Abstract
A recombinant macrophage infectivity potentiator (rMIP) protein of Neisseria meningitidis induces significant serum bactericidal antibody production in mice and is a candidate meningococcal vaccine antigen. However, bioinformatics analysis of MIP showed some amino acid sequence similarity to human FK506-binding proteins (FKBPs) in residues 166 to 252 located in the globular domain of the protein. To circumvent the potential concern over generating antibodies that could recognize human proteins, we immunized mice with recombinant truncated type I rMIP proteins that lacked the globular domain and the signal leader peptide (LP) signal sequence (amino acids 1 to 22) and contained the His purification tag at either the N or C terminus (C-term). The immunogenicity of truncated rMIP proteins was compared to that of full (i.e., full-length) rMIP proteins (containing the globular domain) with either an N- or C-terminal His tag and with or without the LP sequence. By comparing the functional murine antibody responses to these various constructs, we determined that C-term His truncated rMIP (−LP) delivered in liposomes induced high levels of antibodies that bound to the surface of wild-type but not Δmip mutant meningococci and showed bactericidal activity against homologous type I MIP (median titers of 128 to 256) and heterologous type II and III (median titers of 256 to 512) strains, thereby providing at least 82% serogroup B strain coverage. In contrast, in constructs lacking the LP, placement of the His tag at the N terminus appeared to abrogate bactericidal activity. The strategy used in this study would obviate any potential concerns regarding the use of MIP antigens for inclusion in bacterial vaccines.
INTRODUCTION
Neisseria meningitidis (meningococcus) infections contribute significantly to mortality and morbidity worldwide (1). Implementation of capsular polysaccharide-protein conjugate vaccines against serogroups A, C, Y, and W into the routine immunization schedules of developed countries has been successful (2–5), but this approach cannot be used for serogroup B strains. The polysaccharide capsule of serogroup B meningococci (MenB) shows structural mimicry of human fetal brain neural cell adhesion molecules (6). Licensed MenB vaccines based on lipooligosaccharide (LOS)-depleted outer membrane (OM) vesicles (V) have been used to control serosubtype strain-specific clonal outbreaks of MenB infection, e.g., in Norway (7), Cuba (8), Brazil (9), and New Zealand (10), but they do not provide cross-strain protection (11). Recently, the 4CMenB (Bexsero) vaccine, developed using a genome-based reverse-vaccinology approach (12), has received a license from the European Union and has been recommended by the Joint Committee for Vaccination and Immunization for the routine vaccination of infants in the United Kingdom since 2014. The vaccine consists of the factor H binding protein (fHbp, fused to GNA2091 carrier protein), neisserial heparin binding protein (NHBA, fused to GNA1030 carrier protein), and an adhesin, NadA, mixed with the MenZB OMV vaccine from the New Zealand MenB outbreak strain (NZ98/254, P1.7-2,4, sequence type 41 [ST-41]/ST-44) (13, 14). Another vaccine, bivalent in nature, consists of two recombinant LP2086 (rLP2086) (fHbp) subfamily proteins and is currently in phase III trials. This first generation of MenB vaccines, however, shows incomplete strain coverage of meningococcal strains in the populations examined. For example, it has been predicted using a meningococcal antigen typing system that approximately 73% to 78% of all MenB strains in several European countries would be killed by postvaccination sera induced by the 4CMenB vaccine (15); in Canada, the estimate was only 66% (95% confidence interval [CI], 46% to 78%) (16). Estimating the breadth of strain coverage afforded by the bivalent rLP2086 vaccine is a complex task, but, using a methodology based on a killing assay, it has been reported that for toddlers and adolescents-young adults, protective bactericidal titers ranged from 44% to 100% and from 68% to 98%, respectively, against MenB strains expressing heterologous fHbp proteins (17).
In order to develop effective vaccines, it is critical to identify novel conserved antigens capable of inducing cross-protective antibody responses. Many individual OM and secreted proteins have been investigated for their ability to induce serum bactericidal antibodies (SBA) (18), which is a generally accepted laboratory correlate of protection for serogroup B meningococci (19). Proteomic studies carried out in our laboratory identified the high abundance of a 29-kDa meningococcal MIP protein (the product of gene NMB1567, NEIS1487) in the OM (20). It has been also reported that the gonococcal homologue Neisseria gonorrhoeae macrophage infectivity potentiator (Ng-MIP) was a surface-exposed lipoprotein in N. gonorrhoeae (21). We showed in a previous study (22) that MIP is highly conserved, and in a collection of well-characterized meningococcal isolates (differing in serogroup, serotype, and serosubtype), isolated from carriers or patients, we found only three distinct MIP sequence types (designated I, II, and III). MIP is also surface exposed on meningococci, and the protein is expressed at similar levels among different strains studied thus far (22). We also examined MIP allelic information and sequence diversity in an early version of the http://pubmlst.org/neisseria/ database containing 200 sequenced Neisseria strains (23) and found that 76%, 8%, or 7% of serogroup B meningococci express a type I, II, or III MIP, respectively (to provide 91% strain coverage). Significantly, a recombinant type I meningococcal MIP protein was able to induce high levels of functional and cross-MIP-type reactive bactericidal antibodies when delivered in liposomes and even saline solution alone. Thus, MIP may be an important candidate for inclusion in novel meningococcal vaccines (22).
The Neisseria MIP shares homology with the surface-exposed MIP protein from Legionella pneumophila (24) and other bacteria (22). However, the L. pneumophila MIP shares some amino acid sequence similarity with the immunophilin family of human FK506-binding proteins (FKBPs), which are a family of conserved, widely distributed eukaryotic proteins (25, 26). FKBPs are active as peptidyl-prolyl-cis-trans-isomerases (PPIases) (27), an enzymatic activity also shown by bacterial MIP proteins, and they are targeted by the macrolide immunosuppressants FK506 (Tacrolimus) (28, 29) and rapamycin (Sirolimus) (30). In the current study, we examined the amino acid sequence similarity between meningococcal MIP and human FKBP proteins using bioinformatics analyses and, based on this information, rationally designed recombinant truncated MIP proteins for immunization studies. Comparing the vaccine properties of these proteins with those of full (i.e., full-length) recombinant MIP (22), we identified an antigen formulation without human sequence similarity, capable of generating cross-protective bactericidal antibody responses.
MATERIALS AND METHODS
Bacteria, growth conditions, and preparation of meningococcal OM.
Neisseria meningitidis strains H44/76 (B:15:P1.7,16) and MC58 (B:15:P1.7,16b) and other meningococcal strains expressing different serogroup capsular polysaccharide, PorB protein serotypes, and PorA protein serosubtypes, originally isolated from patients or colonized individuals, have been described previously (22, 31). N. meningitidis serogroup A (Z1534; P1.5-2,10:F3-9, ST-21; identification no. [ID] 90 [http://pubmlst.org/neisseria/]) was provided by D. Caugant, Norwegian Institute of Public Health, Norway. N. meningitidis serogroup W (M11 240441; W:P1.22,26:F3-7, ST-2977 [clonal complex 174 {cc174}]; ID 20458) and serogroup Y (M12 240717; Y:P1.22,9:F3-7, ST-1466 [cc174]; ID 28173) were provided by R. Borrow, Public Health England, Manchester, United Kingdom. Neisseria gonorrhoeae strain P9-17 (Pil+ Opa+) has been described previously (32). Meningococci and gonococci were grown on supplemented GC agar plates (33) and/or Mueller-Hinton agar plates, incubated at 37°C in an atmosphere containing 5% (vol/vol) CO2. Escherichia coli strains TOP10, BL21(DE3) pLysS (Invitrogen), and BLR(DE3) (Novagen) were used for cloning and protein expression and were grown on Luria-Bertani (LB) agar and in LB broth.
To generate a Δmip isogenic deletion mutant in strain MC58, the target gene was truncated by replacing the gene sequence with a kanamycin (Kan) resistance cassette (following the method described by Echenique-Rivera et al. [34], with modifications). Approximately 800-bp fragments of the flanking regions of the target gene were amplified by PCR from MC58 genomic DNA. A fragment upstream of the target gene was created using a forward primer (UMIPk-FOR; 5′-GTTCGGGCAGCTTCAGGA-3′) and a reverse primer (UMIPk-REV; 5′-GCCGGTACCAATGGTGTTCATGATGGAT-3′), carrying the restriction site for KpnI (underlined). A downstream fragment was amplified using a forward primer (DMIPk-FOR; 5′-GAGGGTACCAAAGTAAATTAAGTCCGAATC-3′), also incorporating a restriction site for KpnI, and a reverse primer (DMIPk-REV; 5′-GACGCGGTTTCCCTCAAT-3′). Amplification of the target DNA sequences was done using 2× Phusion PCR master mix (Finnzymes) under the following PCR conditions: initial denaturation (95°C, 2 min) followed by 30 cycles of denaturation (95°C, 30 s), annealing (54°C, 30 s), and extension (68°C, 1 min) and a final extension at 68°C for 10 min. The PCR products were purified using a Wizard PCR cleanup system (Promega) and then digested with KpnI restriction enzyme (Promega) at 37°C for 4 h. The purified PCR fragments were coligated (at 22°C for 3 h) and deactivated at 70°C for 10 min, and the ligation product was amplified using UMIPk-FOR and DMIPk-REV primers under the PCR conditions described above (with an elongated extension time of 1.5 min per cycle). The PCR product was purified using a Wizard SV gel and PCR cleanup system (Promega) and cloned into the pGEM-T Easy vector (Promega). Next, the plasmid was digested with KpnI to insert the Kan cassette and amplified from pCRII TOPO vector (Invitrogen) using a forward primer (KANTOPO-FOR; 5′-CAGGTACCTTCAGACGGCTTTAACAAAATTCAGGGCGCA-3′) containing a DNA uptake sequence (DUS) (in italics) and a reverse primer (KANTOPO-REV; 5′-CAGGTACCTCAGAAGAACTCGTCAAGAAG-3′), both primers carrying the restriction site for KpnI (underlined). After the ligation, the plasmid carrying the Kan cassette was transformed into E. coli TOP10 and subsequently into the naturally competent MC58 strain. Mutagenesis was achieved by heterologous allelic exchange. Transformants were screened by PCR, and the selected MC58 MIP− strain was confirmed by Western blotting using rabbit anti-MIP antisera. Identically to the wild-type organism, the mutant expressed pili, Opa, Opc, PorA, and PorB.
Meningococcal OM were prepared by extraction of whole cells by the use of lithium acetate as described previously (35).
Cloning and expression of the meningococcal mip gene in E. coli and purification and properties of recombinant proteins. (i) N-term His full rMIP (LP).
Genomic DNA of MC58 was extracted by alkaline lysis, as described previously (22), and used as the PCR template. Amplification of the target DNA sequence from MC58 genomic DNA was done by PCR with 2× Phusion PCR master mix (Finnzymes) using primers designed from the gene sequence encoding MIP (NMB1567) accessed from the NCBI website, as described previously (22). Methods for gene cloning into the pRSETA system and expression and purification of the N-terminal (N-term) His full rMIP protein containing the signal leader peptide (LP) by nickel-nitrilotriacetic acid (Ni-NTA) metal-affinity chromatography (Qiagen) have been described previously (22).
(ii) Full and truncated rMIP proteins.
Based on bioinformatics analyses, four constructs were generated by amplifying the mip gene encoding type I MIP from H44/76 (identical to type I MIP from MC58) using primers listed in Table 1 and cloning into the pET system. Truncated constructs (from amino acid [aa] position 143) without the globular domain, thus bypassing any homology with human protein, were prepared with a His tag sequence at either the N or C terminus. Recombinant proteins without the signal leader peptide including the cysteine residue (amino acids 1 to 21) consisted of full (250 amino acids) or truncated (121 amino acids) NMB1567 protein and contained a His tag sequence composed of MHHHHHHGG located at the N terminus or a His tag sequence composed of GGHHHHHH or GHHHHHH at the C terminus of the protein. PCR amplifications were made from genomic DNA of the N. meningitidis H44/76 strain, and the fragments were inserted into the pET24 or pET26 expression vector (Novagen). Expression of recombinant proteins was done in E. coli strain BLR(DE3), and proteins were found in the soluble fraction. Immobilized metal ion affinity chromatography (IMAC) purification was done under native conditions, and proteins were dialyzed against phosphate-buffered saline (PBS; pH 7.4).
TABLE 1.
Oligonucleotides used to create full and truncated rMIP proteins that lack the leader sequencea
| Primer | Sequence (5′–3′) | Use |
|---|---|---|
| 1567-sens-NdeI | CGCGGCCATATGCATCATCATCATCATCATGGCGGCGGCAAAAAAGAAGCCGCCC | For constructing N-term His full rMIP (His-GG-NMB1567; aa 22–272) |
| 1567-rev-XhoI | CCTCCGCTCGAGTTAATTTACTTTTTTGATGTCGA | |
| 1567-sens-NdeI | CGCGGCCATATGCATCATCATCATCATCATGGCGGCGGCAAAAAAGAAGCCGCCC | For constructing N-term His truncated rMIP (His-GG-Trunc-NMB1567; aa 22–143) |
| AS-1567-trunc-rev-stop-XhoI | CCTCCGCTCGAGTCAGCCGTCTTTGGCGGCATTTTCTTTC | |
| 1567-NdeI-sens-woHis | GGAATTCCATATGGGCAAAAAAGAAGCCGCCC | For constructing C-term His full rMIP (NMB1567-GG-His; aa 22–272) |
| 1567-His-C-term-XhoI-antisens | CCGCTCGAGTCAGTGGTGGTGATGATGATGGCCGCCATTTACTTTTTTGATGTCGAC | |
| 1567-NdeI-sens-woHis | GGAATTCCATATGGGCAAAAAAGAAGCCGCCC | For constructing C-term His truncated rMIP (Trunc-NMB1567-G-His; aa 22–143) |
| 1567-trunc-real-his-C-term | CCGCTCGAGTCATCAGTGGTGGTGATGATGATGGCCGCCGTCTTTGGCGGCATTTTCTTTC |
Restriction sites are underlined. aa, amino acid.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis performed with anti-His and anti-rMIP antibodies of the purified recombinant proteins confirmed protein identities and expected molecular weights. Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Pierce Thermo Scientific). Levels of contaminating lipopolysaccharide (LPS) in all the protein samples were negligible (<0.002% [wt/wt]) as quantified with the Limulus amebocyte lysate (LAL) assay (Lonza).
(iii) Predictive structural modeling.
SwissModel (web server) and the visualization tool Swiss PDBViewer were used to predict the three-dimensional (3D) structure of the MIP (NMB1567) protein, using the crystal structure of the MIP dimer protein of Legionella pneumophila (Protein Data Bank [PDB] code 1FD9) as a template. PyMOL graphics software (DeLano Scientific LLC; http://www.pymol.org) was used for visualization of the meningococcal MIP protein structure.
Immunization of animals.
Groups of five BALB/c (H-2d haplotype) mice of approximately equal sizes and weights (6 to 7 weeks of age) were immunized intraperitoneally on days 0, 14, and 28 with N-term His full rMIP, N-term His truncated rMIP, C-term His full rMIP, C-term His truncated rMIP, and N-term His full rMIP (LP). Each mouse received 20 μg of protein/dose, and the groups of animals were immunized with proteins delivered in both saline solution and liposomes alone. The method for preparing liposomes has been described previously (22). Groups of five mice were also injected with control preparations consisting of saline solution alone and empty liposomes, and one group was maintained for access to normal serum. Terminal bleeding of mice by cardiac puncture under anesthesia was carried out on day 42. Polyclonal rabbit antisera to N-term His full rMIP (LP) were raised as described previously (22). All sera were stored at −20°C until required. This study complied with the animal experimentation guidelines of the Home Office and the authors' institutions, and no animals suffered significant adverse effects.
Characterization of biological and functional properties of antibodies to rMIP constructs. (i) ELISA.
Individual antisera from mice immunized with N-term His full rMIP, N-term His truncated rMIP, C-term His full rMIP, C-term His truncated rMIP, and N-term His full rMIP (LP) were reacted in an enzyme-linked immunosorbent assay (ELISA) against their immunizing antigens and against H44/76 and MC58 OM, as described previously (36). Enzyme substrate was added for 10 min, and absorbance was measured at 450 nm (iMark; Bio-Rad). The ELISA titer was extrapolated from the linear portion of the serum titration curve and then taken as the reciprocal dilution which gave an increase in absorbance of 0.1 U after 10 min. A two-sample t test was used to compare differences between mean values for ELISA data sets as described previously (36).
(ii) Western immunoblotting.
Whole-cell lysate preparations from the H44/76 and MC58 wild-type strains and the MC58Δmip mutant were separated by SDS-PAGE and then transferred to nitrocellulose by semidry blotting. After incubation with murine or rabbit sera (1/100 dilution), immunological reactivity was detected by using anti-mouse/rabbit immunoglobulin-alkaline phosphatase conjugate (Bio-Rad) as described previously (36).
(iii) Fluorescence-activated cell sorter (FACS) analysis.
This was done as described previously (22). Briefly, overnight-grown bacteria were centrifuged, and after addition of cold 70% (vol/vol) ethanol (2 ml), the bacterial pellet was stored at −20°C for 1 h to permeabilize the capsule. Bacteria were washed twice with sterile PBS containing 1% (wt/vol) bovine serum albumin (BSA) and suspended to 2 ×108 CFU/ml. Next, 1 ml of bacterial suspension was centrifuged (2,200 × g for 3 min) and the pellet was suspended in 200 μl pooled murine sera (1/10) and incubated at 37°C for 1 h. After being washed with PBS, bacteria were incubated with 100 μl of fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG (Dako) (1/50 dilution) at room temperature for 1 h. Bacteria were fixed with a 0.4% (wt/vol) paraformaldehyde solution at room temperature for 30 min, and samples were analyzed on a FACSAria flow cytometer (BD Biosciences).
(iv) IF and confocal microscopy.
Immunofluorescence (IF) and confocal microscopy were done as previously described (37). Briefly, pooled murine antisera (1/50 dilution) were reacted with fixed whole meningococci. Antibody bound to meningococci was detected by reaction with anti-mouse immunoglobulin–FITC conjugate (Dako; diluted 1/50 in PBS–1% [wt/vol] BSA), and the samples were then viewed and photographed with a Leica SP5 confocal microscope. Three different random fields of each sample preparation were photographed, and quantitative evaluation of the intensities visible in the selected fields was obtained using Leica software (Leica Microsystems Las-AF-Lite_2.6.0_7266). For each image, the regions of interest (ROI) were defined by drawing three lines of similar lengths (40 μm) randomly distributed across each field. The gray-scale values of one color channel (channel 1) of a spatial image series that lay within an ROI area were selected in this way (channel/ROI), which resulted in obtaining the mean values of the intensities for each ROI. Mean values of ROIs (n = 3) from each of the three fields per sample preparation were averaged to give quantitative evaluation of the immunofluorescence (IF) intensities of each sample investigated. Antiserum raised to MC58 OM was used as a positive control for the labeling experiments.
(v) Complement-activated killing of meningococci.
The bactericidal activity of pooled murine antisera was determined as described previously (36), using 5% (vol/vol) and 2% (vol/vol) baby rabbit serum (AbD Serotec) as a source of exogenous complement with meningococci and gonococci, respectively. Murine antisera raised against OM served as an assay positive control. Complement-dependent bactericidal activity was determined from the numbers of bacteria surviving in the presence of serum and complement compared to the numbers surviving with complement but without test serum. Sera that showed bactericidal activity (>50%) in two or more dilutions were considered positive, as judged with a Student's t test, with P values of <0.05 considered significant. Bactericidal assays were not done with the Δmip (MIP−) mutant, which showed increased sensitivity to exogenous complement.
(vi) Examining the reactivity of anti-rMIP antisera with human FKBP protein.
Human Chang conjunctiva epithelial cells (European Tissue Culture Collection, United Kingdom) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% (vol/vol) decomplemented fetal calf sera and a cell lysate prepared by sonication (MSE Soniprep 150 sonicator) as described previously (38). The reactivity of pooled murine antisera to rMIP proteins and of sham-immunized sera, as well as of rabbit anti-rMIP antiserum, was examined initially by Western blot analysis of denatured human cell lysate containing FKBP2, using polyclonal rabbit anti-human FKBP2 (GenBank accession no. NP_004461.2) antibody (Abcam) (142 amino acids [aa], 15.6 kDa) as a positive control.
In order to examine the reactivity of anti-rMIP antisera against nondenatured FKBP2 protein, an ELISA was developed using as the coating antigen (i) a Chang cell lysate, prepared by suspension of cells from a T75 75-cm2 flask into PBS (0.5 ml) followed by three cycles of freezing in liquid nitrogen and thawing at 37°C in a water bath, and (ii) purified active human FKBP2 recombinant full protein (Abcam ab93681). Wells of Nunc MaxiSorp ELISA plates were coated with Chang cell lysate (50 μg/ml, 100 μl/well) or pure human FKBP2 protein (1 μg/ml, 100 μl/well) at 4°C overnight, blocked, and then reacted in triplicate with each of the pooled antisera from mice immunized with rMIP proteins and sham preparations and with rabbit anti-rMIP serum and matching preimmune serum. The polyclonal rabbit anti-human FKBP2 antibody (Abcam) was used as a positive control. Plates were incubated at 37°C for 2 h, washed, and reacted with the respective anti-mouse IgG-horseradish peroxidase (HRP) (1/3,000) and anti-rabbit IgG-HRP (1/1,000) conjugates for 1 h at 37°C. Enzyme substrate was added for 10 min, and absorbance was measured at λ450 nm (iMark; Bio-Rad). Student's t test was used to compare the mean absorbance values between groups, with P values of <0.05 considered significant.
RESULTS
Similarity between meningococcal MIP protein and human FKBP family proteins as the basis for generating recombinant MIP proteins for immunization.
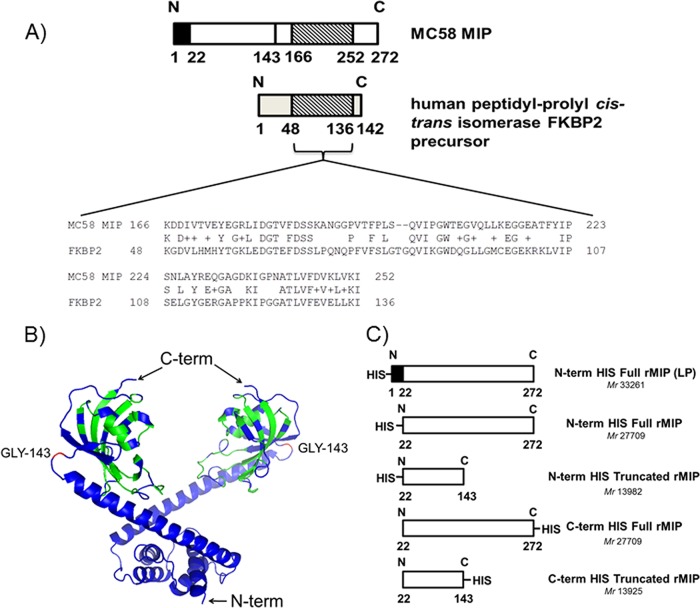
Initially, comparisons were done with the Basic Local Alignment Search Tool (NCBI website), in order to investigate the degree of homology between the 272-amino-acid Neisseria meningitidis MIP protein (NMB1567) and the immunophilin family of human FKBP proteins. We identified the presence of some amino acid sequence similarity of meningococcal MIP to human proteins located between amino acids 166 and 252 (Fig. 1A). The human peptidyl-prolyl cis-trans isomerase FKBP2 precursor and meningococcal MIP share ∼49% amino acid similarity within this region. In addition, the murine homologue of FKBP2, which is 97% identical to the human protein, shares the same areas of amino acid identity with meningococcal MIP as the human FKBP2 protein. Based on the dimer structure of L. pneumophila MIP (29), a putative 3D model of meningococcal MIP was constructed, with the dimerization domain located in the N-terminal portion of the protein (Fig. 1B), which is probably lipidated to enable anchoring of MIP in the membrane (29). Examination of this putative model showed that amino acids 166 to 252 are included within the globular fragment. Each globular fragment (the C-terminal portion of the protein) covers amino acids 143 to 253 and contains a PPIase FKBP-type domain.
FIG 1.
Comparison of the meningococcal MIP protein and human FKBP family proteins and preparation of recombinant MIP proteins. (A) BLAST analysis of MC58 MIP protein against human peptidyl-prolyl cis-trans isomerase FKBP2 precursor. N, N terminus; C, C terminus. (B) Putative 3D model of the MIP (NMB1567) dimer. The amino acids colored in green show similarity (identical and positive scores obtained using NCBI protein BLAST tool) to human protein IPI00002535 (= FKBP2 peptidyl-prolyl cis-trans isomerase). Position GLY143 of the MIP dimer, indicated in red, illustrates the C-term truncation site. (C) Diagram comparing recombinant type I MIP proteins prepared in this study.
Based on the BLAST analyses and the putative MIP model construction, two recombinant truncated MIP constructs without the globular domain, to bypass homology with the human protein, and two recombinant full MIP proteins were expressed in E. coli. The recombinant proteins were prepared with a His purification tag at either the N or C terminus and without the signal sequence leader peptide (LP; amino acids 1 to 21) (Fig. 1C), in order to examine the effect of the position of the His tag and the influence of the LP sequence on immunogenicity. The N-term His full rMIP protein containing the signal LP has been described previously (22).
Antigenicity of the recombinant MIP proteins.
Purified recombinant N-term His full rMIP, N-term His truncated rMIP, C-term His full rMIP, and C-term His truncated rMIP were used for immunization in saline solution and liposomes without additional adjuvants, based on the observation that this protocol previously induced cross-protective bactericidal antibodies with N-term His full rMIP (LP) (22). Individual antisera from mice immunized with the recombinant proteins were reacted in ELISA against their immunizing proteins (Fig. 2A) and also against the N-term His full rMIP protein (LP) (Fig. 2B). Immunization with the truncated and full MIP proteins in saline solution alone induced low mean levels of antibodies that reacted with their homologous immunizing proteins (reciprocal titers ranging from ∼500 to 2,000, with no significant differences between the responses to the antigens, P > 0.05) and against the N-term His full rMIP (LP) (reciprocal mean titers of ∼6 to 52,000). These responses were significantly lower than those induced by immunization with N-term His full rMIP (LP) (reciprocal mean titer of ∼370,000 tested against the homologous immunizing antigen) (Fig. 2A and B). However, when delivered in liposomes, the truncated and full MIP proteins induced mean antibody ELISA titers (P < 0.05) that were significantly higher, ranging from ∼7,000 to 440,000 tested against the immunizing antigen (Fig. 2A) and from ∼6,000 to 35,000 tested against the N-term His full rMIP (LP) protein (Fig. 2B), than the responses in saline solution. In comparison, the mean reciprocal titer response to liposomal N-term His full rMIP (LP) was ∼3 × 106 (Fig. 2A and B).
FIG 2.
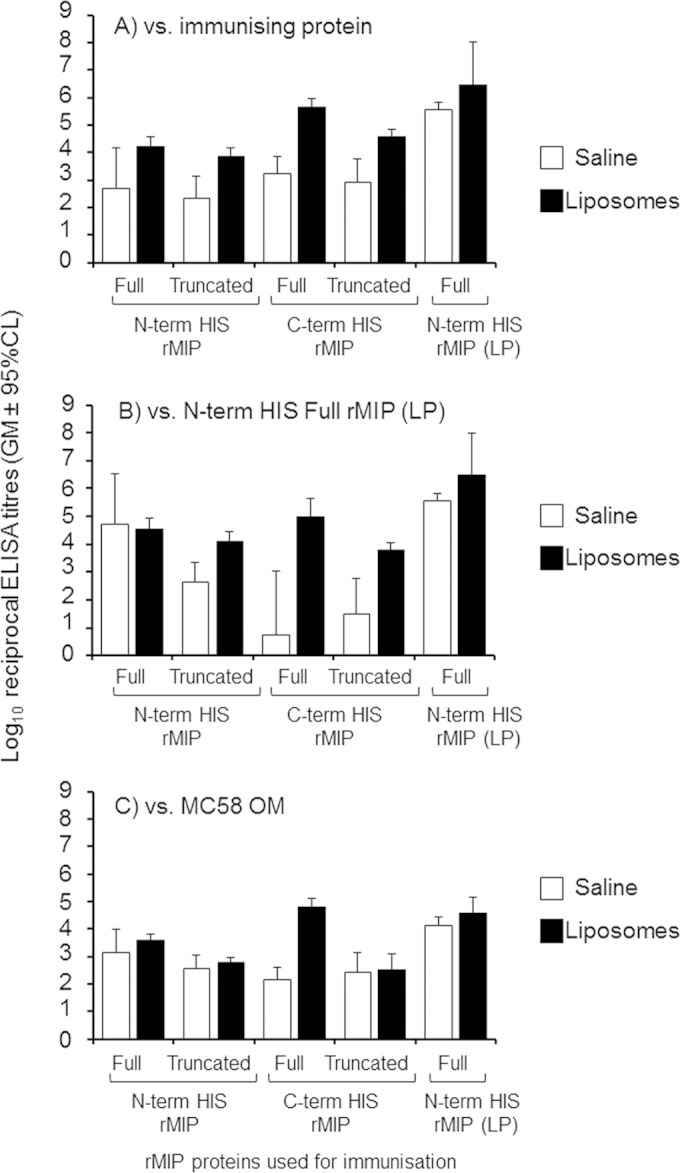
ELISA reactivity of antisera raised against different recombinant MIP proteins. Antisera from individual animals raised against the rMIP proteins in either saline solution or liposomes were reacted against their immunizing protein (A), the N-term His full rMIP (LP) (B), and MC58 OM (C). The columns represent the geometric mean reciprocal ELISA titers [n = 5 animals per group, with the exception of the N-term His full rMIP (LP), where n = 15 animals were used], and the error bars represent the 95% confidence limits. Similar reactivity was observed with the H44/76 OM preparation, expressing identical type I MIP (data not shown). No reactivity (absorbance of <0.1) was observed with sera from sham-immunized animals.
Murine antisera were also tested in ELISA against OM from strain MC58 containing the homologous type I MIP (Fig. 2C). Low mean levels of antibodies were induced by immunization with truncated and full MIP proteins in saline solution (reciprocal titers from ∼150 to 1,400), and they were significantly lower (P < 0.05) than the levels observed following immunization with N-term His full rMIP (LP) (reciprocal titer of ∼14,000). Presentation of proteins in liposomes increased the amount of antibody induced by the truncated and full MIP proteins (reciprocal titers of from ∼300 to 4,000), but these were again significantly lower (P < 0.05) than those induced by liposomal N-term His full rMIP (LP) (reciprocal titer of ∼37,000). The reactivity of antisera against H44/76 OM was similar to that observed against MC58, as the two strains express identical type I MIPs (data not shown).
The specificity of the immune response against rMIP proteins was investigated by Western blotting with a whole-cell lysate preparation of strain MC58 expressing homologous type I MIP. Antisera raised to all rMIP proteins in liposomes showed strong reactivity, with a band with an apparent molecular mass of ∼29 kDa (Fig. 3). In contrast, antisera raised to N- and C-terminal His full and truncated rMIP proteins delivered in saline solution showed very weak or no reactivity, compared to the strong reactivity of antisera to the N-terminal His full rMIP (LP) in saline solution. Specificity was confirmed by the lack of reactivity of any of the anti-MIP sera with the MC58Δmip mutant (data not shown).
FIG 3.
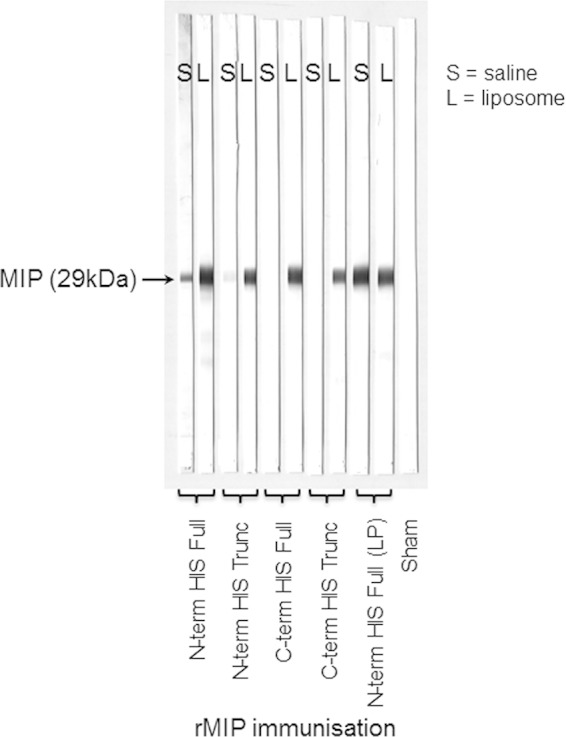
Western blot analysis of the reactivity of murine antisera raised to rMIP proteins with whole-cell lysate of homologous type I MIP strain. Pooled murine antisera (1/100 dilution) were reacted against whole-cell lysate of strains MC58 and H44/76 (type I MIP+) and MC58Δmip mutant (MIP−). Positive antiserum reactivity on Western blot is indicated with a band of ∼29 kDa (MIP) with both strains (MC58 strip blots are shown). Identical results were obtained using whole-cell lysate of strain H44/76 (data not shown). No reactivity to the MC58Δmip mutant was observed (not shown). Sham-immunized mice also show no reactivity (a representative strip blot is shown). S, saline solution; L, liposomes.
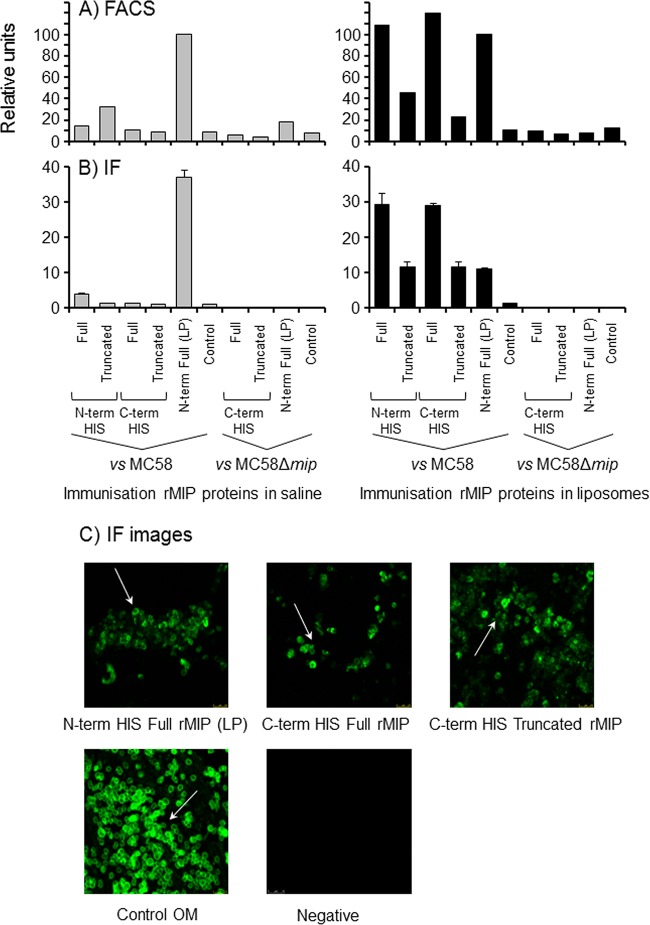
Antibody IF and FACS analysis were used to investigate the ability of anti-rMIP protein antibodies to recognize and bind to MIP on meningococcal cells. Antisera raised to the full and truncated rMIP proteins delivered in saline solution showed low or no binding to wild-type MC58, compared to baseline control levels (P > 0.05); by contrast, antisera to the N-term His full rMIP (LP) in saline solution showed a high level of binding to MC58 cells (P < 0.05 compared to all other sample values), which was specific, as no reactivity was observed with the MC58Δmip mutant strain (Fig. 4A and B). In contrast, antisera raised to the full and truncated rMIP proteins in liposomes showed significant (P < 0.05) binding to MC58 cells, as judged by IF and FACS analysis, compared to controls. Significantly, the signals generated by the full rMIP proteins in liposomes were generally higher than those generated by the truncated rMIP proteins (P < 0.05): it is likely that antisera to the full rMIP proteins contain antibodies directed against both the globular and nonglobular domains, thereby providing more antibody at the surface of the bacteria. Furthermore, to demonstrate specificity, antisera raised to the C-term His full, C-term His truncated, and N-term His full rMIP (LP) proteins in liposomes did not react with the MC58Δmip mutant strain (Fig. 4A and B). Representative IF images show the characteristic “doughnut-like” OM staining by antisera that were raised to full and truncated proteins (Fig. 4C).
FIG 4.
Antibodies to rMIP proteins recognize MIP on the surface of meningococci. (A) FACS analysis demonstrates anti-rMIP antibody binding to the surface of meningococci. Pooled murine antisera to rMIP constructs in either saline solution or liposomes were reacted (1/10) with fixed whole MC58 cells and the Δmip mutant. The column data are presented as a percentage of the reactivity of the N-term His full rMIP (LP) protein, which has been shown previously to induce bactericidal antibodies and to bind to meningococcal cells (22). Data are representative of the results of experiments done with the pooled samples (n ≥ 2 experiments). (B) Immunofluorescence (IF) staining confirms binding of anti-rMIP antibodies to the surface of meningococci. Pooled murine antisera to rMIP constructs were reacted (1/50) with fixed whole MC58 cells and the Δmip mutant. IF staining was quantified as described in Materials and Methods. The columns represent the mean ROI values, and the error bars represent the standard errors of the means (of 3 independent areas of staining measured in triplicate). The data are from a representative experiment (n ≥ 3 experiments for pooled samples). (C) Representative confocal images of immunofluorescence staining of MC58 meningococcal surfaces with antisera raised to N-term His full rMIP (LP), C-term His full rMIP, and C-term truncated rMIP, delivered in liposomes. Similar staining patterns were observed with antisera raised to N-term His full and truncated rMIP proteins in liposomes. The positive control was antisera raised to whole MC58 OM, and a representative negative-control (sham immunization) image is shown. Images were captured at ×60 magnification.
Truncated recombinant MIP proteins elicit cross-type strain bactericidal antibodies.
Initially, murine antisera were tested for their ability to promote complement-mediated killing of homologous meningococcal strain MC58 (Table 2). No bactericidal activity was shown by antisera raised to recombinant full or truncated MIP constructs that lack the signal LP when delivered in saline solution, whereas the N-term His full rMP (LP) induced a median bactericidal titer of 128, similar to previously published activity (22). The C-term His full rMIP and C-term His truncated rMIP were able to induce bactericidal antibodies when delivered in liposomes, with median titers of 128 to 256, which were similar to those observed with the N-term His full rMP (LP) (Table 2) (22). However, no bactericidal activity was observed for antisera raised to the N-term His full rMIP or N-term His truncated rMIP proteins that lacked the LP, delivered in liposomes. Similar data were obtained when the antisera were tested against another meningococcal strain MC168, expressing an identical type I MIP protein (Table 2).
TABLE 2.
Serum bactericidal activity of murine antisera raised against rMIP proteinsa
| Delivery vehicle | Protein for immunization | Reciprocal SBA titer (50% endpoint) against: |
|||
|---|---|---|---|---|---|
| MC58 (I) | MC168 (I) | MC90 (II) | MC54 (III) | ||
| Saline solution | N-term His Full rMIP | <4 | <4 | 64 | <4 |
| N-term His Truncated rMIP | <4 | <4 | 64 (16, 64) | 4 | |
| C-term His Full rMIP | <4 | <4 | 64 (32, 64) | <4 | |
| C-term His Truncated rMIP | <4 | <4 | 64 (32, 64) | <4 | |
| N-term His Full rMIP (LP) | 128 (64, 128) | 256 (64, 512) | 256 (128, 256) | 128 (64, 256) | |
| Liposomes | N-Term His Full rMIP | <4 | <4 | 128 | 512 |
| N-term His Truncated rMIP | <4 | <4 | 128 (64, 256) | 512 | |
| C-term His Full rMIP | 128 (64, 256) | 512 | 256 | 256 (256, 512) | |
| C-term His Truncated rMIP | 128 (128, 256) | 256 (128, 256) | 512 (128, 512) | 512 (128, 512) | |
| N-term His Full rMIP (LP) | 256 (256, 512) | 256 (128, 512) | 256 | 256 (128, 256) | |
| Controls | Saline solution only | <4 | <4 | <4 | <4 |
| Liposomes only | <4 | <4 | 64 (32, 64) | <4 | |
| Normal mouse serum | 8 | 8 | 64 (32, 64) | 8 | |
Antisera were tested against the homologous strains expressing type I MIP protein (MC58 and MC168) and against heterologous strains expressing type II (MC90) and type III (MC54) MIP proteins. The titers are expressed as the reciprocal of the highest dilution at which 50% killing was observed, using baby rabbit complement (5%, vol/vol) as an exogenous source. The titer for mice immunized with native LOS-replete MC58 OM was 20,000 (internal positive control). Data are the median values, with the range of values in parentheses, for SBA from between a minimum of 3 and a maximum of 11 independent measurements of bactericidal activity of all pooled serum samples. Single values denote that the SBA titers from the independent experiments were identical. Sera from sham-immunized mice showed no significant bactericidal activity (<4 to 64, depending on the strain tested).
Murine antisera were tested for their ability to promote complement-mediated killing of meningococcal strains expressing heterologous type II or III MIP protein (Table 2). Against the type II MIP MC90 strain, some bactericidal activity was observed with antisera raised against the full and truncated rMIP proteins constructs in saline solution (median titer of 64), but the data were not significant (P > 0.05) compared to control results. In contrast, bactericidal activity against MC90 was observed for some of the antisera raised to rMIP proteins delivered in liposomes. The C-term full and truncated rMIP protein antisera showed significant median SBA titers of 256 to 512, which were similar to that observed for N-term His full rMIP (LP), whereas the median titer for antisera to the N-term His full and truncated rMIP proteins of 128 was only 1 dilution more than the value determined for the control liposomes (64) and probably not significant (P > 0.05).
No significant bactericidal activity against type III strain MC54 was observed with antisera raised to the full or truncated rMIP proteins delivered in saline solution, whereas the N-term His full rMIP (LP) protein induced high levels of SBA (median titer, 128), as observed previously (22). Interestingly, all of the proteins delivered in liposomes induced high levels of cross-reactive bactericidal antibodies (median titer of 256 or 512) against the heterologous type III MIP strain which were similar to the titer obtained with the N-term His full rMIP (LP) protein (256) (Table 2).
Antisera raised to rMIP proteins in liposomes, which showed bactericidal activity against the heterologous serogroup B strains, were tested also against meningococci expressing other serogroup capsules and against gonococci (Table 3). All of the different meningococcal serogroup isolates expressed type I MIP, whereas the P9-17 gonococcal MIP protein showed ∼97% similarity to meningococcal type I protein between amino acids 1 and 143. Antisera raised to the C-term truncated rMIP protein in liposomes showed significant SBA against strains of serogroups A, C, Y, and W (P < 0.05), whereas antisera to the full rMIP proteins were nonbactericidal (titers of <16). In contrast, antisera to all three recombinant proteins killed gonococcal strain P9-17, with a significant 4-fold increase in the SBA titer (2,048) above the background SBA titer (512).
TABLE 3.
Serum bactericidal activity of murine antisera raised against rMIP proteins in liposomes for other serogroup meningococci and for gonococcia
| Immunization protein | Reciprocal SBA titer (50% endpoint) against: |
||||
|---|---|---|---|---|---|
| A | C | W | Y | P9-17 Ngo | |
| C-term His Truncated rMIP | 128 (64, 256) | 256 | 256 (32, 1024) | 32 (16, 128) | 2,048 |
| C-term His Full rMIP | <16 | <16 | <16 | <16 | 2,048 |
| N-term His Full rMIP (LP) | <16 | <16 | <16 | <16 | 2,048 |
| Control NMS | <4 | <4 | <4 | <4 | 512b |
Antisera raised to rMIP proteins in liposomes which showed bactericidal activity against heterologous serogroup B strains were tested against other serogroup meningococci and against P9-17 gonococci (Ngo) (see Materials and Methods for details; the serogroup C strain is MC173, which was described previously [22]). All the meningococcal strains expressed type I MIP, whereas the Ngo MIP showed ∼95% similarity to complete meningococcal type I MIP. The titers are expressed as the reciprocal of the highest dilution at which 50% killing was observed, using baby rabbit complement (5% [vol/vol]) as an exogenous source. Data are the median values, with the range of values in parentheses where shown, for SBA from between 2 and 6 independent measurements of bactericidal activity of pooled serum samples. Single values denote that the SBA titers from the independent experiments were identical. NMS, normal mouse serum.
Strain P9-17 is serum sensitive.
Reactivity of antisera to rMIP proteins with human FKBP2 protein.
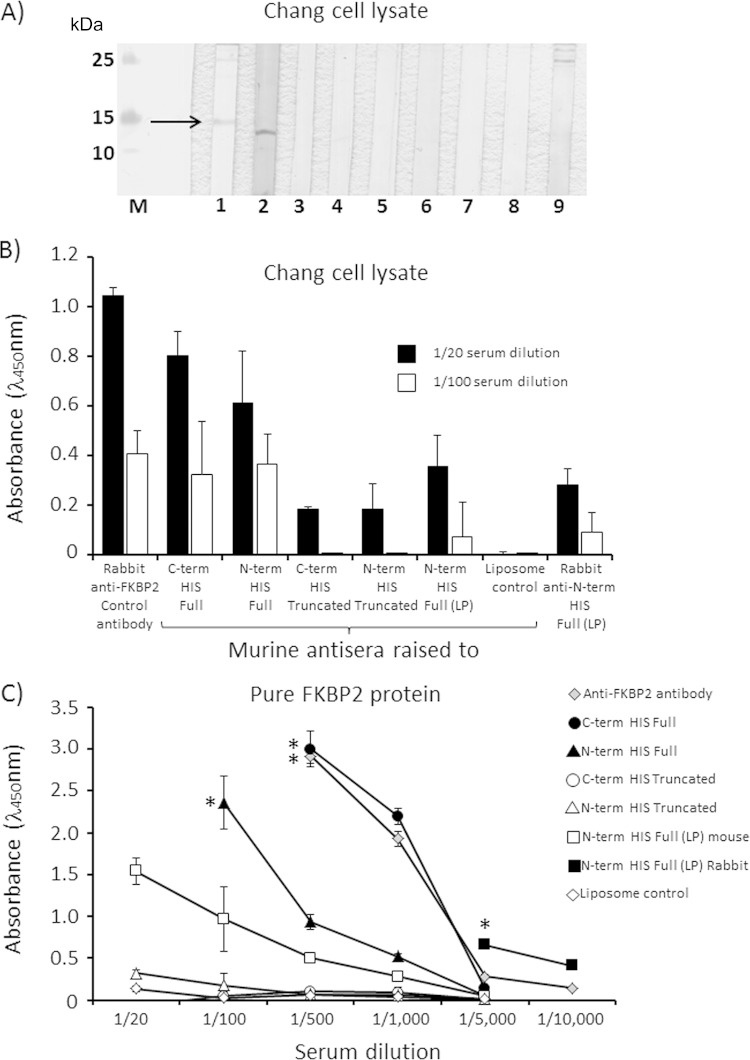
Initially, bactericidal antisera raised to rMIP proteins delivered in liposomes were tested for their ability to recognize human FKBP2 protein by Western blot analysis. No reactivity with denatured 15-kDa human FKBP2 protein, present in lysate of human Chang conjunctival cells, was observed for murine antisera raised to any of the full or truncated recombinant proteins, with or without the signal LP, or for rabbit antisera raised to the full rMIP protein (Fig. 5A).
FIG 5.
Reactivity of anti-rMIP sera with human FKBP protein. (A) For Western blot analysis of denatured protein reactivity, strip blots were prepared from a sonicated lysate of human Chang conjunctiva epithelial cells and reacted with polyclonal rabbit anti-human FKBP2 antibody (1/20 dilution), reacting with a protein band with a molecular mass of ∼15 kDa, identified by an arrow (lane 1). Reactions were performed with murine antisera (1/20 dilution) raised to the following proteins in liposomes: N-terminal full rMIP (lane 2), N-terminal truncated rMIP (lane 3), C-terminal full rMIP (lane 4), C-terminal truncated rMIP (lane 5), N-terminal full rMIP (LP) (lane 6), murine serum to a liposome control (no protein) (lane 7), normal mouse serum (lane 8), and rabbit anti-N-terminal full rMIP (LP) serum (lane 9) (22). M denotes the protein Mr markers. The single band observed in lane 2 does not correspond to human FKBP2 protein and is an unknown protein present in the Chang cell lysate that is recognized by the N-terminal full rMIP-liposome serum pool. (B and C) Reactivity of antisera with native FKBP2 protein in Chang cell lysate (B) and with pure active human FKBP2 protein (C), examined by ELISA. Serial dilutions of the same murine and rabbit antisera tested in Western blot analysis were reacted against a Chang cell lysate and against pure active human FKBP2 protein. The polyclonal rabbit anti-human FKBP2 antibody was used as a positive control. The columns in panel B and symbols in panel C represent the mean absorbance values from triplicate wells, and the error bars represent the standard deviations (n = 3 independent experiments). For murine sera, data are shown after subtraction of background absorbance values for the no-antibody control and for normal mouse serum; for rabbit anti-rMIP serum, background absorbance values for the no-antibody control and preimmunization bleed are subtracted. The asterisks in panel C denote that absorbance values for the preceding serum dilutions exceeded the upper detectable range of the microplate reader (>3.0).
However, in order to examine the reactivity of anti-rMIP antisera against native, nondenatured FKBP2 protein, an ELISA was developed in which either a Chang cell lysate or purified active human FKBP2 recombinant full protein was reacted with the same murine and rabbit antisera as tested in Western blot analysis. Specificity of the ELISA was demonstrated by reactivity of the positive-control commercial rabbit polyclonal antibody to human protein present in the Chang cell lysate and purified FKBP2 protein (Fig. 5B and C). In addition, the pure FKBP2 protein ELISA showed greater sensitivity than the Chang cell lysate ELISA, which was likely due to differences in the relative amounts of FKBP2 used to coat the wells, and was reflected by the higher serum dilutions required to titer out the anti-FKBP2 responses using pure protein as the capture antigen. Significant reactivity with FKBP2 protein in the Chang cell lysate and with the pure FKBP2 protein was shown by murine antisera raised to N-term His full and C-term His full rMIP proteins and by both murine and rabbit antisera raised to N-term His full rMIP (LP). In contrast, the reactivity of murine antisera to the C-term His and N-term His truncated rMIP proteins was significantly lower than that to the full rMIP proteins, showing reductions in ELISA absorbance values of ∼70% (1/20 dilution, P < 0.05) and >95% (1/100 dilution, P < 0.05) with the protein present in Chang cell lysate (Fig. 5B) and >95% (at all dilutions tested, P < 0.05) with pure FKBP2 protein (Fig. 5C).
DISCUSSION
The MIP protein of Neisseria meningitidis can induce cross-strain bactericidal antibodies (22), thus fulfilling a major criterion for a potential antigen for inclusion in defined serogroup B meningococcal vaccines. However, we determined that meningococcal MIP shows some amino acid sequence similarity to human FKBP proteins, which could potentially preclude its use. In order to circumvent the possibility of inducing antibody responses that recognize human proteins, using a structural prediction model based on the L. pneumophila MIP protein, we constructed recombinant MIP proteins that were truncated after the extended α-helix, thus removing the putative globular domains that contain PPIase activity and sequence similarity. The abilities of these truncated proteins to induce bactericidal antibodies were compared alongside those of the recombinant full proteins that contained the putative globular domains.
Immunization in saline solution with full or truncated rMIP constructs that lack the LP did not induce bactericidal antibodies. This lack of SBA correlated with low levels of antibody production as judged by ELISA against both the immunizing protein and the full protein containing the LP and the absence of both Western blot reactivity and of antibody binding to MIP on meningococci. In contrast, and confirming the findings of our previous study (22), immunization in saline solution with the N-term full rMIP (LP) fusion protein induced high levels of bactericidal antibodies that correlated with reactivity with MIP protein in ELISA, Western blot analysis, and surface labeling of meningococci.
Immunization in liposomes did enable some of the rMIP constructs to induce bactericidal antibodies, and the position of the His tag appeared to influence SBA. With His at the N terminus, the full and truncated rMIP proteins lacking the LP did not induce SBA against the homologous type I strain, which could possibly be due to induced and deleterious steric conformational changes within potential protective epitopes near the N terminus. No significant SBA was observed against the heterologous type II strain, although some SBA was observed against the type III strain, which may reflect increased sensitivity of this strain to serum antibodies. In comparison, the N-term full rMIP (LP) protein was capable of inducing homologous bactericidal antisera and it is possible that retaining the LP and fusion peptide sequence from the expression vector provides sufficient distance between the His tag and the mature protein to facilitate folding to a more native conformation. It is interesting that Tsolakos et al. were unable to generate bactericidal antibodies to a recombinant MIP protein that was produced without the LP sequence and delivered with monophosphoryl lipid A (MPLA) in saline solution (39). Despite the ability of MIP-specific antisera to bind to the surface of meningococci (22) and to facilitate the deposition of complement factors and promote opsonophagocytosis (39), the inability of Tsolakos et al. to induce SBA in their study may have been the consequence of a lack of self-adjuvanticity and the deleterious effects of MPLA on the conformation of meningococcal proteins (38, 40).
A contributory explanation for how inclusion of the LP sequence impacts the immunogenicity of pathogen-associated antigens could be related to the influence of the protein isoelectric point (IP), charge, and hydrophobicity on protein-protein interactions and antigen presentation and processing. Several studies have demonstrated a role for LP sequences in enhancing immunogenicity: for example, comparison of the antigenicity of a recombinant mature low-Mr antigen secreted by Mycobacterium tuberculosis with the equivalent full protein containing the LP showed that the full protein elicited stronger in vitro proliferative responses of peripheral blood mononuclear cells than the mature protein (41). Those authors suggested that the hydrophobic LP may have contributed to the antigenicity of the full molecule. This observation was reproduced subsequently for a panel of LP peptides derived from M. tuberculosis proteins, which similarly showed higher proliferative responses than the same peptide antigens lacking the LP (42).The advantage of including the LP has also been shown in a study of coccidioidomycosis infection, in which vaccination of mice with an 18-amino-acid LP derived from a specific antigen, Ag2/panel reactive antibody (PRA), induced protective immunity to the pathogen that was superior to that provided by the mature protein lacking the LP (43). In our current study, the LP sequences among different meningococci showed a high degree of similarity (see Fig. S1 in the supplemental material), and the IP value for LP amino acid sequence 1 to 21 is 7.98 (http://web.expasy.org/compute_pi/). The IP values for the full and truncated N-term and C-term His proteins are similar (i.e., between 6.17 and 6.21), and these proteins start with a glycine (position 22) and two positively charged lysine residues. However, inclusion of the fusion-LP sequence (IP value of 5.99) reduces the IP value to 5.73 for the full N-term His (LP) protein and it is possible that these changes in IP and hydrophobicity influence the immunogenicity of this protein in the absence of adjuvants, although the mechanisms involved are not known and are outside the scope of the current article. While it would be interesting to examine whether an N-term and/or C-term His truncated construct containing the LP, alone or as part of the fusion protein, has self-adjuvant properties and is capable of inducing SBA in saline solution alone, this issue is academic and unlikely to be important for human immunization, where liposomal incorporation not only would provide the adjuvant effect in lieu of protein self-adjuvanticity but also would allow slow antigen release from a depot and the potential for mimicking in vivo protein conformation (44, 45).
The possibility cannot be excluded that the presence of the LP might also allow some lipidation of rMIP during expression in E. coli, thus potentially contributing to the inherent self-adjuvanticity that enabled generation of bactericidal antibodies by this protein delivered in saline solution alone (22). In our current study, the N-term His full rMIP (LP) protein was expressed in E. coli strain BL21(DE3) and the other proteins (full and truncated) in a derivative of the BL21 strain lacking recA, E. coli BLR(DE3). However, it has been shown that recombinant protein expression using E. coli BL21(DE3) results in little or no lipidation and that to obtain lipidated protein, other E. coli BL21(DE3) derivative strains, e.g., C41 or C43, should be used for overexpression of membrane proteins (46, 47).
The presence of the His tag at the C terminus was preferential for generating SBA, with the full and truncated rMIP proteins in liposomes inducing similar and high levels of SBA against the homologous type I MIP strain and also showing cross-protection against the heterologous type II and III strains. Significantly, these SBA titers were identical to those induced by the N-term His full rMIP (LP). However, capsule serogroup did appear to influence the bactericidal activity of anti-rMIP sera, with only antisera raised to the C-term truncated rMIP protein in liposomes showing bactericidal activity against meningococci expressing different capsule polysaccharides. One possible explanation is that sera raised to the C-term truncated rMIP protein contain antibodies that bind more avidly to surface-exposed epitopes on the nonglobular domain, whereas sera raised to the full rMIP proteins could contain a lower proportion of avidly binding antibodies relative to the proportion of antibodies directed against both the globular and nonglobular domains of the whole protein. Capsule structure could play a role in hindering the binding of bactericidal antibodies, and it is interesting that antisera raised to these full and truncated rMIP proteins showed similar levels of bactericidal activity against gonococci that do not express capsule. However, since only single strains representative of these other serogroups could be tested due to serum limitations, definitive confirmation of these observations would require testing against a much larger panel of strains, alongside comparison with sera raised to licensed capsule polysaccharide-conjugate vaccines.
In our previous study, we had identified the type I, II, and III MIP proteins in a small collection of strains (22). However, the expanding http://pubmlst.org/neisseria/ database of sequenced Neisseria strains has allowed us to examine the distribution of MIP alleles and proteins among 3,716 pathogenic Neisseria isolates (database accessed 27 October 2014) (23). We have generated a Clustal alignment of the nonredundant amino acid sequences for MIP protein in both meningococci and gonococci as well as the corresponding dendrogram and determined the distribution and number of isolates for each allele and protein (see Fig. S1 and S2 and Table S1 in the supplemental material). There are 37 and 6 nonredundant alleles expressing different MIP proteins for meningococci and gonococci, respectively. For serogroup B meningococci, the previously identified type I (allele 2), II (allele 1), and III (allele 6) MIP proteins together provide 82% coverage of the sequenced isolates and 78% coverage if all other serogroups are included (see Table S1). However, it is likely that other meningococci expressing other MIP types would also be killed by cross-type antibodies, so these figures probably represent a minimum percentage of susceptible strains. Thus, immunization with a type I C-term His full, C-term His truncated, or N-term His full rMIP (LP) protein in a liposomal formulation could potentially provide at least 78% to 82% coverage of meningococcal strains. Notably, anti-rMIP sera could also kill the gonococcal P9 strain; examination of the complete amino acid sequences of the gonococcal MIP proteins encoded by the 6 different alleles and the type I, II, and III meningococcal MIP proteins shows ∼95% sequence identity, which increases to ∼97% identity within amino acid sequence 1 to 143 (see Fig. S1 in the supplemental material).
In the current study, the concept of a truncation strategy was validated by the observation that bactericidal antisera raised to the truncated rMIP proteins did not cross-react with native pure human FKBP2 protein or with a nondenatured human epithelial cell lysate, whereas significant reactivity was observed with antisera raised to the full rMIP proteins. Thus, this successful strategy used with meningococcal rMIP to obviate any concerns regarding cross-reactivity with human proteins could be applied also to other bacterial MIP antigens proposed as vaccine candidates, e.g., an immunodominant chlamydial MIP protein that has been shown to induce protective immune responses during murine intravaginal infection (48). Our current study results suggest also that a functional bactericidal epitope(s) possibly resides within surface-exposed N-terminal α-helix amino acid sequence 21 to 143. Surface exposure has been reported for the whole meningococcal MIP (22, 39), the homologous gonococcal MIP (21), the Legionella MIP (49), and a MIP lipoprotein from Chlamydia trachomatis (50). Interestingly, it has been suggested that the long α1-helix located at the N terminus of the MIP protein from Trypanosoma cruzi is exposed to the exterior and could play a role in host cell interactions (51). However, the crystal structure of the meningococcal MIP and how the protein anchors to the bacterial membrane remain to be determined.
In summary, immunization with a C-term His truncated rMIP protein is capable of inducing cross-type reactive bactericidal antibodies while circumventing potential cross-reactivity with human FKBP proteins and deserves serious consideration for inclusion in a defined serogroup B meningococcal antigen vaccine.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by GlaxoSmithKline Biologicals (GSK) S.A., who covered all costs.
M.C. and J.E.H. are named inventors on meningococcal vaccine patents owned by the University of Southampton and GSK. V.W., N.D., and M.G. are, or were at the time of the study, employees of the GSK group of companies. V.W. is a designated inventor on patents owned by GSK and owns shares and options to shares in GSK.
We acknowledge the generosity of Dominic Caugant, the National Institute of Public Health, Oslo, Norway, and Ray Borrow, Public Health England, for providing the serogroup A and W and Y meningococci, respectively. We are grateful to Richard Jewell and Carolann McGuire, University of Southampton Faculty of Medicine, for assistance in FACS analyses; to David A Johnston, Biomedical Imaging Unit for confocal microscopy imaging; and to Neville Wright, Biophysics Facility Centre, School of Biological Sciences, University of Southampton, for technical discussions.
This publication made use of the Neisseria Multi Locus Sequence Typing Website (http://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford. The development of this site has been funded by the Wellcome Trust and the European Union.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01815-14.
REFERENCES
- 1.Rouphael NG, Stephens DS. 2012. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol 799:1–20. doi: 10.1007/978-1-61779-346-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow R, Miller E. 2006. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev Vaccines 5:851–857. doi: 10.1586/14760584.5.6.851. [DOI] [PubMed] [Google Scholar]
- 3.Djingarey MH, Barry R, Bonkoungou M, Tiendrebeogo S, Sebgo R, Kandolo D, Lingani C, Preziosi MP, Zuber PLF, Perea W, Hugonnet S, Tolve NDD, Tevi-Benissan C, Clark TA, Mayer LW, Novak R, Messonier NE, Berlier M, Toboe D, Nshimirimana D, Mihigo R, Aguado T, Diomande F, Kristiansen PA, Caugant DA, Laforce FM. 2012. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine 30:B40–B45. doi: 10.1016/j.vaccine.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 4.Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, Anemona A, Halperin SA, Dobson S, Pollard AJ. 2008. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 299:173–184. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 5.Tan LK, Carlone GM, Borrow R. 2010. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med 362:1511–1520. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 6.Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355–357. [DOI] [PubMed] [Google Scholar]
- 7.Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Holstfredriksen J, Halstensen A, Holten E, Lindbak AK, Nokleby H, Rosenqvist E, Solberg LK, Closs O, Eng J, Froholm LO, Lystad A, Bakketeig LS, Hareide B. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093–1096. doi: 10.1016/0140-6736(91)91961-S. [DOI] [PubMed] [Google Scholar]
- 8.Sierra GVG, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Annals 14:195–210. [PubMed] [Google Scholar]
- 9.de Moraes JC, Perkins BA, Camargo MCC, Hidalgo NTR, Barbosa HA, Sacchi CT, Gral IML, Gattas VL, Vasconcelos HD, Plikaytis BD, Wenger JD, Broome CV. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 10.Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191–2196. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 11.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27:B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 12.Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, Galeotti CL, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo PJ, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood DW, Jeffries AC, Saunders NJ, Granoff DM, Venter JC, Moxon ER, Grandi G, Rappuoli R. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani MM, du-Bobie J, Comanducci M, Arico B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidit E, Welsch JA, Granoff D, Rappuoli R, Pizza M. 2006. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30(Suppl 2):B87–B97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, Carannante A, Deghmane AE, Fazio C, Frosch M, Frosi G, Gilchrist S, Giuliani MM, Hong E, Ledroit M, Lovaglio PG, Lucidarme J, Musilek M, Muzzi A, Oksnes J, Rigat F, Orlandi L, Stella M, Thompson D, Pizza M, Rappuoli R, Serruto D, Comanducci M, Boccadifuoco G, Donnelly JJ, Medini D, Borrow R. 2013. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 13:416–425. doi: 10.1016/S1473-3099(13)70006-9. [DOI] [PubMed] [Google Scholar]
- 16.Bettinger JA, Scheifele DW, Halperin SA, Vaudry W, Findlow J, Borrow R, Medini D, Tsang R. 2013. Diversity of Canadian meningococcal serogroup B isolates and estimated coverage by an investigational meningococcal serogroup B vaccine (4CMenB). Vaccine 32:124–130. doi: 10.1016/j.vaccine.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 17.McNeil LK, Zagursky RJ, Lin SL, Murphy E, Zlotnick GW, Hoiseth SK, Jansen KU, Anderson AS. 2013. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev 77:234–252. doi: 10.1128/MMBR.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granoff DM. 2010. Review of meningococcal group B vaccines. Clin Infect Dis 50:S54–S65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermont C, van den Dobbelsteen G. 2002. Neisseria meningitidis serogroup B: laboratory correlates of protection. FEMS Immunol Med Microbiol 34:89–96. doi: 10.1111/j.1574-695X.2002.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams JN, Skipp PJ, Humphries HE, Christodoulides M, O'Connor CD, Heckels JE. 2007. Proteomic analysis of outer membranes and vesicles from wild-type serogroup B Neisseria meningitidis and a lipopolysaccharide-deficient mutant. Infect Immun 75:1364–1372. doi: 10.1128/IAI.01424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuzzi R, Serino L, Scarselli M, Savino S, Fontana M, Monaci E, Taddei A, Fischer G, Rappuoli R, Pizza M. 2005. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol 58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- 22.Hung MC, Salim O, Williams JN, Heckels JE, Christodoulides M. 2011. The Neisseria meningitidis macrophage infectivity potentiator protein induces cross-strain serum bactericidal activity and is a potential serogroup B vaccine candidate. Infect Immun 79:3784–3791. doi: 10.1128/IAI.05019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolley KA, Maiden MC. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cianciotto NP, Eisenstein BI, Mody CH, Toews GB, Engleberg NC. 1989. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun 57:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dornan J, Taylor P, Walkinshaw MD. 2003. Structures of immunophilins and their ligand complexes. Curr Top Med Chem 3:1392–1409. doi: 10.2174/1568026033451899. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber SL. 1991. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science 251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 27.Siekierka JJ, Wiederrecht G, Greulich H, Boulton D, Hung SHY, Cryan J, Hodges PJ, Sigal NH. 1990. The cytosolic-binding protein for the immunosuppressant Fk-506 is both a ubiquitous and highly conserved peptidyl-prolyl cis-trans isomerase. J Biol Chem 265:21011–21015. [PubMed] [Google Scholar]
- 28.Fischer G, Bang H, Ludwig B, Mann K, Hacker J. 1992. MIP protein of Legionella pneumophila exhibits peptidyl-prolyl-cis trans isomerase (PPlase) activity. Mol Microbiol 6:1375–1383. doi: 10.1111/j.1365-2958.1992.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 29.Riboldi-Tunnicliffe A, Konig B, Jessen S, Weiss MS, Rahfeld J, Hacker J, Fischer G, Hilgenfeld R. 2001. Crystal structure of Mip, a prolylisomerase from Legionella pneumophila. Nat Struct Biol 8:779–783. doi: 10.1038/nsb0901-779. [DOI] [PubMed] [Google Scholar]
- 30.Fretz H, Albers MW, Galat A, Standaert RF, Lane WS, Burakoff SJ, Bierer BE, Schreiber SL. 1991. Rapamycin and Fk506 binding-proteins (immunophilins). J Am Chem Soc 113:1409–1411. http://www.broadinstitute.org/chembio/lab_schreiber/pubs/pdffiles/090.pdf. [Google Scholar]
- 31.McGuinness BT, Clarke IN, Lambden PR, Barlow AK, Poolman JT, Jones DM, Heckels JE. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514–517. doi: 10.1016/0140-6736(91)91297-8. [DOI] [PubMed] [Google Scholar]
- 32.Virji M, Heckels JE. 1986. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leukocytes. J Gen Microbiol 132:503–512. [DOI] [PubMed] [Google Scholar]
- 33.Zak K, Diaz JL, Jackson D, Heckels JE. 1984. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis 149:166–173. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- 34.Echenique-Rivera H, Muzzi A, Del Tordello E, Seib KL, Francois P, Rappuoli R, Pizza M, Serruto D. 2011. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS Pathog 7:e1002027. doi: 10.1371/journal.ppat.1002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JN, Skipp PJ, O'Connor CD, Christodoulides M, Heckels JE. 2009. Immunoproteomic analysis of the development of natural immunity in subjects colonized by Neisseria meningitidis reveals potential vaccine candidates. Infect Immun 77:5080–5089. doi: 10.1128/IAI.00701-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christodoulides M, McGuinness BT, Heckels JE. 1993. Immunization with synthetic peptides containing epitopes of the class 1 outer-membrane protein of Neisseria meningitidis: production of bactericidal antibodies on immunization with a cyclic peptide. J Gen Microbiol 139:1729–1738. doi: 10.1099/00221287-139-8-1729. [DOI] [PubMed] [Google Scholar]
- 37.Jolley K, Appleby L, Wright JC, Christodoulides M, Heckels JE. 2001. Immunization with recombinant Opc outer membrane protein from Neisseria meningitidis: influence of sequence variation and levels of expression on the bactericidal immune response against meningococci. Infect Immun 69:3809–3916. doi: 10.1128/IAI.69.6.3809-3816.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung MC, Heckels JE, Christodoulides M. 2013. The adhesin complex protein of Neisseria meningitidis is a new adhesin with vaccine potential. mBio 4:e00041-13. doi: 10.1128/mBio.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsolakos N, Brookes C, Taylor S, Gorringe A, Tang CM, Feavers IM, Wheeler JX. 2014. Identification of vaccine antigens using integrated proteomic analyses of surface immunogens from serogroup B Neisseria meningitidis. J Proteomics 101:63–76. doi: 10.1016/j.jprot.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Christodoulides M, Brooks JL, Rattue E, Heckels JE. 1998. Immunization with recombinant class 1 outer-membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144:3027–3037. doi: 10.1099/00221287-144-11-3027. [DOI] [PubMed] [Google Scholar]
- 41.Webb JR, Vedvick TS, Alderson MR, Guderian JA, Jen SS, Ovendale PJ, Johnson SM, Reed SG, Skeiky YAW. 1998. Molecular cloning, expression, and immunogenicity of MTB12, a novel low-molecular-weight antigen secreted by Mycobacterium tuberculosis. Infect Immun 66:4208–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovjazin R, Volovitz I, Daon Y, Vider-Shalit T, Azran R, Tsaban L, Carmon L, Louzoun Y. 2011. Signal peptides and trans-membrane regions are broadly immunogenic and have high CD8+ T cell epitope densities: implications for vaccine development. Mol Immunol 48:1009–1018. doi: 10.1016/j.molimm.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang CY, Magee DM, Ivey FD, Cox RA. 2002. Role of signal sequence in vaccine-induced protection against experimental coccidioidomycosis. Infect Immun 70:3539–3545. doi: 10.1128/IAI.70.7.3539-3545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haensler J. 2010. Liposomal adjuvants: preparation and formulation with antigens. Methods Mol Biol 626:73–90. doi: 10.1007/978-1-60761-585-9_6. [DOI] [PubMed] [Google Scholar]
- 45.Wetzler LM, Blake MS, Gotschlich EC. 1988. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J Exp Med 168:1883–1897. doi: 10.1084/jem.168.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HW, Liu SJ, Liu HH, Kwok Y, Lin CL, Lin LH, Chen MY, Tsai JP, Chang LS, Chiu FF, Lai LW, Lian WC, Yang CY, Hsieh SY, Chong P, Leng CH. 2009. A novel technology for the production of a heterologous lipoprotein immunogen in high yield has implications for the field of vaccine design. Vaccine 27:1400–1409. doi: 10.1016/j.vaccine.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 47.Tseng CL, Leng CH. 2012. Influence of medium components on the expression of recombinant lipoproteins in Escherichia coli. Appl Microbiol Biotechnol 93:1539–1552. doi: 10.1007/s00253-011-3516-8. [DOI] [PubMed] [Google Scholar]
- 48.Lu C, Peng B, Li Z, Lei L, Li Z, Chen L, He Q, Zhong G, Wu Y. 2013. Induction of protective immunity against Chlamydia muridarum intravaginal infection with the chlamydial immunodominant antigen macrophage infectivity potentiator. Microbes Infect 15:329–338. doi: 10.1016/j.micinf.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helbig JH, Luck PC, Steinert M, Jacobs E, Witt M. 2001. Immunolocalization of the MIP protein of intracellularly and extracellularly grown Legionella pneumophila. Lett Appl Microbiol 32:83–88. doi: 10.1046/j.1472-765x.2001.00861.x. [DOI] [PubMed] [Google Scholar]
- 50.Neff L, Daher S, Muzzin P, Spenato U, Guelacar F, Gabay C, Bas S. 2007. Molecular characterization and subcellular localization of macrophage infectivity potentiator, a Chlamydia trachomatis lipoprotein. J Bacteriol 189:4739–4748. doi: 10.1128/JB.01889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira PJB, Vega MC, González-Rey E, Fernández-Carazo R, Macedo-Ribeiro S, Gomis-Rüth FX, González A, Coll M. 2002. Trypanosoma cruzi macrophage infectivity potentiator has a rotamase core and a highly exposed alpha-helix. EMBO Rep 3:88–94. doi: 10.1093/embo-reports/kvf009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.