Abstract
Diabetic complications involve inflammation-mediated microvascular and macrovascular damage, disruption of lipid metabolism, glycosylation of proteins, and abnormalities of neutrophil-mediated events. Resolution of inflamed tissues to health and homeostasis is an active process mediated by endogenous lipid agonists, including lipoxins and resolvins. This proresolution system appears to be compromised in type 2 diabetes (T2D). The goal of this study was to investigate unresolved inflammation in T2D. Wild-type (WT) and genetically engineered mice, including T2D mice (db/db), transgenic mice overexpressing the human resolvin E1 (RvE1) receptor (ERV1), and a newly bred strain of db/ERV1 mice, were used to determine the impact of RvE1 on the phagocytosis of Porphyromonas gingivalis in T2D. Neutrophils were isolated and incubated with fluorescein isothiocyanate-labeled P. gingivalis, and phagocytosis was measured in a fluorochrome-based assay by flow cytometry. Mitogen-activated protein kinase (MAPK) (p42 and p44) and Akt (Thr308 and Ser473) phosphorylation was analyzed by Western blotting. The mouse dorsal air pouch model was used to evaluate the in vivo impact of RvE1. Results revealed that RvE1 increased the neutrophil phagocytosis of P. gingivalis in WT animals but had no impact in db/db animals. In ERV1-transgenic and ERV1-transgenic diabetic mice, phagocytosis was significantly increased. RvE1 decreased Akt and MAPK phosphorylation in the transgenic animals. In vivo dorsal air pouch studies revealed that RvE1 decreases neutrophil influx into the pouch and increases neutrophil phagocytosis of P. gingivalis in the transgenic animals; cutaneous fat deposition was reduced, as was macrophage infiltration. The results suggest that RvE1 rescues impaired neutrophil phagocytosis in obese T2D mice overexpressing ERV1.
INTRODUCTION
Excessive inflammation is now recognized as a central component of the most prevalent diseases in developed societies. The complications of diabetes mellitus, particularly type 2, include periodontitis and cardiovascular disease. Fifty percent of the U.S. population has at least some periodontal disease; type 2 diabetes (T2D) doubles the risk of periodontitis (1). A major link between T2D and its complications is inflammation (2, 3). Enhanced inflammation is well characterized in T2D (4), and prolonged inflammation is an important aspect of periodontitis complications (5). Tumor necrosis factor alpha (TNF-α), which has been implicated as a proinflammatory adipokine in T2D and obesity, seems to play an important role. Specific inhibition of TNF-α in diabetic-animal experiments reverses the upregulation of proinflammatory cytokine genes, leukocyte infiltration into the periodontium, and associated bone loss (5).
In periodontitis, after acute infection, the shift to chronicity and persistence of pathogens may be the result of increased inflammation (6–10) and leads to leukocyte-mediated tissue destruction. The increase in inflammation induced by T2D directly contributes to the increased prevalence and severity of periodontitis in T2D (11).
The active endogenous mediators of resolution of inflammation are now known (12–14). It is also well established that sufficient proresolution agonist (lipoxin and resolvin) concentrations in inflammation are necessary to prevent tissue damage (7, 15) and that these pathways are deficient in T2D (16). The actions of these molecules support their potential use in inflammatory diseases. For example, in a sepsis model (cecal ligation and puncture), a resolvin reduced local and systemic bacterial burdens, cytokine production, and polymorphonuclear neutrophil (PMN) accumulation and increased peritoneal mononuclear cell recruitment and macrophage phagocytosis (17). Mouse survival was significantly increased by resolvin treatment. These findings also suggest that excess inflammation impedes bacterial clearance. In T2D, the observed increased susceptibility to infection is associated with impaired phagocytosis and bacterial killing by cells of the innate immune system. This impairment has been shown to be due to the chronic hyperglycemia in poorly controlled type 2 diabetics that primes neutrophils and monocytes, resulting in an exaggerated inflammatory response and tissue damage (18–20).
Genetically engineered animals have been used to study the impact of T2D on the inflammatory response. For example, the leptin receptor-deficient db/db knockout mouse provides a monogenic model of obesity and T2D (21). The hallmark phenotypic change in db/db mice is insulin resistance; after 8 weeks of age, db/db mice are severely obese and hyperglycemic (22). db/db mice with periodontitis exhibit more aggressive disease with aggravated bone loss (23).
Resolvins, such as resolvin E1 (RvE1), are biosynthesized from the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid. RvE1, a derivative of EPA, shows remarkable potency in resolving inflammation-related diseases such as asthma (24), retinopathy (25), and periodontal disease (15, 26, 27). Emerging evidence suggests that nonresolving inflammation is a critical underlying component of many prevalent chronic diseases such as arthritis, diabetes, and periodontal and cardiovascular diseases (26) and is sustained in part by a deficiency of mediators that normally resolve inflammation (28–30). RvE1 binds to G protein-coupled receptors such as BLT1 (a leukotriene B4 receptor) and ERV1 (also known as chemR23, CMKLR1; for a review, see reference 31). It has been demonstrated that activation of the ERV1 receptor (32, 33) decreases neutrophil migration (34), diminishes inflammatory cytokines, and increases phagocytosis of apoptotic neutrophils by macrophages (35). ERV1-overexpressing transgenic mice are protected from Porphyromonas gingivalis-induced periodontitis (36).
The purpose of these investigations was to begin to unravel the complexities of deficient resolution of inflammation in T2D and a major comorbidity, periodontitis. We report the engineering of two transgenic animals, one normoglycemic and one type 2 diabetic, that overexpress the receptor for the proresolution agonist RvE1 and the impact of RvE1 on neutrophil phagocytosis by exogenous administration of RvE1. We also report the actions of RvE1 on the modification of neutrophil signaling pathways induced my proinflammatory infections.
MATERIALS AND METHODS
Animals.
Male db/db (homozygous) and db/− (heterozygous) mice with a strain FVB [FVB.BKS(D)-Leprdb/ChuaJ] background and age-matched nondiabetic wild-type (WT) control mice were obtained from Jackson Laboratories (Bar Harbor, ME).
Preparation of ERV1 and db/ERV1 transgenic mice.
ERV1 mice were engineered as previously described (36). FVB mice were bred with db/− (heterozygous) mice in order to produce the F1 generation, ERV1+ db/−. The F1 generation mice were bred in, yielding db/ERV1 transgenic mice (db−/−/db−/− with overexpression of ERV1). Four experimental groups included WT, ERV1 transgenic (ERV1), db/db, and db/ERV1 mice. All animal experiments were in conformity with the standards of the Public Health Service policy on the humane care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of The Forsyth Institute.
Genotyping of mice.
Genomic DNA was isolated from tail biopsy specimens of mice and screened by PCR with primers directed to mouse ERV1 (forward primer 5′-CTCGGTCTCCTAGGCAAC-3′) and human ERV1 (forward primer 5′-GTCTTCCTCCCAATCCAT-3′). The mouse and human ERV1 amplicons shared the same reverse primer (5′-TAGAAAGCCAGGACCCAG-3′). For the db/db mice, we used the protocol provided by Jackson Laboratories with restriction enzyme digestion by RsaI and forward primer 5′-AGAACGGACACTCTTTGAAGTCTC-3′ and reverse primer 5′-CATTCAAACCATAGTTTAGGTTTGTGT-3′. The db/db mice showed double bands (108 and 27 bp), and the WT mice showed a single band (135 bp).
Blood glucose levels.
Blood Glucose Test Strips and a Blood Glucose Monitoring System (QSTEPS Biometer Dual Monitoring System; Biomedix, St. Paul, MN) were used to determine the glucose level in a drop of whole blood collected from each mouse.
Resolvin synthesis.
RvE1 was prepared by total organic synthesis as described by Arita et al. (32). The structural integrity of RvE1 was monitored by liquid chromatography-UV-tandem mass spectrometry. Immediately before use, RvE1 was diluted in phosphate-buffered saline (PBS) to a final ethanol concentration of <1%.
P. gingivalis phagocytosis and killing by neutrophils.
P. gingivalis strain A7436 was cultured as previously described (37, 38). After 48 h of anaerobic growth in Wilkins-Chalgren broth in an anaerobic chamber with 85% N2, 5% H2, and 10% CO2, bacteria were harvested by centrifugation; washed three times with sterile, pyrogen-free saline; incubated; and labeled with fluorescein isothiocyanate (FITC; 100 μg/ml of PBS) as previously described (39).
Neutrophils were extracted from peritoneal exudates collected 12 h after the intraperitoneal injection of zymosan-A (1 mg/ml of PBS). The neutrophils were seeded into 24-well plates (1 ml of medium containing 106 cells/well), and bacteria were added at a multiplicity of infection of 20. Four different conditions were assigned to each animal strain: (i) control (neutrophils alone), (ii) vehicle (neutrophils plus P. gingivalis plus PBS), (iii) neutrophils plus P. gingivalis plus RvE1 at 10 ng/ml, and (iv) neutrophils plus P. gingivalis plus RvE1 at 100 ng/ml. For the RvE1 groups, the neutrophils were pretreated with RvE1 15 min before a bacterial challenge. After 2 h of incubation, the neutrophils were collected, quenched with 0.2% trypan blue, and analyzed by flow cytometry (FACScan with CellQuest software; BD Bioscience). Phagocytosis was quantified as the percentage of cells containing bacteria (percent fluorescent cells) and the number of bacteria per cell (events per cell).
In separate experiments, the bactericidal activity of neutrophils against phagocytosed P. gingivalis was assessed by the CFU method of Amano et al. (40). Data are reported as a killing index as described by Kobayashi et al. (41).
Western blot analysis of Akt and mitogen-activated protein kinase (MAPK) phosphorylation.
Protein samples for Western blotting were prepared as previously described (18). Neutrophils were lysed and fractionated by adding 40 μl of 6× SDS sample buffer to 200 μl of the reaction mixture and boiling the samples for 10 min. The final composition of SDS sample buffer after mixing was 2% (wt/vol) SDS, 58.3 mM Tris-HCl (pH 6.8), 6% (vol/vol) glycerol, 5% (vol/vol) 2-mercaptoethanol, 0.002% (wt/vol) bromophenol blue, 1% (vol/vol) protease inhibitors (Sigma, St. Louis, MO), and 1 mM phenylmethylsulfonyl fluoride (Sigma, St. Louis, MO). Aliquots of these samples were separated on 10% (vol/vol) SDS polyacrylamide slab gels, and separated proteins were immediately transferred electrophoretically to polyvinylidene difluoride membranes. The membranes were blocked for 1 h at room temperature with 5% skim milk in Tris-buffered saline (TBS), pH 7.6. The blocking buffer was removed by washing three times with TBS plus Tween 20 (1%), and the membranes were incubated with Akt antibodies (total, phospho-Thr 308, and phospho-Ser473; Cell Signaling Technology, Danvers, MA) and MAPK phosphospecific antibodies (p42 and p44; Cell Signaling Technology, Danvers, MA) overnight. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies (Santa Cruz Biotechnology, Dallas, TX) served as an internal control. The membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibodies for Akt and GAPDH (Santa Cruz Biotechnology, Dallas, TX) and a horseradish peroxidase-conjugated goat anti-rat secondary antibody for MAPK (Santa Cruz Biotechnology, Dallas, TX) in TBS for 1 h at room temperature. Immunoreactive bands detected by chemiluminescence (Immuno-Star; Bio-Rad). Band intensity was determined by densitometric analysis (ChemImager 5500 system; Alpha Innotech Corp.). Protein content was measured by the Bradford method. Akt phosphorylation was normalized to total Akt, and MAPK phosphorylation was normalized to GAPDH.
Dorsal air pouch protocol.
In order to study neutrophil phagocytosis and killing in vivo, air pouches were raised on the dorsa of mice by the subcutaneous injection of 3 ml of sterile air on days 0 and 3, and all experiments were carried out on day 6 (42). Each animal was treated with 50 ng of RvE1 intraperitoneally, followed by 500 μl of sterile PBS or 105 cells of P. gingivalis strain A7436 in PBS (optical density at 600 nm [OD600], 0.9 to 1.0) 15 min later. Mice were sacrificed 4 h postinjection, and individual air pouches were lavaged three times with sterile PBS (3 ml per lavage) as previously described (42, 43). The exudates were centrifuged at 1,000 × g for 5 min, cell pellets were suspended in PBS (200 μl) and counted, and 50 μl of each cell suspension was mixed with 150 μl of 30% bovine serum albumin in PBS, centrifuged onto microscope slides at 500 rpm for 5 min with a cytospin centrifuge, air dried, and Wright-Giemsa stained for identification of individual cell types. An additional sample of 100 μl was incubated in 10 ml of Wilkins-Chalgren broth in an anaerobic chamber, and after 24 h, the bacterial concentration was measured spectrophotometrically (OD600).
Accumulation of fat containing activated macrophages in db/db animals is thought to be a source of increased TNF-α and a potential contributor to the enhanced inflammatory phenotype of T2D (44, 45). In order to evaluate the tissue lining, the air pouches were excised, soaked in 10% formalin solution for 72 h, and embedded in paraffin. Sections 5 μm thick were cut, washed, and placed in a water bath. The sections were stained with hematoxylin and eosin and mounted on permanent slides for subsequent analysis with an optical microscope (Zeiss Axiovert). For immunohistochemistry, the sections were blocked with 1.5% goat serum (Vector Laboratories, Burlingame, CA) and then incubated with anti-CD68 antibody (1/2,000 in goat serum; Abcam, Cambridge, MA) overnight at 4°C. The following day, slides were brought to room temperature and biotinylated secondary anti-rabbit IgG (1/200; Vector Laboratories, Burlingame, CA) was applied for 30 min, followed by ABC reagent (Vector Laboratories, Burlingame, CA) for 30 min. Diaminobenzidine solution (Vector Laboratories, Burlingame, CA) was applied and counterstained with hematoxylin. Standardized photos of all groups were taken at a magnification of ×200, and the images were saved on a computer. The thicknesses of the epithelium, connective tissue, and adipose tissue were calculated with ImageJ software (Image Processing and Analysis in Java). In addition, macrophage infiltration, quantified as CD68+ cell counts, was analyzed as total cell counts and as macrophage density (cells/μm2).
Statistical analysis.
Data are expressed as mean values ± the standard errors of the means. The differences between groups in all comparisons were tested by one-way analysis of variance (ANOVA) with Bonferroni corrections for multiple comparisons (α = 0.05). All analyses were performed with SPSS statistical software, version 19 (IBM SPSS Statistics; IBM Corporation, Somers, NY).
RESULTS
Glycemic index.
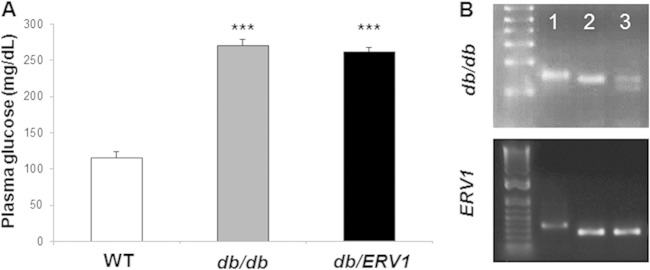
Glycemic-index measurements revealed that the WT animals were normoglycemic and db/db mice were severely hyperglycemic (Fig. 1). The overexpression of ERV1 in db/db mice did not change the circulating glucose concentrations (WT, 115.2 ± 8.6 mg/dl; db/db, 270 ± 8.8 mg/dl; db/ERV1, 262.2 ± 5.8 mg/dl).
FIG 1.
Mouse genotypes and phenotypes. (A) Baseline plasma glucose levels (mg/dl) show that db/db mouse plasma glucose levels are out of the normal range and that overexpression of ERV1 does not impact glycemic control. ***: db/db, P < 0.001 compared to WT; db/ERV1, P < 0.0001 compared to WT (n = 6). (B) Genotyping results. Lanes: 1, WT, 2, ERV1 transgenic (ERV1), 3, db/db-ERV1 transgenic (db/ERV1).
RvE1 increases P. gingivalis phagocytosis and killing by neutrophils when ERV1 is overexpressed.
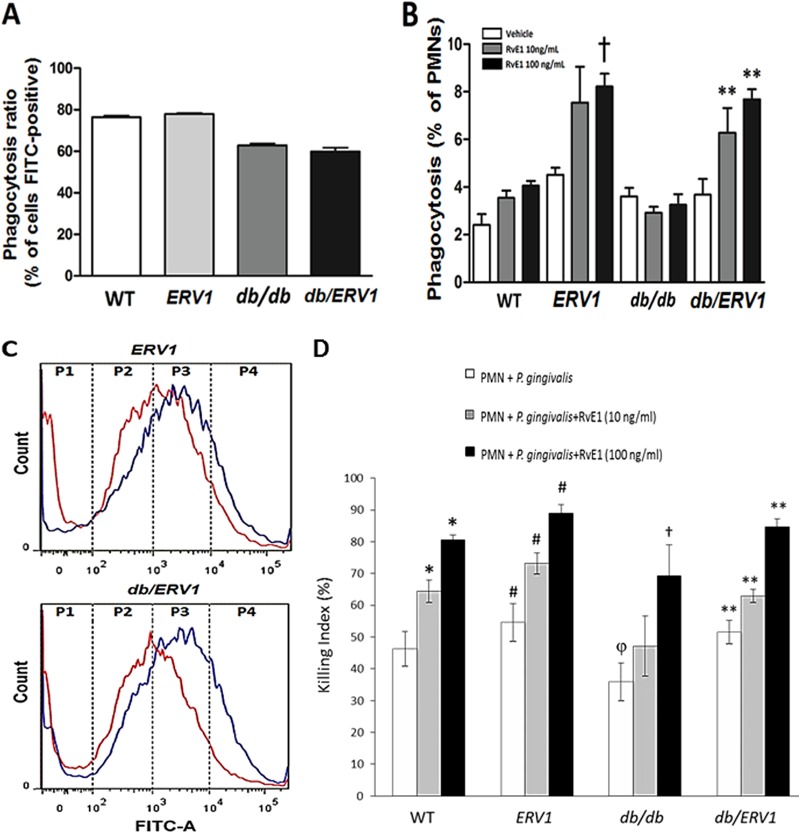
The percentage of neutrophils containing bacteria (FITC positive) was lower in db/db animals (with or without ERV1 overexpression) than in WT and ERV1 mutant animals (Fig. 2A). Phagocytosis was also quantified as the number of bacteria (events per cell) as a measure of phagocytosis efficiency. RvE1 ligand, at both concentrations, significantly increased phagocytosis efficiency in the transgenic animals (ERV1 and db/ERV1) (Fig. 2B and C). db/db neutrophil phagocytosis was impaired compared to WT and ERV1 neutrophil phagocytosis. RvE1 rescued the phagocytosis response in the db/ERV1 animals but not in the db/db animals.
FIG 2.
RvE1 increases P. gingivalis phagocytosis by neutrophils overexpressing ERV1. P. gingivalis phagocytosis by neutrophils was quantified by flow cytometry as the percentage of neutrophils containing bacteria (FITC positive) and the number of bacteria per neutrophil. (A) Percentages of FITC-positive neutrophils. Diabetic mice exhibit significantly fewer phagocytizing neutrophils (n = 9). (B) Percentages of FITC-positive neutrophils with more than 10,000 events (†, P < 0.05 compared to ERV1 with vehicle; **, P <0.01 compared to db/ERV1 with vehicle. (C) Distribution of bacteria per neutrophil by quartile. P4 = >10,000 events per cell. Note the shift to more bacteria per neutrophil with RvE1 treatment (red, no treatment; blue, 10 ng/ml RvE1; n = 8 nondiabetic and 6 diabetic mice). (D) Neutrophil bactericidal activity is enhanced by RvE1. With a CFU killing assay for P. gingivalis, the actions of two doses of RvE1 (10 and 100 ng/ml) were assessed in WT, ERV1, db/db, and db/ERV1 mice. Differences within and between groups were determined by ANOVA with Bonferroni corrections for multiple comparisons. Between-group comparisons revealed that bactericidal activity was lower in db/db mice than in WT animals (or all other strains) (φ, P < 0.05). Treatment of db/db neutrophils with RvE1 restored the killing response to the level of untreated WT mice at 10 ng/ml, and the effect was significantly greater at 100 ng/ml (†, P < 0.05). Overexpression of ERV1 in diabetic db/ERV1 mice completely normalized the killing response (**, P < 0.05). It is interesting that the db/db killing response was significantly lower than that of the WT at both doses of RvE1 and that this response deficiency was eliminated by the overexpression of ERV1. *, P < 0.05 compared to PMNs plus P. gingivalis; #, P <0.05 compared to the WT under all conditions (n = 4).
P. gingivalis killing by neutrophils was significantly increased by RvE1 at both doses in WT animals (Fig. 2D; P < 0.05). ERV1 animals exhibited significantly more killing than the WT without added RvE1 and significantly more killing than the WT at both doses of RvE1. Killing was lower in db/db mice than in WT animals without added RvE1, and the addition of 10 ng/ml RvE1 did not significantly improve killing. High-dose RvE1 (100 ng/ml) rescued the killing response to a level approaching the WT killing response. Overexpression of ERV1 in db/ERV1 animals rescued the killing response under all conditions.
P. gingivalis induces phosphorylation of Akt and MAPK that is reversed by RvE1.
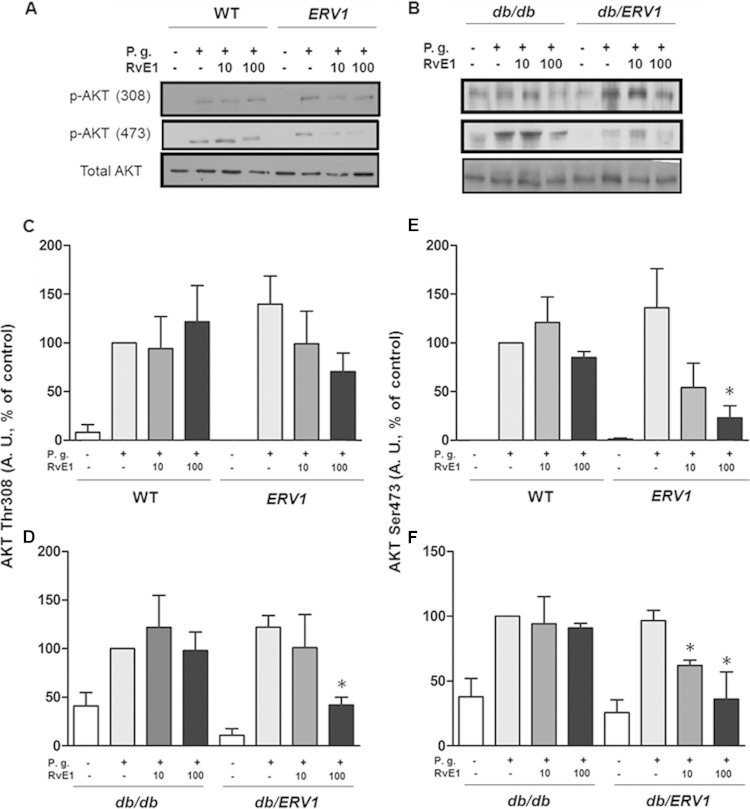
Intracellular signaling through Akt and p38-MAPK was activated by P. gingivalis in protein phosphorylation assays, demonstrating that the neutrophils in all groups of mice are responsive to bacterial stimulation. However, phagocytosis by db/db neutrophils is impaired. In order to determine the impact of RvE1 on activated signaling pathways, Akt and p38-MAPK phosphorylation was assessed after RvE1 treatment in all groups. RvE1 (10 ng/ml) induced a small increase in Akt phosphorylation in db/db mice, while a significant reduction was observed in db/ERV1 mice. When RvE1 administration was increased to 100 ng/ml, a significant decrease in Akt phosphorylation (threonine 308, serine 473) was observed in both db/ERV1 and ERV1 mice. Quantification of phosphorylated Akt bands in Western blot assays demonstrated that P. gingivalis stimulation induced Akt phosphorylation at threonine 308 and serine 473 in all of the groups. Preincubation with 100 ng/ml RvE1 decreased phosphorylation at both thr308 and ser478 (Fig. 3; P < 0.05), while 10 ng/ml RvE1 decreased the phosphorylation of only ser478 in the db/ERV1 group (P < 0.05).
FIG 3.
P. gingivalis (P.g.) induces phosphorylation of Akt that is reversed by RvE1. (A, B) Representative Western blot images quantified in panels C to F. (C, D) Densitometric quantification of Akt phosphorylation at threonine 308. (E, F) Densitometric quantification of Akt phosphorylation at serine 473. A. U., arbitrary units; *, P < 0.05 compared to vehicle control (n = 4).
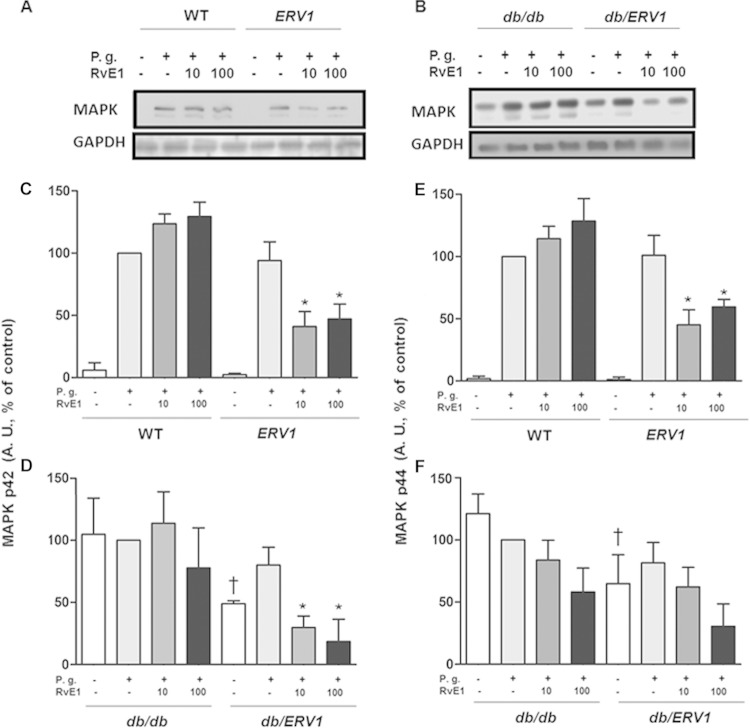
A significant decrease in MAPK phosphorylation was also evident when cells were treated with RvE1 (Fig. 4). A decrease in protein phosphorylation occurred in ERV1 and db/ERV1 mice when cells were treated with RvE1. db/db and db/ERV1 mice showed greater phosphorylation at both sites prior to incubation with P. gingivalis, suggesting preactivation of neutrophils in situ. As observed for Akt phosphorylation, preincubation with RvE1 decreased the phosphorylation of ERK at both p42 and p44 when ERV1 was overexpressed.
FIG 4.
P. gingivalis (P.g.) induces phosphorylation of MAPK that is reversed by RvE1. (A, B) Representative Western blot images quantified in panels C to F. (C, D) Densitometric quantification of ERK phosphorylation (p42). (E, F) Densitometric quantification of MAPK phosphorylation (p44). A. U., arbitrary units; *, P < 0.05 compared to ERV1 plus P. gingivalis plus vehicle; †, P < 0.05 compared to db/db without P. gingivalis plus vehicle; *, P < 0.05 compared to db/db plus P. gingivalis plus vehicle; (n = 4).
RvE1 decreases inflammatory cell influx into the air pouch and increases P. gingivalis clearance.
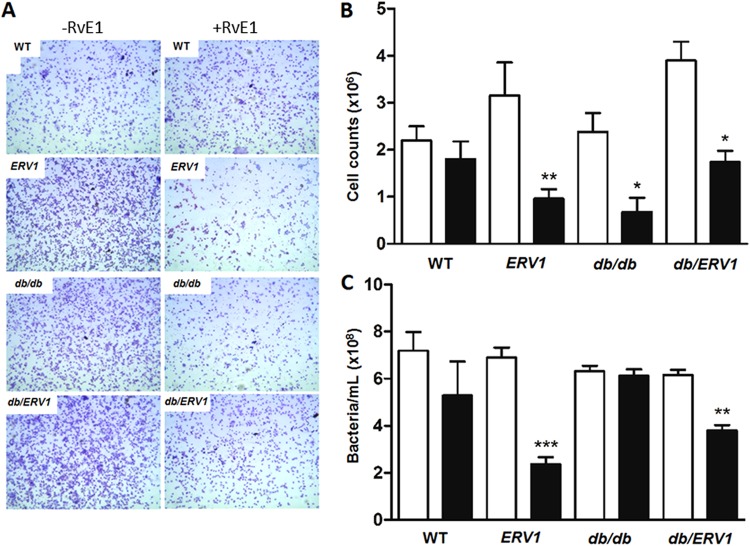
In order to provide an in vivo correlate to the cell-based observations, the impact of RvE1 on cell accumulation, phagocytosis, and killing of P. gingivalis by neutrophils was performed in whole-animal experiments using the dorsal air pouch model. In this model, injection of P. gingivalis into the lumen of the dorsal air pouches of WT animals is known to induce a significant influx of neutrophils that are gradually cleared. Pretreatment with RvE1 decreased neutrophil influx into the dorsal air pouch after 4 h of stimulation with P. gingivalis in all of the groups except db/db mice (Fig. 5A and B). RvE1 pretreatment increased neutrophil phagocytosis and clearance of P. gingivalis in the animals that overexpress ERV1 (ERV1 and db/ERV1), but there was no impact of RvE1 on the db/db animals, confirming the in vitro assay. There was a clear trend to increased P. gingivalis clearance in WT animals that did not reach statistical significance (P = 0.08).
FIG 5.
RvE1 decreases cell influx and increases P. gingivalis clearance. Neutrophils and bacteria were harvested from the air pouch after 4 h by lavage. (A) Representative images of the cellular infiltrate (Wright-Giemsa, <95% neutrophils) with or without RvE1 treatment in all four strains of mice. (B) Total number of neutrophils in the pouch after 4 h (n = 4). (C) Bacteria in the air pouch after 4 h. Bacterial numbers were quantified densitometrically (OD600) after 24 h of growth in Wilkins-Chalgren broth and compared to a standard curve. Bars: black, RvE1; white, control. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (n = 6).
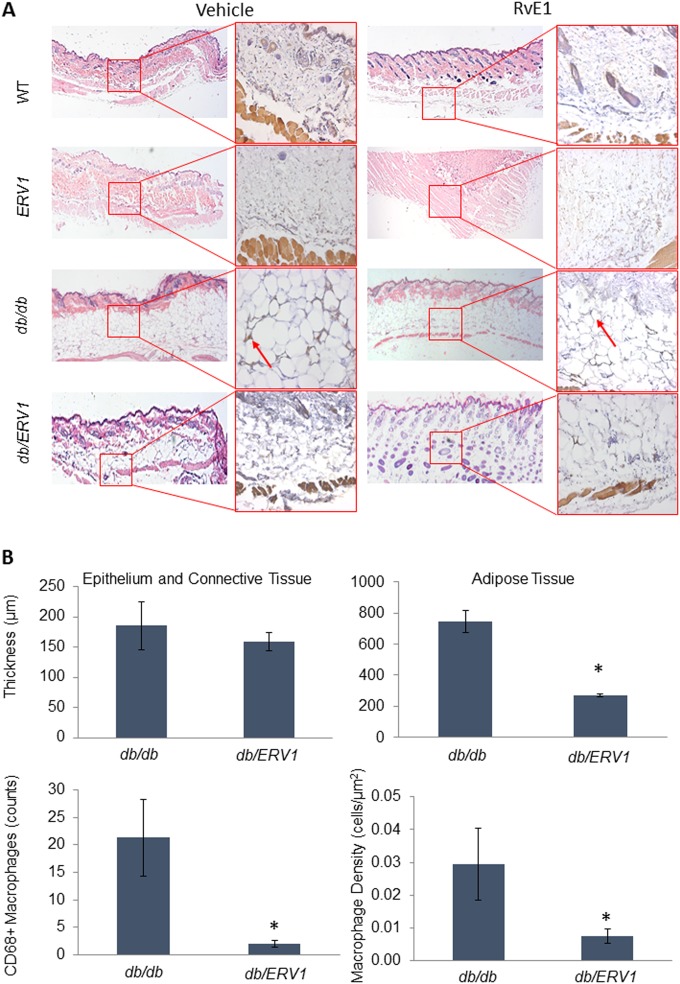
Accumulation of macrophage-laden fat in cutaneous tissue is a well-known characteristic of diabetic mice (44–46). In order to determine the impact of overexpression of ERV1, we compared the dorsal air pouch linings of db/db and db/ERV1 mice (Fig. 6). Overexpression of ERV1 clearly reduces adipose tissue deposition and macrophage infiltration into the fat (Fig. 6A). While the thickness of the epithelium and connective tissue is not significantly affected, the thickness of the adipose tissue is reduced by more than half in db/ERV1 animals. The number and density of macrophages (cells/μm2) in the adipose tissue layer were significantly reduced (Fig. 6B; P < 0.05).
FIG 6.
ERV1 overexpression reduces cutaneous fat accumulation and macrophage accumulation in adipose tissue of diabetic mice. Dorsal air pouches were raised on the backs of all strains of mice. Tissues were stained with hematoxylin and eosin. Tissue macrophages were identified by immunohistochemical detection of CD68 expression. (A) Epithelium, connective tissue, and adipose tissue distribution is demonstrated in each group (ERV1, transgenic mice overexpressing ERV1; db/db, diabetic mice; db/ERV1, diabetic mice overexpressing ERV1) with or without RvE1 treatment. Larger panels demonstrate hematoxylin-and-eosin staining (magnification, ×2.5). CD68+ macrophages in the adipose tissue layer are shown in inserts (arrows; magnification, ×20). Histological analysis of the air pouch lining reveals significant cutaneous adipose tissue accumulation with significant accumulation of CD68+ macrophages in db/db mice that is not evident in diabetic ERV1-overexpressing (db/ERV1) mice. (B) Epithelium, connective tissue, and adipose tissue thicknesses were measured, CD68+ macrophages were counted, and macrophage density in adipose tissue was calculated. Quantification of tissue thickness and macrophage numbers in the adipose tissue reveals significant reductions of both in db/ERV1 animals. *, P < 0.01 [ANOVA] compared to the db/db group.
DISCUSSION
We report here that the immunoresolvent RvE1 increases the phagocytosis, killing, and clearance of the periodontal pathogen P. gingivalis in healthy (WT) mice but does not rescue deficient phagocytosis and clearance in T2D model db/db mice and marginally rescues deficient P. gingivalis killing. Overexpression of the RvE1 receptor gene (ERV1) induced significantly greater phagocytosis and killing in response to RvE1 (P < 0.01) in vitro in both strains of overexpressing mice, i.e., normoglycemic and diabetic (ERV1 and db/ERV1) mice (Fig. 2). Accordingly, in vivo experiments measuring P. gingivalis clearance in the dorsal air pouch model revealed that RvE1 increased the clearance of bacteria with a concomitant decreased influx of neutrophils, suggesting markedly increased efficiency of phagocytosis with a reduced potential for collateral tissue damage. Phagocytosis, killing, and clearance were impaired both in vitro and in vivo in neutrophils of db/db mice. RvE1 had no impact on phagocytosis and a marginal impact on killing by db/db neutrophils unless ERV1 was overexpressed (db/ERV1). We further investigated the actions of RvE1 on signaling pathways in neutrophils preactivated by contact with P. gingivalis and observed that preincubation of neutrophils with RvE1 decreased the phosphorylation of Akt at both threonine 308 and serine 478 when ERV1 was overexpressed; RvE1 stimulation was accompanied by a significant decrease in Akt and MAPK phosphorylation that was more profound in transgenic animals.
Accumulation of cutaneous fat and marked infiltration of the adipose tissue with active macrophages are characteristics of T2D thought to contribute to elevation of systemic inflammation, particularly as a source of the proinflammatory adipokine TNF-α (44–46). Interestingly, overexpression of ERV1 reduces fat accumulation and markedly reduces macrophages in the adipose tissue of diabetic animals. These data suggest a failure of resolution pathways in T2D that can be modified by enhancing resolution. The implication is that agonists of resolution of inflammation may be useful as therapeutics for inflammatory complications of T2D in the future.
An uncontrolled innate immune response is central to chronic inflammatory diseases, including T2D and its complications, including periodontitis. Physiologic changes induced by hyperglycemia and metabolic dysregulation in T2D result in chronic inflammation. The prevailing hypothesis suggests that the hyperglycemic state drives the formation of excess advanced glycation end products, known as AGE, and the expression of its cognate receptor, RAGE (47). This, in turn, induces a proinflammatory cytokine profile contributing to elevation of inflammation and cellular dysfunction systemically. In both metabolic syndrome and T2D, chronic inflammation is associated with insulin resistance, impaired metabolism, and impaired wound healing. In health, an injury induced by pathogens or trauma induces acute inflammation that is characterized on a cellular level by rapid neutrophil infiltration that peaks within hours and is thereafter rapidly cleared to be replaced by monocyte/macrophage infiltration to clear the wound. These events are mediated to a great extent by specialized lipid mediators of resolution of inflammation that induce neutrophil apoptosis and nonphlogistic macrophage activation. Specialized proresolving mediators, such as resolvin E1, greatly influence the fate of innate immune cells. Our group and others have previously demonstrated that RvE1 enhances macrophage phagocytosis and efferocytosis (48).
An important concept that is supported by these data is that excess inflammation actually inhibits bacterial clearance and promotes bacterial growth. This was first demonstrated in murine colitis. In these experiments, neutrophil infiltration was inhibited, but phagocyte clearance and zymosan phagocytosis were enhanced in vitro and in vivo by RvE1 (49, 50). Later work with another member of the resolvin family, resolvin D2 (RvD2), in a murine sepsis model confirmed this concept, showing that in a cecal ligature and puncture model, animals receiving RvD2 exhibited greater survival and enhanced clearance of bacteremia (17). We also reported that treatment of periodontitis with RvE1 induced spontaneous clearance of periodontal pathogens (7). We questioned whether the resolution agonist RvE1 impacts the neutrophil response in vitro and in vivo with induced peritoneal neutrophils and an air pouch model, respectively, in diabetic and normoglycemic ERV1 transgenic mice. After RvE1 treatment, phagocytosis was greater in db/ERV1 and ERV1 animals than in db/db and WT animals. Thus, RvE1 was more efficient in modulating the phagocytic phenotype in ERV1 positive genotype mice (db/ERV1, ERV1) that resulted in damped accumulation of neutrophils with more efficient bacterial clearance.
Previous observations have showed that ω-3 polyunsaturated fatty acids (PUFAs) enhance metabolism in both diabetic and obese mice. Improved whole-body insulin sensitivity and increased activity of resolvin pathways were observed upon dietary supplementation of ω-3 PUFA (51, 52). Consistent with the actions of ω-3 PUFAs, RvD1 and RvD2 each rescued impaired expression and secretion of adiponectin in a time- and concentration-dependent manner. ω-3 PUFA supplementation also modulated proinflammatory signals (TNF-α, interleukin-6 [IL-6], and IL-1β) and cellular function in lean and obese mice. Treatment of animals with exogenous RvD1 prevented hepatic steatosis and reversed insulin resistance, upregulating insulin response genes and increasing insulin tolerance (53). It is interesting that neutrophil functional abnormalities were not likewise corrected by RvE1 treatment. We did not follow blood glucose longitudinally in our experiments, but ERV1 transgenic animals did not show improvements in glycemic control cross-sectionally. However, it is clear that overexpression of ERV1 was necessary to improve the neutrophil response in db/db mice. There are at least four possible explanations for this. (i) The leptin mutation in db/db mice directly affects ERV1 expression and function. (ii) There may be a threshold effect in which resolvin administration is sufficient to upregulate insulin response genes but insufficient to reverse neutrophil functional abnormalities. (iii) RvE1 treatment in our model was acute. Longer treatment regimens may be necessary to affect change. (iv) Overexpression of ERV1 in myeloid cells replaces nonfunctional BLT-1 on db/db neutrophils.
Intracellular signaling after stimulation with RvE1 has been shown to be through extracellular binding to at least two receptors (ERV1 and BLT-1). RvE1 has been shown to positively activate Akt phosphorylation in HeLa cells and human macrophages through ERV1 (54). In our previous studies of RvE1 and osteoclasts, we observed that RvE1 attenuates the nuclear translocation of NF-κB, as well as the phosphorylation of Akt (55). Interestingly, the data suggest that when neutrophils, where the major receptor for RvE1 has been reported to be BLT-1 (33), are stressed, functional ERV1 is expressed, which seems to dominate the response to anti-inflammatory mediators. This was demonstrated for chemerin (an anti-inflammatory protein ligand for ERV1) in myocardial infarction (56). The nature of ERV1 expression and function by neutrophils from db/db animals remains to be elucidated, but it is clear that the phagocytosis and killing response to RvE1 is impaired. Overexpression of ERV1 by db/ERV1 transgenic animals rescues the response, implicating ERV1 and also suggesting the BLT-1 is not functioning on db/db neutrophils.
The mechanism of the interactions between T2D and its complications, such as periodontitis, is unclear. It is, however, well documented that type 2 diabetic (db/db) mice exhibit more periodontal destruction than the WT (23) and that ERV1 transgenic mice are resistant to periodontitis (36). Our work and the work of others provide further insights revealing that phagocyte interactions are dysregulated in people with diabetes and likewise with periodontitis (26, 57). Thus, mechanisms related to inflammation in periodontitis may be linked with the primary cause of T2D, inflammation. Without a clear understanding of these pathways, we cannot identify targets for the development of small molecules for pharmacologic intervention to predictably harness natural pathways to treat these important noncommunicable diseases. The characterization of the impact of RvE1 on the complete cycle of inflammation resolution at the phagocyte level will address major clinical complications of T2D. The relationship between diabetes and periodontal disease is reciprocal. Infections, including periodontal infections, have a significant impact on diabetic control, and diabetes is a significant risk factor for the development and severity of periodontal disease (58). New data clearly demonstrate that type 2 diabetics are refractory to standard periodontal therapy, which further emphasizes this interrelationship (59, 60).
ACKNOWLEDGMENTS
We thank Charles N. Serhan for providing and validating the structure of RvE1.
This study was supported by USPHS grants DE015566, DE018917, and DE023584 from the National Institutes of Dental and Craniofacial Research. S.K. was supported by an unrestricted educational grant from Sunstar Americas.
REFERENCES
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, Beck J, Douglass G, Page R. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. 2002. Atherosclerosis: the new view. Sci Am 286:46–55. doi: 10.1038/scientificamerican0502-46. [DOI] [PubMed] [Google Scholar]
- 3.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. 2005. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 4.Graves DT, Kayal RA. 2008. Diabetic complications and dysregulated innate immunity. Front Biosci 13:1227–1239. doi: 10.2741/2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, Graves DT. 2012. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J 26:1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champion OL, Valdez Y, Thorson L, Guttman JA, Menendez A, Gaynor EC, Finlay BB. 2008. A murine intraperitoneal infection model reveals that host resistance to Campylobacter jejuni is Nramp1 dependent. Microbes Infect 10:922–927. doi: 10.1016/j.micinf.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. 2007. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol 179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 8.Braun J, Wei B. 2007. Body traffic: ecology, genetics, and immunity in inflammatory bowel disease. Annu Rev Pathol 2:401–429. doi: 10.1146/annurev.pathol.1.110304.100128. [DOI] [PubMed] [Google Scholar]
- 9.Andoh A, Benno Y, Kanauchi O, Fujiyama Y. 2009. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Curr Pharm Des 15:2066–2073. doi: 10.2174/138161209788489186. [DOI] [PubMed] [Google Scholar]
- 10.Shih DQ, Targan SR, McGovern D. 2008. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep 10:568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacopino AM. 2001. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol 6:125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 12.Gilroy DW, Lawrence T, Perretti M, Rossi AG. 2004. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN. 2010. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol 177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. 2006. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J 20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 16.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. 2010. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. 2009. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL Jr, Yagi M, Iwata T, Liu H, Hasturk H, Kantarci A, Van Dyke TE. 2008. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol 84:292–301. doi: 10.1189/jlb.1207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierusz-Wysocka B, Wysocki H, Siekierka H, Wykretowicz A, Szczepanik A, Klimas R. 1987. Evidence of polymorphonuclear neutrophils (PMN) activation in patients with insulin-dependent diabetes mellitus. J Leukoc Biol 42:519–523. [DOI] [PubMed] [Google Scholar]
- 20.Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE. 2006. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol 177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman DL. 1982. Diabetes-obesity syndromes in mice. Diabetes 31(Suppl 1 Pt 2):1–6. [DOI] [PubMed] [Google Scholar]
- 22.Coleman DL, Hummel KP. 1974. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia 10(Suppl):607–610. doi: 10.1007/BF01221993. [DOI] [PubMed] [Google Scholar]
- 23.Um YJ, Jung UW, Kim CS, Bak EJ, Cha JH, Yoo YJ, Choi SH. 2010. The influence of diabetes mellitus on periodontal tissues: a pilot study. J Periodontal Implant Sci 40:49–55. doi: 10.5051/jpis.2010.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hisada T, Ishizuka T, Aoki H, Mori M. 2009. Resolvin E1 as a novel agent for the treatment of asthma. Expert Opin Ther Targets 13:513–522. doi: 10.1517/14728220902865622. [DOI] [PubMed] [Google Scholar]
- 25.Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. 2009. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci 50:3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 26.Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. 2011. Impaired phagocytosis in localized aggressive periodontitis: rescue by resolvin E1. PLoS One 6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dyke TE. 2008. The management of inflammation in periodontal disease. J Periodontol 79:1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C, Ding A. 2010. Nonresolving inflammation. Cell 140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. 2010. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. 2008. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freire MO, Van Dyke TE. 2013. Natural resolution of inflammation. Periodontol 2000 63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. 2005. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. 2007. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN, Chiang N, Van Dyke TE. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. 2011. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest 121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, Van Dyke TE, Gyurko R. 2013. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol 190:689–694. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genco CA, Cutler CW, Kapczynski D, Maloney K, Arnold RR. 1991. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun 59:1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genco CA, Schifferle RE, Njoroge T, Forng RY, Cutler CW. 1995. Resistance of a Tn4351-generated polysaccharide mutant of Porphyromonas gingivalis to polymorphonuclear leukocyte killing. Infect Immun 63:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polak D, Shapira L, Weiss EI, Houri-Haddad Y. 2012. The role of coaggregation between Porphyromonas gingivalis and Fusobacterium nucleatum on the host response to mixed infection. J Clin Periodontol 39:617–625. doi: 10.1111/j.1600-051X.2012.01889.x. [DOI] [PubMed] [Google Scholar]
- 40.Amano A, Ishimoto T, Tamagawa H, Shizukuishi S. 1992. Role of superoxide dismutase in resistance of Porphyromonas gingivalis to killing by polymorphonuclear leukocytes. Infect Immun 60:712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi T, Yamamoto K, Sugita N, van Spriel AB, Kaneko S, van de Winkel JG, Yoshie H. 2001. Effective in vitro clearance of Porphyromonas gingivalis by Fc alpha receptor I (CD89) on gingival crevicular neutrophils. Infect Immun 69:2935–2942. doi: 10.1128/IAI.69.5.2935-2942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sin YM, Sedgwick AD, Chea EP, Willoughby DA. 1986. Mast cells in newly formed lining tissue during acute inflammation: a six day air pouch model in the mouse. Ann Rheum Dis 45:873–877. doi: 10.1136/ard.45.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hachicha M, Pouliot M, Petasis NA, Serhan CN. 1999. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med 189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. 2008. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu MJ, Bao S, Bolin ER, Burris DL, Xu X, Sun Q, Killilea DW, Shen Q, Ziouzenkova O, Belury MA, Failla ML, Knoell DL. 2013. Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J Nutr 143:1036–1045. doi: 10.3945/jn.113.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonnell ME, Ganley-Leal LM, Mehta A, Bigornia SJ, Mott M, Rehman Q, Farb MG, Hess DT, Joseph L, Gokce N, Apovian CM. 2012. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity 20:1372–1378. doi: 10.1038/oby.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. 1996. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 67:1085–1093. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- 48.Schwab JM, Chiang N, Arita M, Serhan CN. 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. 2010. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis 16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. 2005. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A 102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.González-Périz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Clària J. 2009. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J 23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. 2011. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN. 2012. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol 189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. 2010. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrera BS, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara MN, Serhan CN, Van Dyke TE, Gyurko R. 2008. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol 155:1214–1223. doi: 10.1038/bjp.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cash JL, Bena S, Headland SE, McArthur S, Brancaleone V, Perretti M. 2013. Chemerin15 inhibits neutrophil-mediated vascular inflammation and myocardial ischemia-reperfusion injury through ChemR23. EMBO Rep 14:999–1007. doi: 10.1038/embor.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuttle HA, Davis-Gorman G, Goldman S, Copeland JG, McDonagh PF. 2003. Platelet-neutrophil conjugate formation is increased in diabetic women with cardiovascular disease. Cardiovasc Diabetol 2:12. doi: 10.1186/1475-2840-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Löe H. 1993. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16:329–334. [PubMed] [Google Scholar]
- 59.Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, Seaquist ER, Reddy MS, Lewis CE, Oates TW, Tripathy D, Katancik JA, Orlander PR, Paquette DW, Hanson NQ, Tsai MY. 2013. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 310:2523–2532. doi: 10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chapple IL, Borgnakke WS, Genco RJ. 2014. Hemoglobin A1c levels among patients with diabetes receiving nonsurgical periodontal treatment. JAMA 311:1919–1920. doi: 10.1001/jama.2014.2228. [DOI] [PubMed] [Google Scholar]