired immunity generally protects against disease and not parasitemia per se (24), and prior studies have established that molFOI is a very good marker of malaria transmission (32).
Understanding how antibodies to merozoite antigens contribute to human antimalarial immunity has been complicated by inconsistent results regarding their protective associations in different studies (15). Relatively little is known about the concentration, repertoire, and function of antibodies that are required to mediate immunity or serve as robust correlates of immunity. While a measure of antibody function would be valuable, the lack of robust assays has limited the inclusion of functional antibody measures to assess the contribution of antibody quality to immunity. The growth inhibition assay has yielded inconsistent results with respect to the role of growth inhibitory antibodies in protection (11, 55, 56). More recently, opsonic phagocytosis assays (14) and neutrophil-based antibody-dependent respiratory burst assays (13) have been developed and applied to a limited number of cohort studies with results suggesting that these assays may function as correlates of immunity.
There is a large body of evidence suggesting that protection from malaria is dependent on high antibody concentrations (22–24, 49, 57–59). We previously demonstrated that high levels of antibodies to AMA-1, MSP1-19, and MSP2 were significantly associated with protection from symptomatic malaria in older children in PNG and that the nature of the IgG subclass was important (24). This suggests that the use of these antigens in the present study to assess acquisition of immunity was appropriate. IgG responses to EBA140, EBA175, and EBA181 were among those most strongly associated with protection from symptomatic malaria in the cohort of older children (23) but were similarly associated with increased risk in the cohort of younger children. Direct comparisons of antibody levels between these two cohorts suggested that a threshold level of antibodies may be required to mediate protective immunity, whereas the IgG subclass profiles of the two cohorts were similar. IgG responses were substantially lower in the younger cohort. The concept of a quantitative correlate of protection for malaria-specific antibodies has been briefly explored in African populations (22, 44, 57–59). Our results support those of a recent study demonstrating the requirement for a “protective threshold” concentration of merozoite-specific antibodies for protection from clinical episodes of malaria (57). These findings have important implications for understanding and assessing human immunity to malaria, for identifying populations at risk, and for the design of future immunoepidemiological studies that aim to assess the importance of humoral responses in protection from malaria.
Our findings highlight how antibodies to merozoite antigens can be biomarkers of both increased malaria risk and protective immunity, depending on the population and the level of immunity (Fig. 6). In populations with limited cumulative exposure (e.g., young children or those living in areas of low transmission), levels of antibodies may be below a protective threshold and may be useful as biomarkers of past exposure and higher future malaria risk but poor biomarkers of clinical immunity. Such biomarkers may be particularly useful where exposure is heterogeneous. In some low-transmission settings, the protective threshold may never be reached because of insufficient or infrequent exposure to malaria infection. With increasing cumulative exposure, the value of these antibodies as biomarkers of exposure and/or risk of symptomatic malaria declines. Antibodies may reach a protective threshold level and evolve to become better biomarkers of immunity but poor biomarkers of increased risk of symptomatic malaria. Further studies in different settings and populations are needed to better define optimal antibody biomarkers of malaria (38), and it should be noted that a protective threshold level of antibodies has not yet been defined. However, we believe that this model provides a framework for understanding divergent antibody associations. The utility of antibodies in serosurveillance is also dependent on the longevity of antibody responses in the absence of infection, which is currently poorly understood and appears to be highly variable among individuals (38, 60).
FIG 6.
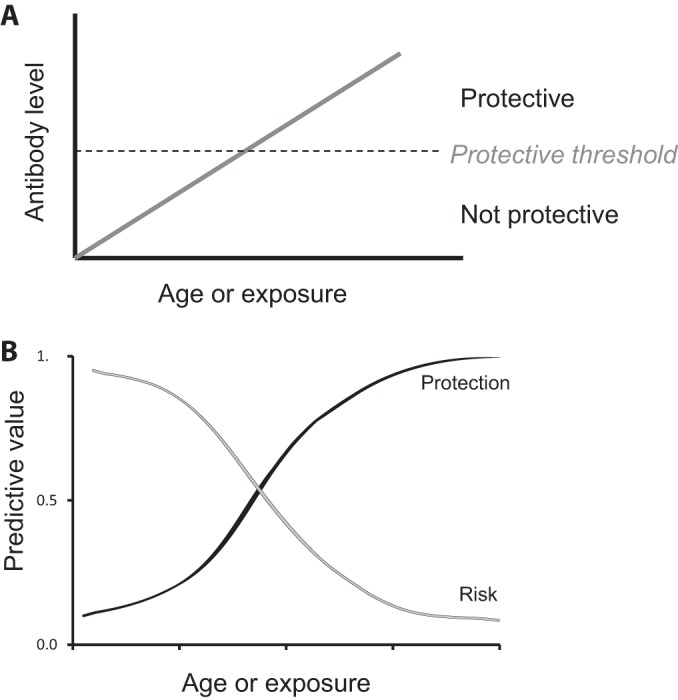
Models of the evolving role of antibodies to P. falciparum merozoite antigens with changing malaria exposure and antibody levels. (A) Low antibody levels are not protective against malaria, but as antibody levels increase (with age and/or exposure) and reach a theoretical threshold, antibodies contribute to protective immunity and serve as biomarkers of malaria immunity. (B) Antibody levels may be valuable as biomarkers to predict malaria risk or protective immunity because they identify individuals who are being exposed to infection. In young children, or those with limited exposure, antibodies have a high predictive value for an increased risk of malaria and have a poor predictive value for protective immunity. As age or cumulative exposure or both increase, the predictive value of antibodies for increased risk of malaria declines to a point at which antibodies become better markers of protection from malaria.
The IgG subclass profiles observed in this cohort were in agreement with those seen in previous studies (17, 18, 20, 21, 24, 50, 61–65), with an IgG1-dominant response observed for MSP1-19 and AMA-1 compared with the IgG3-dominant response observed for MSP2. Our analyses suggest that the nature of the IgG subclass response appeared to be mainly influenced by antigen properties rather than host factors, as we have reported previously (24). The IgG subclass profile was very similar to that seen in our prior study of older PNG children and suggests that differences in the nature of the IgG subclass response do not account for the differences in the associations between antibodies and protective immunity in the two cohorts. Of further interest, the strong correlations observed between IgG and IgM responses to MSP2 suggest coacquisition of these responses, whereas this was not seen with MSP1-19 and AMA-1. These differences seen with IgM responses may reflect inherent structural differences in the antigens and/or the relative conservation of the epitopes that are targeted (MSP2 is intrinsically unstructured, in contrast to MSP1-19 and AMA-1), and this warrants further investigation.
In conclusion, these findings have implications for understanding human immunity to malaria, identifying targets of protective immune responses to facilitate vaccine development, and developing immune biomarkers of malaria exposure that could be used to enhance malaria control and elimination efforts. Our findings suggest that antibody responses can evolve from being biomarkers of malaria risk in populations with low immunity to being biomarkers of, and contributors to, protection from malaria in older individuals or in those with greater cumulative exposure. Furthermore, our data suggest that differing associations with protection between population-based studies may be related to differences in antibody levels between cohorts with a threshold antibody level required for protective immunity, which is supported by recent findings in an African population (57). Our findings highlight the utility of serological approaches, and of molFOI, in identifying populations at risk and assessing ongoing transmission of malaria in populations with low levels of immunity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council of Australia (project grant, program, and career development award to J.G.B.; training award to F.J.I.F. and J.S.R.; postgraduate research fellowship to J.S.R.; Infrastructure for Research Institutes Support Scheme); Australian Research Council (Future Fellowship to J.G.B.); National Institutes of Health (United States); and the Victorian State Government Operational Infrastructure Support grant, Australia.
We thank all participants in the study and their parents or guardians, staff members at the Maprik and Mugil field sites, Moses Lagog and Anslem Masalan, who helped with the processing of the samples, and Robin Anders for providing recombinant MSP2 and AMA-1 proteins and helpful advice and comments.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02398-14.
REFERENCES
- 1.Schofield L, Mueller I. 2006. Clinical immunity to malaria. Curr Mol Med 6:205–221. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 2.Beeson J, Osier F, Engwerda C. 2008. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol 24:578–584. doi: 10.1016/j.pt.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Richards JS, Beeson JG. 2009. The future for blood-stage vaccines against malaria. Immunol Cell Biol 87:377–390. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, McGregor I, Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 5.McGregor IA, Carrington SP, Cohen S. 1963. Treatment of East African P. falciparum malaria with West African human γ-globulin. Trans R Soc Trop Med Hyg 57:170–175. doi: 10.1016/0035-9203(63)90058-0. [DOI] [Google Scholar]
- 6.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45:297–308. [DOI] [PubMed] [Google Scholar]
- 7.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S, Butcher G, Crandall R. 1969. Action of malarial antibody in vitro. Nature 223:368–371. doi: 10.1038/223368a0. [DOI] [PubMed] [Google Scholar]
- 9.Egan A, Burghaus P, Druilhe P, Holder A, Riley E. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol 21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 10.Khusmith S, Druilhe P, Gentilini M. 1982. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun 35:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCallum F, Persson K, Mugyenyi C, Fowkes F, Simpson J, Richards J, Williams T, Marsh K, Beeson J. 2008. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One 3:e3571. doi: 10.1371/journal.pone.0003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson K, McCallum F, Reiling L, Lister N, Stubbs J, Cowman A, Marsh K, Beeson J. 2008. Variation in use of erythrocyte pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Investig 118:342–351. doi: 10.1172/JCI32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joos C, Marrama L, Polson HE, Corre S, Diatta AM, Diouf B, Trape JF, Tall A, Longacre S, Perraut R. 2010. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One 5:e9871. doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, McCallum FJ, Reiling L, Jaworowski A, Anders RF, Marsh K, Beeson JG. 2014. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 12:108. doi: 10.1186/1741-7015-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med 7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braga E, Barros R, Reis T, Fontes C, Morais C, Martins M, Krettli A. 2002. Association of the IgG response to Plasmodium falciparum merozoite protein (C-terminal 19kD) with clinical immunity to malaria in the Brazilian amazon region. Am J Trop Med Hyg 66:461–466. [DOI] [PubMed] [Google Scholar]
- 17.Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis 173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 18.Metzger W, Okenu D, Cavanagh D, Robinson J, Bojang K, Weiss H, McBride J, Greenwood B, Conway D. 2003. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol 25:307–312. doi: 10.1046/j.1365-3024.2003.00636.x. [DOI] [PubMed] [Google Scholar]
- 19.Perraut R, Marrama L, Diouf B, Sokhna C, Tall A, Nabeth P, Trappe J, Longacre S, Mercereau-Puijalon O. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J Infect Dis 191:264–271. doi: 10.1086/426398. [DOI] [PubMed] [Google Scholar]
- 20.Polley S, Conway D, Cavanagh D, McBride J, Lowe B, Williams T, Mwangi T, Marsh K. 2006. High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 24:4233–4246. doi: 10.1016/j.vaccine.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RR, Allen SJ, Greenwood BM, Riley EM. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg 58:406–413. [DOI] [PubMed] [Google Scholar]
- 22.Courtin D, Oesterholt M, Huismans H, Kusi K, Milet J, Badaut C, Gaye O, Roeffen W, Remarque EJ, Sauerwein R, Garcia A, Luty AJ. 2009. The quantity and quality of African children's IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One 4:e7590. doi: 10.1371/journal.pone.0007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards JS, Stanisic DI, Fowkes FJ, Tavul L, Dabod E, Thompson JK, Kumar S, Chitnis CE, Narum DL, Michon P, Siba PM, Cowman AF, Mueller I, Beeson JG. 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51:e50–e60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 24.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. 2009. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, Lanar DE, Dutta S, Rosenthal PJ, Dorsey G, John CC. 2011. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis 204:19–26. doi: 10.1093/infdis/jir223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousema T, Kreuels B, Gosling R. 2011. Adjusting for heterogeneity of malaria transmission in longitudinal studies. J Infect Dis 204:1–3. doi: 10.1093/infdis/jir225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark TD, Greenhouse B, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Staedke SG, Seto E, Kamya MR, Rosenthal PJ, Dorsey G. 2008. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis 198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 28.Bejon P, Cook J, Bergmann-Leitner E, Olotu A, Lusingu J, Mwacharo J, Vekemans J, Njuguna P, Leach A, Lievens M, Dutta S, von Seidlein L, Savarese B, Villafana T, Lemnge MM, Cohen J, Marsh K, Corran PH, Angov E, Riley EM, Drakeley CJ. 2011. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J Infect Dis 204:9–18. doi: 10.1093/infdis/jir222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cattani JA, Moir JS, Gibson FD, Ginny M, Paino J, Davidson W, Alpers MP. 1986. Small-area variations in the epidemiology of malaria in Madang Province. P N G Med J 29:11–17. [PubMed] [Google Scholar]
- 30.Hii JL, Smith T, Mai A, Mellor S, Lewis D, Alexander N, Alpers MP. 1997. Spatial and temporal variation in abundance of Anopheles (Diptera:Culicidae) in a malaria endemic area in Papua New Guinea. J Med Entomol 34:193–205. [DOI] [PubMed] [Google Scholar]
- 31.Kreuels B, Kobbe R, Adjei S, Kreuzberg C, von Reden C, Bater K, Klug S, Busch W, Adjei O, May J. 2008. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. J Infect Dis 197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- 32.Mueller I, Schoepflin S, Smith TA, Benton KL, Bretscher MT, Lin E, Kiniboro B, Zimmerman PA, Speed TP, Siba P, Felger I. 2012. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci U S A 109:10030–10035. doi: 10.1073/pnas.1200841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook J, Kleinschmidt I, Schwabe C, Nseng G, Bousema T, Corran PH, Riley EM, Drakeley CJ. 2011. Serological markers suggest heterogeneity of effectiveness of malaria control interventions on bioko island, equatorial Guinea. PLoS One 6:e25137. doi: 10.1371/journal.pone.0025137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WM, Lemnge MM, Cox J, Reyburn H, Riley EM. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noor AM, Mohamed MB, Mugyenyi CK, Osman MA, Guessod HH, Kabaria CW, Ahmed IA, Nyonda M, Cook J, Drakeley CJ, Mackinnon MJ, Snow RW. 2011. Establishing the extent of malaria transmission and challenges facing pre-elimination in the Republic of Djibouti. BMC Infect Dis 11:121. doi: 10.1186/1471-2334-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, Masika P, Mosha J, Bousema T, Shekalaghe S, Cook J, Corran P, Ghani A, Riley EM, Drakeley C. 2009. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One 4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG. 2013. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott SR, Fowkes FJ, Richards JS, Reiling L, Drew DR, Beeson JG. 2014. Research priorities for the development and implementation of serological tools for malaria surveillance. F1000Prime Rep 6:100. doi: 10.12703/P6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin E, Kiniboro B, Gray L, Dobbie S, Robinson L, Laumea A, Schoepflin S, Stanisic D, Betuela I, Siba P, Felger I, Schofield L, Zimmerman P, Mueller I. 2010. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One 5:e9047. doi: 10.1371/journal.pone.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara D, Thomson J, Kasehagen L, Zimmerman P. 2004. Development of multiplex PCR-ligase detection reaction assay for diagnosis of infection by the four parasite species causing malaria in humans. J Clin Microbiol 42:2403–2410. doi: 10.1128/JCM.42.6.2403-2410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michon P, Cole-Tobian J, Dabod E, Schoepflin S, Igu J, Susapu M, Tarongka N, Zimmerman P, Reeder J, Beeson J, Schofield L, King C, Mueller I. 2007. The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg 76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 42.Dutta S, Lalitha P, Ware L, Barbosa A, Moch J, Vassell M, Fileta B, Kitov S, Kolodny N, Heppner D, Haynes J, Lanar D. 2002. Purification, characterization and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect Immun 70:3101–3110. doi: 10.1128/IAI.70.6.3101-3110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodder A, Crewther P, Anders R. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun 69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mugyenyi CK, Elliott SR, McCallum FJ, Anders RF, Marsh K, Beeson JG. 2013. Antibodies to polymorphic invasion-inhibitory and non-inhibitory epitopes of Plasmodium falciparum apical membrane antigen 1 in human malaria. PLoS One 8:e68304. doi: 10.1371/journal.pone.0068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coley AM, Parisi K, Masciantonio R, Hoeck J, Casey JL, Murphy VJ, Harris KS, Batchelor AH, Anders RF, Foley M. 2006. The most polymorphic residue on Plasmodium falciparum apical membrane antigen 1 determines binding of an invasion-inhibitory antibody. Infect Immun 74:2628–2636. doi: 10.1128/IAI.74.5.2628-2636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falk N, Maire N, Sama W, Owusu-Agyei S, Smith T, Beck HP, Felger I. 2006. Comparison of PCR-RFLP and Genescan-based genotyping for analyzing infection dynamics of Plasmodium falciparum. Am J Trop Med Hyg 74:944–950. [PubMed] [Google Scholar]
- 47.Schoepflin S, Valsangiacomo F, Lin E, Kiniboro B, Mueller I, Felger I. 2009. Comparison of Plasmodium falciparum allelic frequency distribution in different endemic settings by high-resolution genotyping. Malar J 8:250. doi: 10.1186/1475-2875-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Covell G. 1950. Congenital malaria. Trop Dis Bull 47:1147–1167. [PubMed] [Google Scholar]
- 49.Reiling L, Richards JS, Fowkes FJ, Barry AE, Triglia T, Chokejindachai W, Michon P, Tavul L, Siba PM, Cowman AF, Mueller I, Beeson JG. 2010. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol 185:6157–6167. doi: 10.4049/jimmunol.1001555. [DOI] [PubMed] [Google Scholar]
- 50.Tongren J, Drakeley C, McDonald S, Reyburn H, Manjurano A, Nkya W, Lemnge M, Gowda C, Todd J, Corran P, RIley E. 2006. Target antigen, age and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun 74:257–264. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor RR, Smith DB, Robinson VJ, McBride JS, Riley EM. 1995. Human antibody response to Plasmodium falciparum merozoite surface protein 2 is serogroup specific and predominantly of the immunoglobulin G3 subclass. Infect Immun 63:4382–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egan AF, Chappel JA, Burghaus PA, Morris JS, McBride JS, Holder AA, Kaslow DC, Riley EM. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun 63:456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Persson KE, Fowkes FJ, McCallum FJ, Gicheru N, Reiling L, Richards JS, Wilson DW, Lopaticki S, Cowman AF, Marsh K, Beeson JG. 2013. Erythrocyte-binding antigens of Plasmodium falciparum are targets of human inhibitory antibodies and function to evade naturally acquired immunity. J Immunol 191:785–794. doi: 10.4049/jimmunol.1300444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarra MB, Ayodo G, Sumba PO, Kazura JW, Moormann AM, Narum DL, John CC. 1 December 2011, posting date Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. Pediatr Infect Dis J doi: 10.1097/INF.0b013e31822d1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duncan CJ, Hill AV, Ellis RD. 2012. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum Vaccin Immunother 8:706–714. doi: 10.4161/hv.19712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dent AE, Bergmann-Leitner ES, Wilson DW, Tisch DJ, Kimmel R, Vulule J, Sumba PO, Beeson JG, Angov E, Moormann AM, Kazura JW. 2008. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS One 3:e3557. doi: 10.1371/journal.pone.0003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murungi LM, Kamuyu G, Lowe B, Bejon P, Theisen M, Kinyanjui SM, Marsh K, Osier FH. 2013. A threshold concentration of anti-merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine 31:3936–3942. doi: 10.1016/j.vaccine.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg 73:222–228. [PubMed] [Google Scholar]
- 59.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fowkes FJ, McGready R, Cross NJ, Hommel M, Simpson JA, Elliott SR, Richards JS, Lackovic K, Viladpai-Nguen J, Narum D, Tsuboi T, Anders RF, Nosten F, Beeson JG. 2012. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis 206:1612–1621. doi: 10.1093/infdis/jis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavanagh D, Dobaño C, Elhassan IM, Marsh K, Elhassan A, Hviid L, Khalil EA, Theander TG, Arnot DE, McBride JS. 2001. Differential patterns of human immunogloblin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect Immun 69:1207–1211. doi: 10.1128/IAI.69.2.1207-1211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitua AY, Urassa H, Wechsler M, Smith T, Vounatsou P, Weiss NA, Alonso PL, Tanner M. 1999. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol 21:307–317. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 63.Nebie I, Diarra A, Ouedraogo A, Soulama I, Bougouma E, Tiono A, Konate A, Chilengi R, Theisen M, Dodoo D, Remarque E, Bosomprah S, Milligan P, Sirima S. 2008. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun 76:759–766. doi: 10.1128/IAI.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scopel K, Fontes C, Ferreira M, Braga E. 2006. Factors associated with immunoglobulin G subclass polarization in naturally acquired antibodies in Plasmodium falciparum merozoite surface proteins: a cross-sectional survey in Brazilian Amazonia. Clin Vaccine Immunol 13:810–813. doi: 10.1128/CVI.00095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi YP, Sayed U, Qari SH, Roberts JM, Udhayakumar V, Oloo AJ, Hawley WA, Kaslow DC, Nahlen BL, Lal AA. 1996. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun 64:2716–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.