Abstract
IgG4 responses are considered indicative for long-term or repeated exposure to particular antigens. Therefore, studying IgG4-specific antibody responses against Staphylococcus aureus might generate new insights into the respective host-pathogen interactions and the microbial virulence factors involved. Using a bead-based flow cytometry assay, we determined total IgG (IgGt), IgG1, and IgG4 antibody responses to 40 different S. aureus virulence factors in sera from healthy persistent nasal carriers, healthy persistent noncarriers, and patients with various staphylococcal infections from three distinct countries. IgGt responses were detected against all tested antigens. These were mostly IgG1 responses. In contrast, IgG4 antibodies were detected to alpha-toxin, chemotaxis inhibitory protein of S. aureus (CHIPS), exfoliative toxins A and B (ETA and -B), HlgB, IsdA, LukD, -E, -F, and -S, staphylococcal complement inhibitor (SCIN), staphylococcal enterotoxin C (SEC), staphylococcal superantigen-like proteins 1, 3, 5, and 9 (SSL1, -3, -5, and -9), and toxic shock syndrome toxin 1 (TSST-1) only. Large interpatient variability was observed, and the type of infection or geographical location did not reveal conserved patterns of response. As persistent S. aureus carriers trended toward IgG4 responses to a larger number of antigens than persistent noncarriers, we also investigated sera from patients with epidermolysis bullosa (EB), a genetic blistering disease associated with high S. aureus carriage rates. EB patients responded immunologically to significantly more antigens than noncarriers and trended toward even more responses than carriers. Altogether, we conclude that the IgG4 responses against a restricted panel of staphylococcal antigens consisting primarily of immune modulators and particular toxins indicate important roles for these virulence factors in staphylococcal pathogen-host interactions, such as chronicity of colonization and/or (subclinical) infections.
INTRODUCTION
Staphylococcus aureus is responsible for more deaths annually in the United States than HIV/AIDS and tuberculosis combined (1, 2). S. aureus infections can range from mild skin and soft tissue infections (3) to more severe bacteremia (4) and osteomyelitis (5), and they can be resolving or chronic. S. aureus is able to persistently adhere to the anterior nares of 30% of all humans, while in the remaining population this opportunistic pathogen is never or only incidentally detectable (6–9). It has long been established that nasal colonization is associated with an increased chance of infection (10–12). In patients with epidermolysis bullosa (EB), a genetic blistering disease that leaves patients highly susceptible to S. aureus colonization, nasal carriage rates of 50 to 80% have been reported, and 75 to 100% of their skin wounds are culture positive for S. aureus (13–17). Interestingly, although EB patients interact frequently with S. aureus, bacteremia is seldom reported in these patients (18).
Numerous S. aureus virulence factors have been identified (19–24). However, the precise roles of most of these virulence factors during colonization and pathogenesis in humans have remained largely unclear, as determination of in vivo expression of bacterial virulence factors is technically challenging. Burian et al. showed by a quantitative PCR (qPCR) analysis on samples from 4 persistent nasal carriers that the adhesin genes clfB, fnbA, and isdA and the immune modulator gene chp are expressed in vivo (25). In an artificial inoculation study, clfB proved to be essential for colonization in humans (26).
Instead of direct in vivo detection of virulence factors, the human antibody response can be used as an indicator for the in vivo expression of S. aureus virulence factors (9, 27–29). Our group has published several reports on human immune responses to S. aureus. Persistent carriers have higher IgG titers directed to toxic shock syndrome toxin 1 (TSST-1) than persistent noncarriers (9). During bacteremia, patients developed significantly higher IgG responses than age-matched uninfected controls. These responses were directed to the immune modulators staphylococcal superantigen-like protein 1 (SSL1), SSL5, and staphylococcal complement inhibitor (SCIN) and the toxins γ-hemolysin B (HlgB) and leukocidin F (LukF) (28), indicating that these virulence factors are produced in vivo during infection. Furthermore, Algerian patients suffering from various S. aureus infections showed higher IgG responses directed to exfoliative toxins A (ETA), ETB, HlgB, LukD, E, and -S, staphylococcal enterotoxin A (SEA), SEE, SEH, and SEM than controls (30).
When IgG responses are studied, usually only the total IgG (IgGt) levels are measured. However, IgGt is composed of 4 different subclasses, each with distinct biological functions and induction patterns (31). IgG1 responses, which represent approximately 60% of IgGt, are directed primarily against proteins. They are induced within a week of infection, peak between 1 and 2 weeks, and resolve after 2 to 3 weeks (32). IgG1 functions as a proinflammatory signal inducing Fc receptor-mediated and complement-mediated phagocytosis. IgG4 antibodies, which represent approximately 5% of IgGt, are produced after prolonged exposure, typically taking months or years (32, 33). IgG4 antibodies are reported to not activate or weakly activate complement via the classical pathway, in contrast to IgG1 antibodies. Furthermore, IgG4 antibodies are correlated with tolerance after allergy (31, 33, 34), they have a low binding affinity to Fc receptors on phagocytes, and IgG4-mediated opsonophagocytosis is reportedly less efficient than IgG1-mediated opsonophagocytosis (35).
The aim of our present study was to determine whether analysis of IgGt, IgG1, and IgG4 directed against virulence factors of S. aureus could help elucidate the location and duration of exposure to these bacterial factors during infection. Using a previously developed bead-based flow cytometry assay (xMap; Luminex) (9, 28), we measured total IgGt, IgG1, and IgG4 antibody responses directed to 40 different S. aureus virulence factors in sera from patients suffering from 4 different S. aureus infections originating from 3 geographical locations. In addition, we studied the humoral responses in sera from healthy carriers, noncarriers, and EB patients with well-documented S. aureus colonization status, all from The Netherlands. We looked for infection-specific responses to the virulence factors of S. aureus and the IgG subclasses involved. Furthermore, we determined IgG subclass/total IgG ratios to determine the contributions of the different subclasses to the IgGt response to S. aureus. By studying these responses, we obtained new insights on the (chronicity of) human exposure to S. aureus and the virulence factors involved, which have implications for antistaphylococcal vaccine development.
MATERIALS AND METHODS
Sera from healthy volunteers and patients.
We included sera from 19 Dutch persistent carriers and 26 Dutch persistent noncarriers (9). An individual was defined as a persistent nasal carrier when 3 out of 3 nasal swabs taken 2 weeks apart were positive and as a persistent noncarrier when all swabs were negative for S. aureus (9). All swabs were processed as described by Nouwen et al. (36). In addition, we included sera isolated from 10 Dutch patients with bacteremia at diagnosis and 1, 2, and 3 weeks after diagnosis (37) and sera from 13 EB patients (18). Furthermore, we included serum samples from 10 patients without S. aureus-related infections admitted to the Mustapha Pacha Hospital, Algiers, Algeria, and patients with either S. aureus skin infections (n = 10), joint infections (n = 10), or respiratory infections (n = 10) 14 days (range, 7 to 34) after strain identification (30). Serum samples were collected from 60 healthy Sudanese volunteers at the University of Khartoum and from 25 Sudanese citizens with S. aureus skin infections. The Medical Ethics Committee in the Erasmus Medical Centre approved the study (MEC-2007-106) for work performed in Rotterdam, The Netherlands. The Medical Ethics Committee of the University Medical Center Groningen approved the collection of sera from EB patients (approval no. NL27471,042,09). Local ethical committees reviewed and approved both Algerian (30) and Sudanese (unpublished data) studies. All serum donors provided written informed consent.
Antigens.
The antigens used were isolated upon overexpression and comprised 10 cell wall-associated and 30 secreted S. aureus antigens: alpha-toxin (A-Tox); chemotaxis inhibitory protein of S. aureus (CHIPS); clumping factors A and B (ClfA and ClfB); extracellular fibrinogen-binding protein (Efb); exfoliative toxins A and B (ETA and -B); fibronectin-binding proteins A and B (FnbpA and -B); γ-hemolysin B (HlgB); iron-responsive surface determinants A and H (IsdA and -H); leukocidin (Luk) S-PV, LukF-PV, LukD-PV, and LukE-PV; S. aureus surface protein G (SasG); staphylococcal complement inhibitor (SCIN); serine-aspartate dipeptide repeat proteins D and E (SdrD and SdrE); staphylococcal enterotoxins A to E, G to J, M to O, Q, and R (SEA to SEE, SEG to SEJ, SEM to SEO, SEQ, and SER); staphylococcal superantigen-like proteins 1, 3, 5, 9, and 11 (SSL1, SSL3, SSL5, SSL9, and SSL11), and toxic shock syndrome toxin 1 (TSST-1) (30, 38–50) (see Table S1 in the supplemental material). Besides S. aureus antigens, IgGt (16-16-090707; Athens Research & Technology, Athens, GA, USA), IgG1 (16-16-090707-1M; Athens Research), and IgG4 (16-16-090707-4M; Athens Research) were used to assess whether cross-reactivity existed between subclass-specific detection antibodies. Beads without antigens were used as a negative control.
Measurement of antistaphylococcal antibodies.
Serum IgGt antibodies directed against the different S. aureus antigens were simultaneously quantified in a multiplex assay using a bead-based flow cytometry technique (xMap; Luminex Corporation), following previously described protocols (9, 28, 51). The median fluorescence intensity (MFI) was determined as the median fluorescence of 100 beads and is used as measure of immunoglobulins bound to the antigens coupled the beads. IgGt was detected using goat anti-human IgG–phycoerythrin (PE) from Jackson Immuno Research (Newmarket, Suffolk, United Kingdom). Subclass-specific responses were determined using monoclonal mouse anti-human IgG1 of the IgG1 subclass (05-3300; Zymed, Paisley, United Kingdom) or monoclonal mouse anti-human IgG4 of the IgG1-k subclass (05-3800; Invitrogen, Paisley, United Kingdom). All subclass-specific antibodies were detected using IgG goat anti-mouse–PE (Abcam, Cambridge, United Kingdom). To assess cross-reactivity between subclass-specific detection antibodies, IgG1 and IgG4 were coupled to microspheres and incubated with the anti-IgGt, anti-IgG1, or anti-IgG4 detection antibodies. As a positive control, pooled serum from 36 healthy volunteers was used.
Data analysis.
Coefficient of variation (CV) values were determined by dividing the standard deviation of the measurements by the mean of the measurements. Values with a CV exceeding 25% were excluded from further analysis. Control beads without protein coupled were included in each experiment to determine nonspecific binding. The nonspecific MFI values were subtracted from the antigen-specific results. Groups were compared using a Mann-Whitney U test in IBM SPSS Statistics 20. The Bonferroni correction was applied to P values to correct for multiple testing. The ratios were calculated by dividing the IgG (subclass) signal by the IgGt signal. The median of these ratios is shown in the figures in the supplemental material, to determine subclass-specific contributions to the IgGt signal and to facilitate intergroup comparisons. An increase of signal in the bacteremic patients was defined as a ratio of >1 for all time points, and this ratio was calculated by dividing the signal at the later time points by that at the first time point.
RESULTS
Validation of IgG subclass-specific Luminex assay.
To compare S. aureus antigen-specific IgG levels in serum samples, the bead-based multiplex Luminex assay was applied as described previously (28, 30, 51, 52), and the level of cross-reactivity between our detection antibodies for IgG1 and IgG4 and coupled antibodies was assessed by incubating a bead mixture containing IgGt, IgG1, and IgG4 with either the anti-IgG1 antibody or the anti-IgG4 antibody. IgG1 antibodies showed 2.7% [(291/10,759) × 100] cross-reactivity to IgG4-coupled antibodies. IgG4 antibodies gave 0.4% [(65/16,096) × 100] cross-reactivity to the coupled IgG1 antibodies. Thus, less than 5% cross-reactivity of the detection antibodies was observed. The CV values ranged between 5% and 37%, and on average, 20% of all measurements had to be excluded for exceeding the CV value cutoff of 25%, comparable to results from previous studies (9, 28, 51–55). High CVs were measured mainly for low MFI signals in the IgG4 Luminex assays (MFI of <1000). In all determinations done here, human pooled serum gave median MFI values of 2,883 (range, 49 to 15,655) for IgGt, 794 (range, 6 to 16,700) for IgG1, and 73 (range, 0 to 9,379) for IgG4. Taking the results together, we conclude that our Luminex assay is suitable to determine both total and subclass-specific responses against S. aureus antigens.
IgGt, IgG1, and IgG4 antistaphylococcal antibodies in an Algerian discovery cohort.
To expand our previous IgGt data (30), the IgGt, IgG1, and IgG4 responses directed against 40 S. aureus antigens were determined in a discovery cohort of Algerian patients with either S. aureus joint (10 patients), respiratory (10 patients), or skin (10 patients) infections and in 10 Algerian control patients. Significant differences in IgGt, IgG1, and IgG4 between groups are shown in Table S2A in the supplemental material.
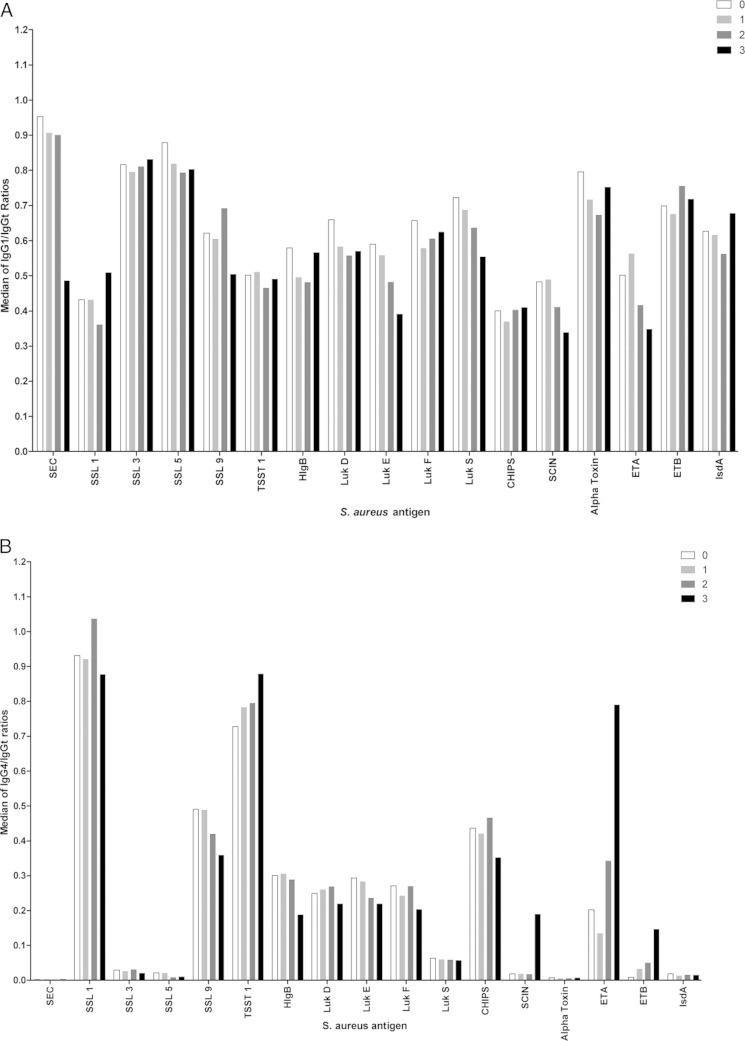
Ratios of IgG subclass to total IgG were calculated to determine the contributions of the different subclasses to the IgGt response to 40 different S. aureus antigens. IgG1, which constitutes approximately 60% of IgGt (31, 32), showed responses directed to almost all tested S. aureus antigens, similar to results obtained for IgGt (Fig. 1A). Strikingly, in all groups we observed IgG4 responses against a restricted panel of antigens, consisting of alpha-toxin, CHIPS, ETA and -B, HlgB, IsdA, LukD, -E, -F, and -S, SCIN, SEC, SSL1, -3, -5, and -9, and TSST-1 (Fig. 1B), with only few individuals showing high responses against alpha-toxin, ETB, IsdA, SEC, and SLL3 and -5 (Fig. 1A and B). These S. aureus virulence factors are almost all secreted immune modulators. No defined patterns of IgG1 or IgG4 responses were observed for the different types of S. aureus infection. Skewed IgG4/IgGt ratios for SER and SSL11 in joint infections and for SEB in respiratory infections were caused by single patients with high responses. The interquartile ranges of all ratios are given in Fig. S1A and B in the supplemental material.
FIG 1.
IgG1/IgGt and IgG4/IgGt ratios in sera from 40 Algerian volunteers. (A) Median of the IgG1/IgGt ratios in sera from 10 Algerian patients with either joint (dark gray bars), respiratory (light gray bars), or skin (white bars) S. aureus infections and in sera from 10 Algerian control patients without S. aureus infection (black bars). On the x axis, the 40 tested S. aureus antigens are listed. The y axis shows the median IgG1/IgGt signal ratios for each particular antigen. Dotted lines mark 60% (the reported IgG1/IgGt ratio), 30% (50% of this reported value), and 90% (150% of this reported value). (B) Same as for panel A, but showing the IgG4/IgGt ratios. The dotted line marks 5%, which is the reported IgG4/IgGt ratio.
Antistaphylococcal IgGt, IgG1, and IgG4 antibodies in an expanded cohort of Sudanese patients with S. aureus skin infections and healthy controls.
To study the antibody responses in skin infections in a larger cohort from a different geographical setting, we measured the relative IgGt, IgG1, and IgG4 levels in sera of 25 Sudanese patients with S. aureus skin infections and 60 healthy Sudanese volunteers. For this purpose, the same antigens were used as in the above-described analysis of the Algerian serum sets (except SasG and SEB). Significant differences in the IgGt, IgG1, and IgG4 levels between groups are shown in Table S2B in the supplemental material. IgG1 responses against all tested antigens were detected in sera from both patients and healthy controls, similar to the case for the Algerian discovery cohort (Fig. 2A). Intriguingly, also for the Sudanese serum sets, IgG4 antibody responses were detected against alpha-toxin, CHIPS, ETA, ETB, HlgB, IsdA, LukD, -E, -F, and -S, SCIN, SEC, SSL1, -3, -5, -9, and -11, and TSST-1 (Fig. 2B). Interquartile ranges of all ratios are given in Fig. S2A and B in the supplemental material. Altogether, the sera from Algerian and Sudanese patients and the respective control sera revealed IgG4 responses to a similar subset of the tested antigens.
FIG 2.
IgG1/IgGt and IgG4/IgGt ratios in sera from 25 Sudanese patients with S. aureus skin infection and 60 healthy Sudanese volunteers. (A) Median of the IgG1/IgGt ratios in sera of 25 Sudanese patients with S. aureus skin infection (white bars) and 60 Sudanese volunteers (black bars). On the x axis, the 38 tested S. aureus antigens are listed (note that SasG and SEB were not included in this particular analysis). The y axis shows the median of the IgG1/IgGt signal ratios for each particular antigen. Dotted lines mark 60% (the reported IgG1/IgGt ratio), 30% (50% of this reported value), and 90% (150% of this reported value). (B) Same as for panel A, but showing the IgG4/IgGt ratios. The dotted line marks 5%, the reported IgG4/IgGt ratio.
Induction of IgGt, IgG1, and IgG4 antistaphylococcal antibodies during progression of bacteremia.
As previous reports have shown that antistaphylococcal IgGt responses reach peak values after a median of 21 days after diagnosis of bacteremia (range, 5 to 50) (28, 37), we studied the contributions of IgG1 and IgG4 to the increase of IgGt. We determined the IgGt, IgG1, and IgG4 levels in response to 40 S. aureus antigens in serum samples taken at the acute phase, and 1, 2, and 3 weeks after diagnosis of bacteremia in 10 Dutch patients. For the last time point, 3 samples were not available. Antigens against which increased responses could be determined were counted. Indeed, the IgGt level was increased during the 3-week observation period with bacteremic patients, specifically showing increased responses to 6 to 23 antigens (median, 16.5) (see Table S3A and B in the supplemental material). Patients showed increased IgG1 responses during the 3-week observation period to 1 to 21 antigens (median, 11.5) (Table S3A and B). IgG1/IgGt ratios were calculated for antigens showing an increase in IgGt, IgG1, and IgG4 signals (Fig. 3A). Notably, the IgG1/IgGt ratios did not vary over time, showing that an increase in IgGt was caused mainly by an increase in IgG1. The IgG4 responses were poorly conserved between patients, and also in these serum sets, these responses were detectable in only a restricted panel of antigens, namely, alpha-toxin, CHIPS, ETA and -B, HlgB, IsdA, LukD, LukE, LukF, LukS, SCIN, SEC, SSL1, -3, -5, and -9, and TSST-1 (Fig. 3B). During a period of 3 weeks after the onset of bacteremia, patients showed increased IgG4 responses to 0 to 13 antigens (median, 6.5) (see Table S3A and B in the supplemental material). IgG4/IgGt ratios were calculated for antigens showing an increase in IgGt, IgG1, and IgG4 signals. This showed that the IgG4/IgGt ratios did not change over time. Thus, the IgG4 signals increased together with IgGt signals. Interquartile ranges of all ratios are given in Fig. S3A and B in the supplemental material.
FIG 3.
IgG1/IgGt and IgG4/IgGt ratios in sera from 10 Dutch bacteremic patients during disease progression. (A) Median of the IgG1/IgGt ratios in 10 Dutch bacteremic patients during disease progression. White bars, IgG1/IgGt ratio at diagnosis. Light gray bars, IgG1/IgGt ratio at 1 week after diagnosis. Dark gray bars, IgG1/IgGt ratio at 2 weeks after diagnosis. Black bars, IgG1/IgGt ratio at 3 weeks after diagnosis. On the x axis, the 17 antigens with increases in either the IgGt, IgG1, or IgG4 signal are depicted. The y axis shows the median of the IgG1/IgGt signal ratios for each particular antigen. The dotted lines mark 60% (the reported IgG1/IgGt ratio), 30% (50% of this reported value), and 90% (150% of this reported value). (B) Same as for panel A, but showing the IgG4/IgGt ratios. The dotted line at 5% marks the reported IgG4/IgGt ratio.
Antistaphylococcal IgGt, IgG1, and IgG4 antibodies in a cohort of Dutch volunteers with long-term S. aureus exposure.
To determine whether carriers and noncarriers differed in their immune responses against S. aureus, sera were collected from 19 persistent nasal carriers and 26 persistent nasal noncarriers. Significantly higher IgGt levels directed to TSST-1 were measured for carriers than for noncarriers. Significantly higher IgG1 levels directed to TSST-1 were measured for carriers than for noncarriers. Finally, carriers showed significantly higher IgG4 levels directed to TSST-1 than noncarriers (see Table S3C in the supplemental material). Also, the numbers of antigens to which responses were detectable were determined. Noncarriers showed IgGt and IgG1 responses to similar numbers of antigens as carriers but trended toward fewer IgG4 responses (mean, 2.68; range, 0 to 11) than carriers (mean, 2.95; range, 0 to 12) (Fig. 4). Based on these findings, we studied sera of EB patients to determine whether long-term exposure to different S. aureus types influenced the numbers of antigens to which IgG4 responses are elicited. Previous studies had shown that up to six different types of S. aureus could be cultured from individual patients with EB (16–18). IgGt showed levels similar to those in a previous study (18). EB patients differed significantly from noncarriers in IgGt responses to ClfA and in IgG4 responses SCIN. EB patients differed significantly from carriers in IgGt responses to ClfA and IgG4 responses to SCIN.
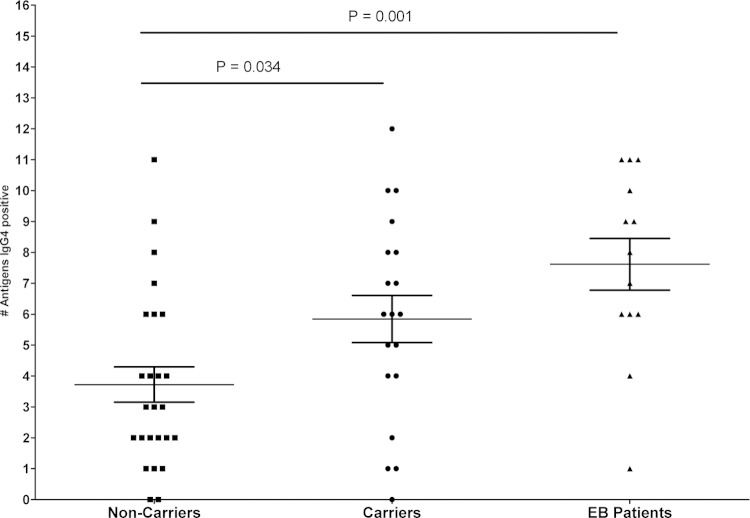
FIG 4.
Numbers of S. aureus antigens to which IgG4 responses were detectable in sera from 19 Dutch carriers (circles), 26 Dutch noncarriers (squares), and 13 Dutch EB patients (triangles). Dutch carriers showed a trend to IgG4 serum responses to more antigens than noncarriers (mean of 2.95 and range of 0 to 12 versus mean of 2.68 range of 0 to 11; P = 0.0339). EB patients showed IgG4 responses to significantly more antigens than noncarriers (mean of 5.38 and range of 1 to 11 versus mean of 2.68 and range of 0 to 11; P = 0.0013) and trended toward more IgG4 responses than carriers (mean of 5.38 and range of 1 to 11 versus mean of 2.98 and range of 0 to 12; P = 0.1275). Mean and standard error of the mean (SEM) are plotted.
Notably, while IgG4 responses to the same restricted panel of antigens were observed in EB patients as for the other groups described above, the EB patients showed IgG4 responses to significantly more antigens than noncarriers (mean of 5.38 and range of 1 to 11 versus mean of 2.68 and range of 0 to 11; P = 0.0013), and they showed a trend toward responding to more antigens than S. aureus carriers (mean of 5.38 and range of 1 to 11 versus mean of 2.68 and range of 0 to 12; P = 0.1275) (Fig. 4). This implies that the intense exposure of EB patients to different S. aureus types results in increased numbers of staphylococcal antigens to which IgG4 responses will develop.
DISCUSSION
In the present study, we investigated the IgG subclass-specific responses directed against 40 different S. aureus virulence factors. These responses were measured in the sera from patients from 3 geographical locations suffering from 4 different types of S. aureus infections. In addition, we studied the humoral response in sera from healthy human carriers, noncarriers, and patients suffering from epidermolysis bullosa, with well-documented S. aureus colonization status, to gain more insights into the bacterial factors involved in pathogen-host interaction. Total IgG responses were detected against almost all antigens in our panel, in agreement with our previous analyses (9, 28, 30, 37). IgGt responses consisted mostly of IgG1 responses, consistent with the previously reported finding that IgG1 composes 60% of IgGt (31, 32). In contrast, in all serum sets analyzed here we observed that IgG4 antibodies, which represent approximately 5% of the IgGt response, were detected to a core panel of S. aureus antigens consisting almost exclusively of secreted immune modulators, irrespective of the type of human-pathogen interaction.
IgG4 responses were observed against alpha-toxin, CHIPS, ETA and -B, HlgB, IsdA, LukD, -E, -F, and -S, SCIN, SEC, SSL1, -3, -5, and -9, and TSST-1. These immune modulators interact with both the human innate and acquired immune systems on many levels. Innate responses affected are chemotaxis, which is modulated by CHIPS (56), extravasation, modulated by SSL3 and SSL5 (57), complement activity, which is modulated by SCIN (58), and Toll-like receptor 2 (TLR2) signaling, which is affected by SSL3 (59). SEC and TSST-1 modulate adaptive responses by non-antigen-directed binding of major histocompatibility complex (MHC) class II with T cell receptors, resulting in polyclonal T cell activation (60). Neutrophils are targeted by the γ-hemolysin family (HlgB and LukD, -E, -F, and -S) (20), desmosomes are targeted by exfoliative toxins (ETA and -B) (61), and alpha-toxin lyses mononuclear immune cells and platelets (62). SSL9 binds to monocytes and dendritic cells and blocks the complement system (63, 64), and no clear function has thus far been described for SSL1. Interestingly, patterns of IgG4 response varied extensively between volunteers, indicating that each person is exposed to different virulence factors and/or reacts differently. The different exposure to virulence factors could be explained by the fact that various genetic backgrounds of S. aureus contain different sets of virulence factors, and variation may also be due to differences in regulators or gene expression in various strains (24, 29, 65–69).
IgG4 responses were found to be directed against more different antigens in EB patients than in healthy noncarriers. EB patients are highly susceptible to blistering upon minor trauma due to mutations in structural proteins of the epidermis and the epidermal-dermal junction. Most likely as a consequence of their fragile skin, 62% to 75% of these patients are nasal S. aureus carriers. EB patients with chronic wounds show higher carriage rates than patients without chronic wounds (16, 18). Importantly, S. aureus wound colonization was detected in 92% of the EB patients with chronic wounds and 69% of the patients without chronic wounds (13, 16). Serial sampling of three wounds, the left and right anterior nares, and the throat revealed that 58.3% of the EB patients with chronic wounds and 43.5% of the EB patients without chronic wounds carried alternating S. aureus types over a period of ∼2 years, and during this period, the same S. aureus type was encountered in only 42.5% of all sampled patients (16–18, 70). This suggests that these patients were exposed to diverse staphylococcal virulence factors over a prolonged period of time. Accordingly, our present IgG4 data indicate that repeated exposure to S. aureus in EB patients has led to IgG4 responses directed against more different staphylococcal antigens, although we cannot exclude that other forms of (previous) exposure might result in the development of IgG4. Intriguingly, our IgG4 data indicate a chronic and repeated exposure for all humans to S. aureus and that repeated exposure as in EB patients leads to higher levels of IgG4 responses directed against more antigens than is the case in healthy volunteers. Not all staphylococcal isolates produce all of the virulence factors tested in the present study, and it therefore seems likely that some of them have a higher potential to elicit an IgG4 response than others. Importantly, the presence of IgG4 levels against S. aureus antigens in human individuals may be an indication of past (chronic) or repeated exposure, possibly in the form of asymptomatic, self-limiting infections or colonization (32, 33).
IgG4 is important in neutralizing antibody responses during tolerance after allergy (34, 71), vaccine development (72), and immune therapy (73). The increased interest in IgG4 is caused predominantly by the fact that IgG4 antibodies activate the immune system to a lesser degree by Fc receptor-mediated and complement-mediated phagocytosis than other IgG subclasses, making IgG4 ideal for passive immunization therapies. Our finding that EB patients have the widest spectrum of IgG4 responses while being fairly resistant to bacteremia may provide interesting clues for further vaccination research: possibly new vaccination strategies should induce neutralizing IgG4 antibodies by repeated exposure, although a protective role of other adaptive immune responses cannot be excluded.
The findings we report here were generated in several cohorts, each from distinct geographical locations. As with many clinical studies, acquiring sufficient samples and finding appropriately matched controls are challenging, laborious, and time-consuming. Therefore, we performed an explorative analysis with descriptive statistics based on study cohorts that were relatively small. Accordingly, no power analysis could be done prior to measurement. In this respect, it has to be noted also that previous reports have shown large interpatient variability (9, 19, 28, 30, 37). Nevertheless, we still observed the restricted panel of antigenic immune modulators to which IgG4 responses were mounted in all cohorts analyzed. This observation that IgG4 responses are mounted to a restricted panel of secreted immune modulators of S. aureus is novel and therefore of value to report. Differences between noncarriers and carriers with respect to the number of antigens responded to, as shown in Fig. 4, reach significance without correction (P < 0.05) but do not remain significant after the Bonferroni correction (P < 0.0167). We observe a clear trend in the number of antigens showing IgG4 responses and exposure to S. aureus, but larger longitudinal follow-up studies based on power analyses and initial results will be needed to further substantiate these findings.
To the best of our knowledge, this is the first report on IgG4 responses directed to S. aureus antigens. As we found little cross-reactivity between the different subclass-specific detection antibodies, we conclude that our Luminex assay is robust and has potential for application with other clinically relevant pathogens. Lastly, our study demonstrates a remarkable variation in the composition of the human subclass-specific antibody responses to various antigens of S. aureus, predominantly secreted immune modulators. This has been consistently observed since the start of measuring such antibodies and is fully in line with the outcomes of our previously published analyses (9, 19, 28, 30, 37). Our present data suggest that there is widespread (asymptomatic) exposure to S. aureus in the community, and this applies to all groups studied here, from infected patients to persistent nasal noncarriers. We therefore hypothesize that interactions between humans and S. aureus occur extensively and repeatedly and are even more diverse than currently appreciated. This might have major implications for research on the respective host-pathogen responses in vivo and for the development of immunotherapeutic strategies such as active and passive vaccination.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following individuals for the kind gifts and use of the recombinant S. aureus antigens or for plasmids for overexpressing and purifying them: John D. Fraser for SSL1, -3, -5, 9, and -11; Jos van Strijp for CHIPS, Efb, and SCIN; Jerome Etienne for alpha-toxin, ETA and -B, HlgB, LukD, -E, -F, and -S, and SEA, -C, -D, -E, -G, -H, -I, -J, -N, and -R; Timothy Foster for ClfA and -B, FnpbA and -B, IsdA and -H, SasG, and SdrD and -E; and Barbara Bröker for SEB, -M, -O, and -Q and TSST-1. We also thank Kenza Antri, Ilhem Boubekri, Mohamed Tazir, Francois Vandenesch, and Gerard Lina for the kind gifts of sera and Magda van der Kooi-Pol and José Duipmans for collecting the sera from EB patients.
J.M.V.D. acknowledges funding through Top Institute Pharma grants T4-213 and T4-502. J.S. acknowledges funding though Stichting Toegepaste Wetenschappen projects 10467 and 10469.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02286-14.
REFERENCES
- 1.Calfee DP. 2012. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, and other Gram-positives in healthcare. Curr Opin Infect Dis 25:385–394. doi: 10.1097/QCO.0b013e3283553441. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R, National Nosocomial Infections Surveillance System . 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis 42:389–391. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 3.Gorwitz RJ. 2008. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J 27:1–7. doi: 10.1097/INF.0b013e31815819bb. [DOI] [PubMed] [Google Scholar]
- 4.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan SL. 2014. Recent lessons for the management of bone and joint infections. J Infect 68(Suppl 1):S51–S56. doi: 10.1016/j.jinf.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 7.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med 10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 8.Eriksen NH, Espersen F, Rosdahl VT, Jensen K. 1995. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol Infect 115:51–60. doi: 10.1017/S0950268800058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van Belkum A, van Wamel WJ. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis 199:625–632. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 10.Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 12.Toshkova K, Annemuller C, Akineden O, Lammler C. 2001. The significance of nasal carriage of Staphylococcus aureus as risk factor for human skin infections. FEMS Microbiol Lett 202:17–24. doi: 10.1111/j.1574-6968.2001.tb10774.x. [DOI] [PubMed] [Google Scholar]
- 13.Brandling-Bennett HA, Morel KD. 2010. Common wound colonizers in patients with epidermolysis bullosa. Pediatr Dermatol 27:25–28. doi: 10.1111/j.1525-1470.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 14.Graber CJ, Shane AL, Weintrub P, Chambers HF. 2011. Clonality of Staphylococcus aureus colonization over time in attendees of a camp for children with chronic dermatoses. Pediatr Dermatol 28:519–523. doi: 10.1111/j.1525-1470.2011.01508.x. [DOI] [PubMed] [Google Scholar]
- 15.Pope E, Lara-Corrales I, Mellerio J, Martinez A, Schultz G, Burrell R, Goodman L, Coutts P, Wagner J, Allen U, Sibbald G. 2012. A consensus approach to wound care in epidermolysis bullosa. J Am Acad Dermatol 67:904–917. doi: 10.1016/j.jaad.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Kooi-Pol MM, Veenstra-Kyuchukova YK, Duipmans JC, Pluister GN, Schouls LM, de Neeling AJ, Grundmann H, Jonkman MF, van Dijl JM. 2012. High genetic diversity of Staphylococcus aureus strains colonizing patients with epidermolysis bullosa. Exp Dermatol 21:463–466. doi: 10.1111/j.1600-0625.2012.01502.x. [DOI] [PubMed] [Google Scholar]
- 17.van der Kooi-Pol MM, Sadaghian Sadabad M, Duipmans JC, Sabat AJ, Stobernack T, Omansen TF, Westerhout-Pluister GN, Jonkman MF, Harmsen HJ, van Dijl JM. 2013. Topography of distinct Staphylococcus aureus types in chronic wounds of patients with epidermolysis bullosa. PLoS One 8:e67272. doi: 10.1371/journal.pone.0067272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Kooi-Pol MM, de Vogel CP, Westerhout-Pluister GN, Veenstra-Kyuchukova YK, Duipmans JC, Glasner C, Buist G, Elsinga GS, Westra H, Bonarius HP, Groen H, van Wamel WJ, Grundmann H, Jonkman MF, van Dijl JM. 2013. High anti-staphylococcal antibody titers in patients with epidermolysis bullosa relate to long-term colonization with alternating types of Staphylococcus aureus. J Investig Dermatol 133:847–850. doi: 10.1038/jid.2012.347. [DOI] [PubMed] [Google Scholar]
- 19.van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 20.Vandenesch F, Lina G, Henry T. 2012. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol 2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. 2012. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bestebroer J, De Haas CJ, Van Strijp JA. 2010. How microorganisms avoid phagocyte attraction. FEMS Microbiol Rev 34:395–414. doi: 10.1111/j.1574-6976.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 23.Foster TJ, Hook M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 6:484–488. doi: 10.1016/S0966-842X(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 24.Sibbald MJ, Ziebandt AK, Engelmann S, Hecker M, de Jong A, Harmsen HJ, Raangs GC, Stokroos I, Arends JP, Dubois JY, van Dijl JM. 2006. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev 70:755–788. doi: 10.1128/MMBR.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burian M, Wolz C, Goerke C. 2010. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5:e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtfreter S, Jursa-Kulesza J, Masiuk H, Verkaik NJ, de Vogel C, Kolata J, Nowosiad M, Steil L, van Wamel W, van Belkum A, Volker U, Giedrys-Kalemba S, Broker BM. 2011. Antibody responses in furunculosis patients vaccinated with autologous formalin-killed Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 30:707–717. doi: 10.1007/s10096-010-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, Tavakol M, Verkaik NJ, van Belkum A, Verbrugh HA, van Wamel WJ. 2013. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One 8:e53391. doi: 10.1371/journal.pone.0053391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtfreter S, Kolata J, Broker BM. 2010. Towards the immune proteome of Staphylococcus aureus—the anti-S. aureus antibody response. Int J Med Microbiol 300:176–192. doi: 10.1016/j.ijmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Verkaik NJ, Dauwalder O, Antri K, Boubekri I, de Vogel CP, Badiou C, Bes M, Vandenesch F, Tazir M, Hooijkaas H, Verbrugh HA, van Belkum A, Etienne J, Lina G, Ramdani-Bouguessa N, van Wamel WJ. 2010. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis 50:61–68. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]
- 31.Maguire GA, Kumararatne DS, Joyce HJ. 2002. Are there any clinical indications for measuring IgG subclasses? Ann Clin Biochem 39:374–377. doi: 10.1258/000456302760042678. [DOI] [PubMed] [Google Scholar]
- 32.Sigal LH. 2012. Basic science for the clinician 58: IgG subclasses. J Clin Rheumatol 18:316–318. doi: 10.1097/RHU.0b013e318269446b. [DOI] [PubMed] [Google Scholar]
- 33.Aalberse RC, van der Gaag R, van Leeuwen J. 1983. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol 130:722–726. [PubMed] [Google Scholar]
- 34.Nirula A, Glaser SM, Kalled SL, Taylor FR. 2011. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol 23:119–124. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 35.Sokol RJ, Booker DJ, Stamps R. 1992. The pathology of autoimmune hemolytic-anemia. J Clin Pathol 45:1047–1052. doi: 10.1136/jcp.45.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, Boelens HA, Hofman A, van Belkum A, Verbrugh HA. 2004. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule.” Clin Infect Dis 39:806–811. doi: 10.1086/423376. [DOI] [PubMed] [Google Scholar]
- 37.Verkaik NJ, Boelens HA, de Vogel CP, Tavakol M, Bode LG, Verbrugh HA, van Belkum A, van Wamel WJ. 2010. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 29:509–518. doi: 10.1007/s10096-010-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas D, Dauwalder O, Brun V, Badiou C, Ferry T, Etienne J, Vandenesch F, Lina G. 2009. Staphylococcus aureus superantigens elicit redundant and extensive human Vbeta patterns. Infect Immun 77:2043–2050. doi: 10.1128/IAI.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prevost G, Cribier B, Couppie P, Petiau P, Supersac G, Finck-Barbancon V, Monteil H, Piemont Y. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect Immun 63:4121–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palma M, Wade D, Flock M, Flock JI. 1998. Multiple binding sites in the interaction between an extracellular fibrinogen-binding protein from Staphylococcus aureus and fibrinogen. J Biol Chem 273:13177–13181. doi: 10.1074/jbc.273.21.13177. [DOI] [PubMed] [Google Scholar]
- 42.Yamasaki O, Yamaguchi T, Sugai M, Chapuis-Cellier C, Arnaud F, Vandenesch F, Etienne J, Lina G. 2005. Clinical manifestations of staphylococcal scalded-skin syndrome depend on serotypes of exfoliative toxins. J Clin Microbiol 43:1890–1893. doi: 10.1128/JCM.43.4.1890-1893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Brien L, Kerrigan SW, Kaw G, Hogan M, Penades J, Litt D, Fitzgerald DJ, Foster TJ, Cox D. 2002. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol 44:1033–1044. doi: 10.1046/j.1365-2958.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 44.Loughman A, Sweeney T, Keane FM, Pietrocola G, Speziale P, Foster TJ. 2008. Sequence diversity in the A domain of Staphylococcus aureus fibronectin-binding protein A. BMC Microbiol 8:74. doi: 10.1186/1471-2180-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke FM, McCormack N, Rindi S, Speziale P, Foster TJ. 2010. Fibronectin-binding protein B variation in Staphylococcus aureus. BMC Microbiol 10:160. doi: 10.1186/1471-2180-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke SR, Foster SJ. 2008. IsdA protects Staphylococcus aureus against the bactericidal protease activity of apolactoferrin. Infect Immun 76:1518–1526. doi: 10.1128/IAI.01530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visai L, Yanagisawa N, Josefsson E, Tarkowski A, Pezzali I, Rooijakkers SH, Foster TJ, Speziale P. 2009. Immune evasion by Staphylococcus aureus conferred by iron-regulated surface determinant protein IsdH. Microbiology 155:667–679. doi: 10.1099/mic.0.025684-0. [DOI] [PubMed] [Google Scholar]
- 48.Corrigan RM, Rigby D, Handley P, Foster TJ. 2007. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 153:2435–2446. doi: 10.1099/mic.0.2007/006676-0. [DOI] [PubMed] [Google Scholar]
- 49.Josefsson E, O'Connell D, Foster TJ, Durussel I, Cox JA. 1998. The binding of calcium to the B-repeat segment of SdrD, a cell surface protein of Staphylococcus aureus. J Biol Chem 273:31145–31152. doi: 10.1074/jbc.273.47.31145. [DOI] [PubMed] [Google Scholar]
- 50.Chung MC, Wines BD, Baker H, Langley RJ, Baker EN, Fraser JD. 2007. The crystal structure of staphylococcal superantigen-like protein 11 in complex with sialyl Lewis X reveals the mechanism for cell binding and immune inhibition. Mol Microbiol 66:1342–1355. doi: 10.1111/j.1365-2958.2007.05989.x. [DOI] [PubMed] [Google Scholar]
- 51.Verkaik N, Brouwer E, Hooijkaas H, van Belkum A, van Wamel W. 2008. Comparison of carboxylated and Penta-His microspheres for semi-quantitative measurement of antibody responses to His-tagged proteins. J Immunol Methods 335:121–125. doi: 10.1016/j.jim.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Martins TB, Augustine NH, Hill HR. 2006. Development of a multiplexed fluorescent immunoassay for the quantitation of antibody responses to group A streptococci. J Immunol Methods 316:97–106. doi: 10.1016/j.jim.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Lal G, Balmer P, Joseph H, Dawson M, Borrow R. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin Diagn Lab Immunol 11:272–279. doi: 10.1128/CDLI.11.2.272-279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lal G, Balmer P, Stanford E, Martin S, Warrington R, Borrow R. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J Immunol Methods 296:135–147. doi: 10.1016/j.jim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Ray CA, Bowsher RR, Smith WC, Devanarayan V, Willey MB, Brandt JT, Dean RA. 2005. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. J Pharm Biomed Anal 36:1037–1044. doi: 10.1016/j.jpba.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 56.Rooijakkers SH, van Kessel KP, van Strijp JA. 2005. Staphylococcal innate immune evasion. Trends Microbiol 13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Bestebroer J, van Kessel KP, Azouagh H, Walenkamp AM, Boer IG, Romijn RA, van Strijp JA, de Haas CJ. 2009. Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood 113:328–337. doi: 10.1182/blood-2008-04-153882. [DOI] [PubMed] [Google Scholar]
- 58.Rooijakkers SH, Ruyken M, van Roon J, van Kessel KP, van Strijp JA, van Wamel WJ. 2006. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol 8:1282–1293. doi: 10.1111/j.1462-5822.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 59.Yokoyama R, Itoh S, Kamoshida G, Takii T, Fujii S, Tsuji T, Onozaki K. 2012. Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infect Immun 80:2816–2825. doi: 10.1128/IAI.00399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fraser JD, Proft T. 2008. The bacterial superantigen and superantigen-like proteins. Immunol Rev 225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 61.Nishifuji K, Sugai M, Amagai M. 2008. Staphylococcal exfoliative toxins: “molecular scissors” of bacteria that attack the cutaneous defense barrier in mammals. J Dermatol Sci 49:21–31. doi: 10.1016/j.jdermsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis 198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Shangiti AM, Nair SP, Chain BM. 2005. The interaction between staphylococcal superantigen-like proteins and human dendritic cells. Clin Exp Immunol 140:461–469. doi: 10.1111/j.1365-2249.2005.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langley RJ, Fraser JD. 2013. The staphylococcal superantigen-like toxins. Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 65.McCarthy AJ, Lindsay JA. 2013. Staphylococcus aureus innate immune evasion is lineage-specific: a bioinfomatics study. Infect Genet Evol 19:7–14. doi: 10.1016/j.meegid.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Monecke S, Luedicke C, Slickers P, Ehricht R. 2009. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. Eur J Clin Microbiol Infect Dis 28:1159–1165. doi: 10.1007/s10096-009-0752-2. [DOI] [PubMed] [Google Scholar]
- 67.Rijnders MI, Deurenberg RH, Boumans ML, Hoogkamp-Korstanje JA, Beisser PS, Antibiotic Resistance Surveillance Group, Stobberingh EE. 2009. Population structure of Staphylococcus aureus strains isolated from intensive care unit patients in the Netherlands over an 11-year period (1996 to 2006). J Clin Microbiol 47:4090–4095. doi: 10.1128/JCM.00820-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boakes E, Kearns AM, Badiou C, Lina G, Hill RL, Ellington MJ. 2012. Do differences in Panton-Valentine leukocidin production among international methicillin-resistant Staphylococcus aureus clones affect disease presentation and severity? J Clin Microbiol 50:1773–1776. doi: 10.1128/JCM.06421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Trijp MJ, Melles DC, Snijders SV, Wertheim HF, Verbrugh HA, van Belkum A, van Wamel WJ. 2010. Genotypes, superantigen gene profiles, and presence of exfoliative toxin genes in clinical methicillin-susceptible Staphylococcus aureus isolates. Diagn Microbiol Infect Dis 66:222–224. doi: 10.1016/j.diagmicrobio.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 70.van der Kooi-Pol MM, Duipmans JC, Jonkman MF, van Dijl JM. 2014. Host-pathogen interactions in epidermolysis bullosa patients colonized with Staphylococcus aureus. Int J Med Microbiol 304:195–203. doi: 10.1016/j.ijmm.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Soyer OU, Akdis M, Akdis CA. 2011. Mechanisms of subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am 31:175–190, vii–viii. doi: 10.1016/j.iac.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Ohlsen K, Lorenz U. 2010. Immunotherapeutic strategies to combat staphylococcal infections. Int J Med Microbiol 300:402–410. doi: 10.1016/j.ijmm.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. 2010. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol 37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.