Abstract
Diarrhea and amebic liver abscesses due to invasive Entamoeba histolytica infections are an important cause of morbidity and mortality in the developing world. Entamoeba histolytica adherence and cell migration, two phenotypes linked to virulence, are both aberrant in trophozoites deficient in the metallosurface protease EhMSP-1, which is a homologue of the Leishmania vaccine candidate leishmanolysin (GP63). We examined the potential of EhMSP-1 for use as a vaccine antigen to protect against amebic liver abscesses. First, existing serum samples from South Africans naturally infected with E. histolytica were examined by enzyme-linked immunosorbent assay (ELISA) for the presence of EhMSP-1-specific IgG. Nine of 12 (75%) people with anti-E. histolytica IgG also had EhMSP-1-specific IgG antibodies. We next used a hamster model of amebic liver abscess to determine the effect of immunization with a mixture of four recombinant EhMSP-1 protein fragments. EhMSP-1 immunization stimulated a robust IgG antibody response. Furthermore, EhMSP-1 immunization of hamsters reduced development of severe amebic liver abscesses following intrahepatic injection of E. histolytica by a combined rate of 68% in two independent animal experiments. Purified IgG from immunized compared to control animals bound to the surface of E. histolytica trophozoites and accelerated amebic lysis via activation of the classical complement cascade. We concluded that EhMSP-1 is a promising antigen that warrants further study to determine its full potential as a target for therapy and/or prevention of invasive amebiasis.
INTRODUCTION
Infectious diarrhea is the second most common cause of death in children under 5 years of age (1). Entamoeba histolytica, the enteric ameba that causes invasive amebiasis, was the most common intestinal parasite identified in children between 2 and 5 years of age in a recent comprehensive study of the causes of life-threatening diarrhea in children in sub-Saharan Africa and the Indian subcontinent (2). While amebiasis is still a significant problem in the first 2 years of life (2), the presence of E. histolytica-specific secretory IgA (sIgA) in breast milk correlates with a reduced risk of amebiasis in breastfeeding infants (3). The true global burden of E. histolytica infection remains unknown, but the frequency of infection can be staggering in areas of endemicity. In the Mirpur region of Dhaka, Bangladesh, greater than 50% of children have serologic evidence of E. histolytica infection by 5 years of age (4), and E. histolytica is the intestinal protozoan most frequently associated with diarrhea in this population (5). Invasive disease is characterized by dysentery and the potential for spread via the portal venous circulation to cause amebic liver abscesses (ALAs), which occur in approximately 1% of symptomatic cases (6).
An effective E. histolytica vaccine could conceivably enable global eradication, since E. histolytica infects only humans and some higher nonhuman primates (7). There is currently no such vaccine, but some children with intestinal E. histolytica infection acquire short-lived protective immunity, giving hope that a vaccine can be developed (8–10). Toward this goal, a number of potential vaccine antigens have been partially validated, including the serine-rich E. histolytica protein (SREHP), a lipoproteophosphoglycan, a 29-kDa cysteine-rich thiol-dependent peroxidase, and a d-galactose/N-acetyl-d-galactosamine-specific amebic adherence lectin (Gal lectin) (11–16). Each of these antigens is protective in rodent liver abscess models, and Gal lectin-based subunit vaccines protect against colitis in both mice and baboons (15, 17). Protective immunity in children naturally infected with E. histolytica correlates with secretory IgA antibodies directed to the Gal lectin, further demonstrating its potential as a vaccine antigen (8, 9). However, natural protective immunity is limited to a subset of ∼20% of children (10), which suggests that improved adjuvants and/or inclusion of multiple antigens will be required to develop a successful E. histolytica vaccine. Thus, there is a need to identify and validate additional E. histolytica vaccine antigens.
Entamoeba histolytica metallosurface protease 1, EhMSP-1, is an amebic M8 family zinc metalloprotease with homology to the Leishmania vaccine candidate leishmanolysin (also called GP63) (18–20). The amino acid sequence of EhMSP-1 is highly conserved in pathogenic E. histolytica isolates for which sequence data currently exist (21). We recently demonstrated that EhMSP-1 is an active metalloproteinase that is present on the cell membrane of E. histolytica trophozoites. Furthermore, EhMSP-1 deficiency dramatically increases amebic adherence to host epithelial cell monolayers and reduces amebic motility (18). Given the likely function of EhMSP-1 in regulation of these two virulence-associated phenotypes, its exposure on the cell surface, and the promise of leishmanolysin as a vaccine target, we hypothesize that EhMSP-1 may be a viable vaccine target.
In this study, we determined if EhMSP-1 is immunogenic during naturally occurring E. histolytica infections and used a hamster liver abscess model as a first step toward determining if EhMSP-1 is a potential vaccine antigen. Our findings show that the majority of adults with anti-E. histolytica IgG antibodies also have EhMSP-1-specific IgG antibodies. Furthermore, vaccination with recombinant EhMSP-1 fragments is protective in the hamster liver abscess model of amebiasis. These studies provide evidence in support of further development of EhMSP-1 as a potential component for inclusion in a vaccine to prevent invasive E. histolytica infections.
MATERIALS AND METHODS
Animals.
Four-week-old golden Syrian hamsters were purchased from the Anilab Bioterium (Campinas, São Paulo, Brazil). The hamsters were housed in a pathogen-free facility under ad libitum access to standard chow and filtered water. The FMRP-USP Animal Research Ethics Committee approved all protocols used in these experiments (CETEA no. 105/2012).
Human anti-EhMSP-1 IgG.
Preexisting, deidentified human serum samples from South Africans with amebic liver abscess (a gift from William A. Petri, University of Virginia) were tested for antiamebic and anti-EhMSP-1 IgG antibodies. Ninety-six-well polystyrene enzyme-linked immunosorbent assay (ELISA) plates (Falcon Pro-Bind; catalog no. 353915) were coated with 100 μl/well of purified recombinant GP63 at 50 μg/ml in phosphate-buffered saline (PBS) and incubated overnight at 4°C. The wells were washed three times with 300 μl PBS and blocked with 300 μl PBS-5% dry milk for 1 h at room temperature. After washing three times with 300 μl PBS, 100 μl of the human serum samples diluted 1:50 in PBS was added to the wells and incubated for 1 h at room temperature. The wells were washed three times with 300 μl PBS-0.1% Tween 20, and 100 μl of goat anti-human IgG-peroxidase conjugate antibodies (Sigma; catalog no. A0293) diluted 1:2,500 in PBS-5% dry milk was added to the wells and incubated for 1 h at room temperature. Wells were washed three times with 300 μl PBS-0.1% Tween 20, and 100 μl o-phenylenediamine dihydrochloride (OPD) (OPD Easy-tablets; Acros Organics) solution was added to the wells. The reactions were stopped by adding 50 μl/well of 2 M sulfuric acid. The absorbance of the samples was measured at 490 nm using a microplate reader (BioTek).
Hamster antibodies.
Pooled serum collected from either immunized or nonimmunized animals was used as the source to purify polyclonal hamster IgG antibodies by sequential caprylic acid and ammonium sulfate precipitation according to a protocol adapted from the work of Perosa et al. (22). Briefly, caprylic acid was added dropwise (1:20, vol/vol) to each serum pool (pH 5.0 with acetic acid) while the mixture was gently homogenized. After incubation for 30 min at room temperature, the samples were centrifuged (10,000 × g, 15 min), the pellets were discarded, and a saturated ammonium sulfate solution was added to the supernatants to a concentration of 40%. Precipitated immunoglobulins were then pelleted by centrifugation (10,000 × g, 10 min), followed by resuspension in 1 ml of ultrapure water and exhaustive dialysis in PBS (pH 7.4). Commercially available antibodies are indicated when describing each experiment for which they were used.
Parasites.
E. histolytica strain HM-1:IMSS trophozoites were axenically cultivated in penicillin-streptomycin-supplemented TYI-S-33 medium (23). Prior to animal experiments, parasite virulence was enhanced by adding small pieces of normal hamster liver to the culture tubes (24). Enhanced virulence was confirmed after 48 h by measuring in vitro resistance to complement-mediated lysis and the speed and number of human erythrocytes engulfed by the trophozoites. Twenty-four hours before use in experiments, the culture medium was replaced with serum-free medium in order to increase EhMSP-1 expression on the cell surface, as previously reported (18).
Antigen preparation.
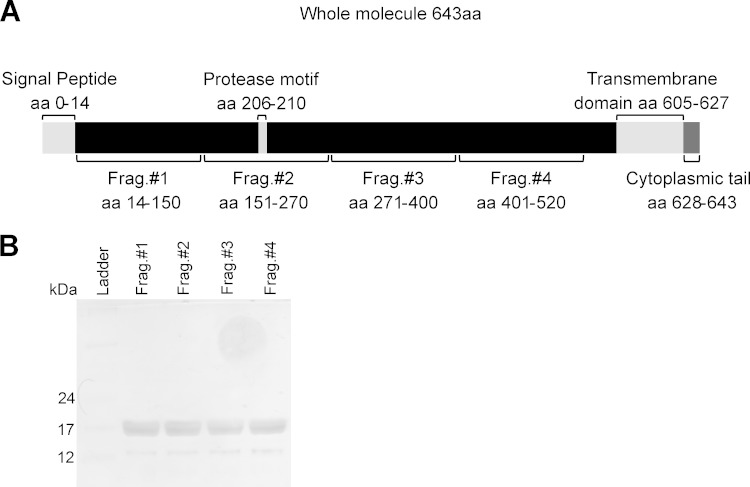
Four recombinant EhMSP-1 fragments comprising the extracellular portion of EhMSP-1 were expressed in Escherichia coli for use as vaccine antigens (Fig. 1A). For this, four approximately 370-bp gene fragments (fragment 1, 73 to 450 bp; fragment 2, 451 to 810 bp; fragment 3, 811 to 1,200 bp; fragment 4, 1,201 to 1,560 bp) were amplified by reverse transcription-PCR (RT-PCR) and cloned into the bacterial expression plasmid pET-DEST42 TOPO (Invitrogen, Carlsbad, CA). Protein fragments were then expressed by induction for 5 h with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) in E. coli strain BL21(DE3) and purified from inclusion bodies by solubilization in accordance with the QIAexpressionist handbook (25) and the work of Rehm et al. (26). Contaminating endotoxin was removed from the protein stocks using polymyxin columns (Pierce, Rockford, IL, USA), and residual endotoxin was quantified using a commercial kit (QCL-1000; Lonza, Williamsport, PA, USA). In accordance with accepted guidelines for preclinical research, all animals received less than 5.0 endotoxin units (EU)/kg of body weight per dose of vaccine antigen (27).
FIG 1.
Expression and purification of recombinant EhMSP-1 fragments. (A) Schematic of EhMSP-1, indicating key features of the protein and locations of the recombinantly expressed protein fragments. aa, amino acids. (B) Silver-stained SDS-PAGE gel showing the purified recombinant EhMSP-1 fragments.
Vaccination regimen.
Three vaccination experiments were conducted, including one to test IgG antibody production following immunization and two to test vaccine efficacy using a hepatic challenge model of amebiasis (described below). The immunizations were performed by adapting a protocol used by Yan et al. (28). Hamsters were immunized with four weekly intraperitoneal injections, each comprised of an emulsion of 100 μl of saline containing 50 μg of each EhMSP-1 protein fragment, 50 μl of complete Freund's adjuvant, and 50 μl of incomplete Freund's adjuvant. Sham-immunized hamsters received the same regimen of immunization with saline (immunization test and first challenge assay) or 200 μg of albumin (second challenge assay) emulsified in adjuvant.
Evaluation of immunization.
To assess the efficiency of immunization with recombinant EhMSP-1, we used both enzyme-linked immunosorbent assay (ELISA) and flow cytometry to compare binding of IgG antibodies from immunized and nonimmunized animals to the recombinant EhMSP-1 fragments or E. histolytica trophozoites. For ELISAs, standard 96-well polystyrene plates (Costar; Corning Inc., New York, NY) were coated with 5 μg of each protein fragment per well. Undiluted immune or nonimmune serum was added, followed by a goat anti-hamster IgG-biotin conjugate (Sigma-Aldrich, St. Louis, MO), and the reactions were completed with an avidin-peroxidase conjugate (BioLegend, San Diego, CA, USA) and OPD (Sigma-Aldrich, St. Louis, MO). The binding of immune or nonimmune purified polyclonal IgG to E. histolytica trophozoites was determined using a FACSCanto II flow cytometer (BD Biosciences). For this, suspensions of 3.5 × 105 log-phase-growth trophozoites were washed twice in PBS, blocked for 15 min on ice with 1.5% bovine serum albumin (BSA) in PBS, incubated with immune or control IgG (100-μg/ml final concentration in PBS with 1.5% BSA) for 30 min on ice, and fixed with 4% paraformaldehyde. Bound IgG was then detected by incubation with a mouse anti-hamster IgG monoclonal antibody cocktail (BD-Pharmingen; 10 μg/ml) followed by an anti-mouse Alexa 488 antibody (Invitrogen; 10 μg/ml).
Hepatic challenge with E. histolytica.
Seven days after the last immunization, hamsters were challenged intrahepatically with an inoculation of 5 × 105 trophozoites in 200 μl of culture medium according to the protocol of Zhang et al. (16). The animals were anesthetized with xylazine and ketamine, and a 2-cm vertical incision was done to externalize the right liver lobe. The parasites were directly injected into the liver using an insulin syringe and a 26-gauge needle. Seven days later, the hamsters were sacrificed by CO2 inhalation and weighed, and the liver was removed and weighed. Serum hepatic transaminase activities were measured using a commercial assay kit (Doles, Goiania, Brazil). Liver samples were fixed in 10% formalin, and the sections were stained by hematoxylin and eosin for histological examination. Tissue lesion scores were determined by a pathologist who was blinded to the sample identity based on both gross pathology and microscopic examination using the following criteria: 0, no lesion; +, a single small sterile lesion with ill-defined borders and dispersed inflammation; ++, a single lesion with defined borders, central necrosis, and focal inflammation; +++, several small abscesses or a single large abscess with associated necrosis, intense inflammation, and amebic trophozoites present; ++++, an abscess involving an entire liver lobe with associated necrosis, intense inflammation, loss of tissue architecture, and amebic trophozoites present. For calculation of vaccine efficacy and statistical analysis, samples with no visible lesion or with small, residual lesions confined to the injection site (scores 0 and +, respectively) were considered negative.
Complement lysis assay.
The abilities of serum and purified IgG from immunized and sham-immunized animals to induce amebic lysis via activation of the classical complement cascade were evaluated using a protocol adapted from the work of Schifferli et al. (29). A suspension of 1 × 106 trophozoites/ml in PBS containing 0.15 mM CaCl2 and 0.5 mM MgCl2 was preincubated with the indicated concentration of purified IgG from immunized and nonimmunized animals for 30 min at 37°C. Normal hamster serum (NHS) served as a source of complement, and following preincubation with IgG, cell lysis resulting from activation of the classical complement cascade was measured by mixing trophozoites with 1 ml of NHS and measuring cell viability by trypan blue exclusion at the indicated time point after the addition of serum. Two types of experiments were conducted: (i) a time course to measure cell lysis after 15, 30, 45, and 60 min following preincubation with 5 μg/ml of purified IgG and (ii) a dose response to measure cell lysis 30 min after addition of serum and preincubation with various concentrations of purified IgG (5.0, 0.5, and 0.05 μg/ml). Pretreatment of some samples for 30 min with 2 mM MgCl2 and 10 mM EGTA was used to block the classical complement pathway and isolate effects of the alternative complement pathway. Heat-inactivated hamster serum (30 min, 56°C) was used as an additional negative control.
Statistics.
Results are expressed as means ± standard errors of the means (SEM). Statistical analyses and graphs were generated using GraphPad Prism 5 software. Statistical significance was determined using the test indicated in each figure legend and a P value cutoff of ≤0.05.
RESULTS
EhMSP-1 is immunogenic in humans and hamsters.
EhMSP-1 is a surface metalloprotease that functions in regulation of E. histolytica adherence and motility (18). To begin assessing if EhMSP-1 might be a useful surface antigen for vaccine development, we used an ELISA to test if people with amebic liver abscesses produce specific anti-EhMSP-1 serum IgG. Measurement of anti-E. histolytica IgG using a commercially available diagnostic kit served as a positive control. Nine of 12 (75%) people with anti-E. histolytica serum IgG antibodies as measured using the commercial RidaScreen assay were positive for anti-EhMSP-1 IgG antibodies (Table 1). We concluded that EhMSP-1 is immunogenic in a large proportion of people during naturally occurring human amebiasis.
TABLE 1.
Prevalence of anti-EhMSP-1 IgG antibodies in South Africans with anti-E. histolytica IgG antibodies as determined by a positive commercial RidaScreen ELISA
| Result typea | No. with anti-EhMSP-1 resultb: |
||
|---|---|---|---|
| Negative | Positive | Total | |
| RidaScreen negative | 2 | 0 | 2 |
| RidaScreen positive | 3 | 9 | 12 |
| Total | 5 | 9 | 14 |
The RidaScreen assay and interpretation were performed according to the manufacturer's instructions.
ELISA for detection of anti-EhMSP-1 IgG was performed using immobilized, recombinant EhMSP-1 as antigen. Positivity was defined as an optical density at 450 nm of ≥2 standard deviations above the mean result for sera that were negative by the RidaScreen test.
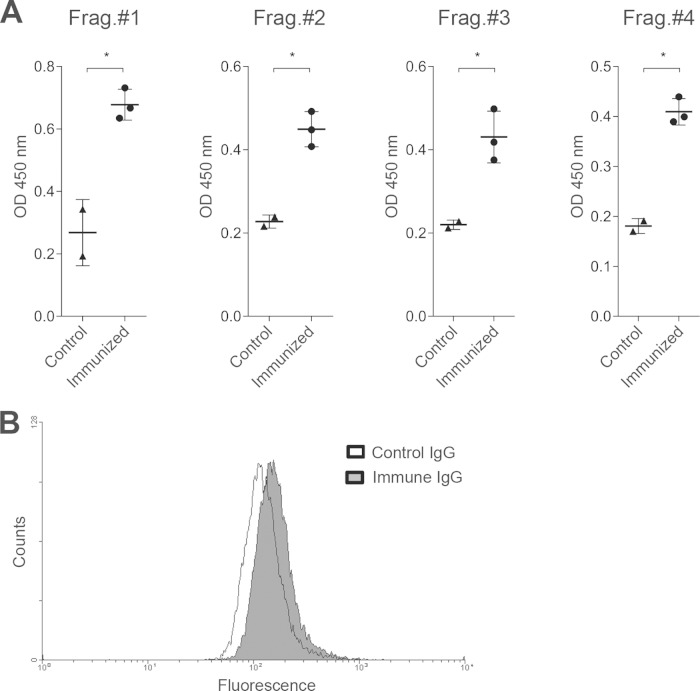
We next used a hamster model and recombinant EhMSP-1 protein fragments to test the immunogenicity of EhMSP-1 in animals. Recombinant protein for immunization was produced in E. coli by cloning gene fragments encoding four EhMSP-1 protein fragments that correspond to the extracellular portion of EhMSP-1 (Fig. 1A). The quality of the purified protein fragments was verified by SDS-PAGE and silver staining (Fig. 1B), and contaminating endotoxin was removed prior to use. Hamsters were immunized with four weekly intraperitoneal injections of 50 μg of each fragment emulsified with Freund's complete and incomplete adjuvant (see also “Vaccination regimen” in Materials and Methods). ELISAs performed on serum collected 7 days after the final immunization demonstrated the presence of specific IgG antibodies to each of the EhMSP-1 fragments (Fig. 2A). Since the recombinant EhMSP-1 protein fragments could be folded aberrantly, we also used immunofluorescence staining and flow cytometry to check if antibodies from the immunized animals recognize native EhMSP-1 present on the surface of E. histolytica trophozoites. For this, purified polyclonal IgG from immunized or control animals was used to stain serum-starved trophozoites. Immune IgG bound the amebic trophozoites more efficiently than did nonimmune IgG (Fig. 2B). Based on these data, we decided to test if EhMSP-1 immunization would protect from amebic liver abscess.
FIG 2.
IgG antibody production in response to EhMSP-1 vaccination. (A) ELISA results demonstrating generation of IgG antibodies to each EhMSP-1 protein fragment. Undiluted immune or control (sham-immunized animal) serum was used to probe each immobilized recombinant protein fragment, followed by development with an anti-hamster IgG-biotin conjugate, avidin-horseradish peroxidase, and OPD. Each data point represents the result for an individual animal, along with the mean optical density (OD) and SEM for each condition. *, P < 0.05 by Student's t test. (B) Binding of purified IgG from immunized and control hamsters to E. histolytica trophozoites. After blocking with bovine serum albumin, E. histolytica trophozoites were incubated with IgG purified from either EhMSP-1-immunized or control animals on ice for 30 min, followed by fixation and detection of bound IgG with a mouse anti-hamster IgG antibody and then anti-mouse Alexa 488 antibody. Fluorescence was analyzed by flow cytometry. Representative fluorescence histograms are shown. The mean fluorescence intensity for trophozoites stained with immune IgG was greater than that for those stained with control IgG (196.2 ± 10.2 versus 167.6 ± 6.1, respectively, mean and SD, n = 3; P = 0.01 by Student's t test).
EhMSP-1 vaccination protects from ALA.
Direct inoculation of E. histolytica trophozoites into hamster livers results in abscesses with pathology that closely resembles the pathology seen in human disease. We selected this model of amebic liver abscess (ALA) for proof-of-concept vaccine studies with EhMSP-1, because the methods for vaccine delivery (e.g., adjuvant selection and intraperitoneal injection) and for scoring vaccine efficacy are well established (30, 31). Hamsters were immunized with EhMSP-1 protein fragments or sham immunized as described in Materials and Methods (see “Vaccination regimen”) and then challenged 7 days after the final immunization by intrahepatic injection of 5 × 105 trophozoites in 200 μl of culture medium. To determine vaccine efficacy, macroscopic and histologic lesions were scored by a blinded pathologist. EhMSP-1 vaccination protected from severe ALA in both of two independent experiments, while the majority of control animals developed severe disease (Table 2 and Fig. 3). When the data from the two experiments were combined, 52% of control animals developed severe liver abscesses, while only 17% of immunized animals developed disease (two-tailed Fisher exact test, P = 0.005); the overall vaccine efficacy was 68%. Liver transaminases are released during liver damage, so we measured liver transaminase levels in the serum to further assess liver damage. Consistent with the protection seen on pathological examination, serum liver transaminase levels were significantly lower in immunized animals than in control animals (Fig. 4).
TABLE 2.
Development of amebic liver abscesses following intrahepatic challenge of EhMSP-1-immunized or control hamsters with E. histolytica
| Trialb | Group | No. with amebic liver abscess/no. challenged (%) | Vaccine efficacy (%) | P valuea |
|---|---|---|---|---|
| Expt 1 | Immunized | 1/9 (11) | 78 | 0.04 |
| Control | 4/8 (50) | |||
| Expt 2 | Immunized | 3/15 (20) | 63 | 0.03 |
| Control | 8/15 (53) | |||
| Combined | Immunized | 4/24 (17) | 68 | 0.005 |
| Control | 12/23 (52) |
Two-tailed Fisher's exact test.
Hamsters were immunized weekly for 4 weeks by intraperitoneal injection of a mixture of 100 μl of saline containing 50 μg each of four EhMSP-1 protein fragments emulsified with 50 μl of complete Freund's adjuvant and 50 μl of incomplete Freund's adjuvant. They were challenged by intrahepatic inoculation of 5 × 105 E. histolytica trophozoites 1 week after the final immunization.
FIG 3.
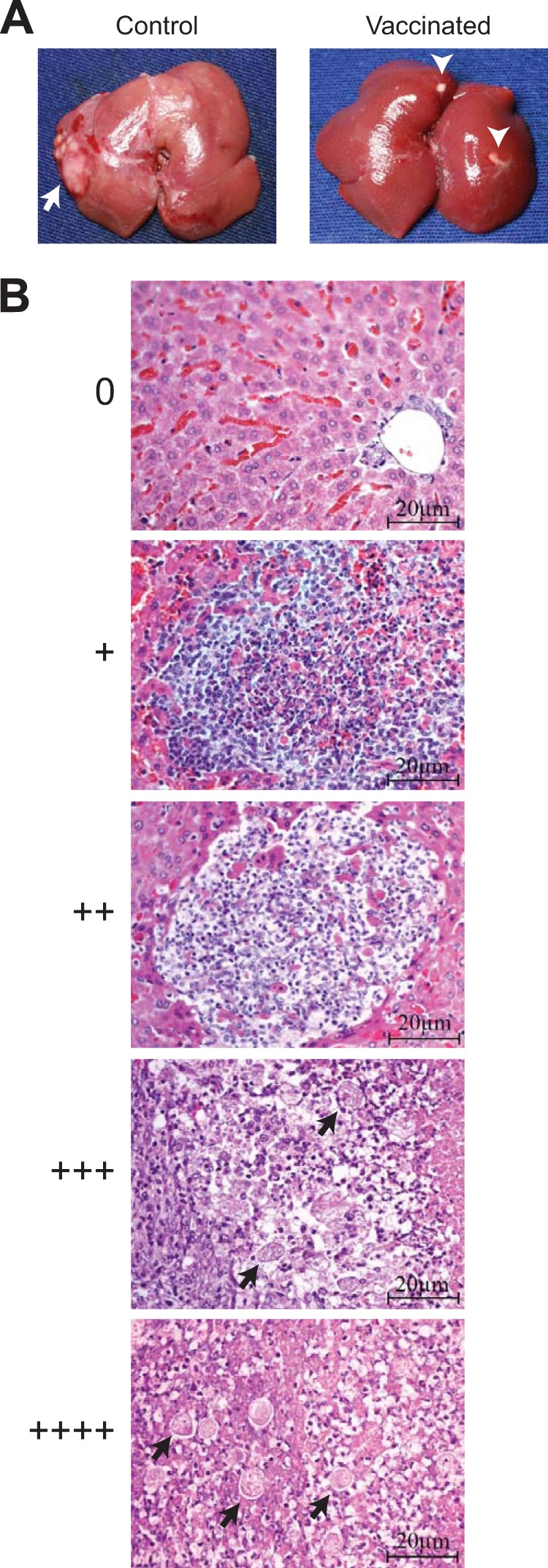
Liver pathology and scoring system for the hamster amebic liver abscess model. (A) Gross liver pathology was scored by a blinded pathologist 7 days after intrahepatic challenge of vaccinated and control hamsters with E. histolytica. Most vaccinated animals had small, sterile lesions visible at the injection site at this time point, while the majority of control (sham-immunized) animals had well-developed liver abscesses (arrow). Note that the arrowheads in the image on the right indicate normal fat deposits on the liver surface. No lesion (0) or a small residual focus (+) was considered negative for calculation of vaccine efficacy. (B) Histopathology and correlation with macroscopic scoring system. Representative photomicrographs of hematoxylin-and-eosin-stained liver tissue are shown, with 20-μm scale bars. Amebic trophozoites (arrows) and tissue necrosis were readily apparent in lesions scored +++ and ++++. Lesions scored ++ were distinguished from lesions scored + by the presence of a defined border and central tissue destruction.
FIG 4.
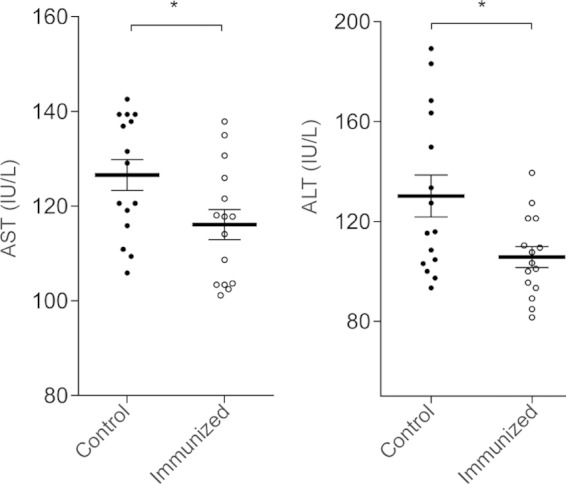
Serum liver transaminase levels in vaccinated and control hamsters challenged by intrahepatic injection of E. histolytica. Serum was collected at the time of sacrifice 7 days after challenge in animal experiment 2. AST (left), aspartate aminotransferase; ALT (right), alanine aminotransferase. Each data point represents the assay results for one animal (n = 15 per experimental group, mean and SEM; *, P < 0.05 by Student's t test).
EhMSP-1 immunization accelerates complement-mediated lysis of E. histolytica.
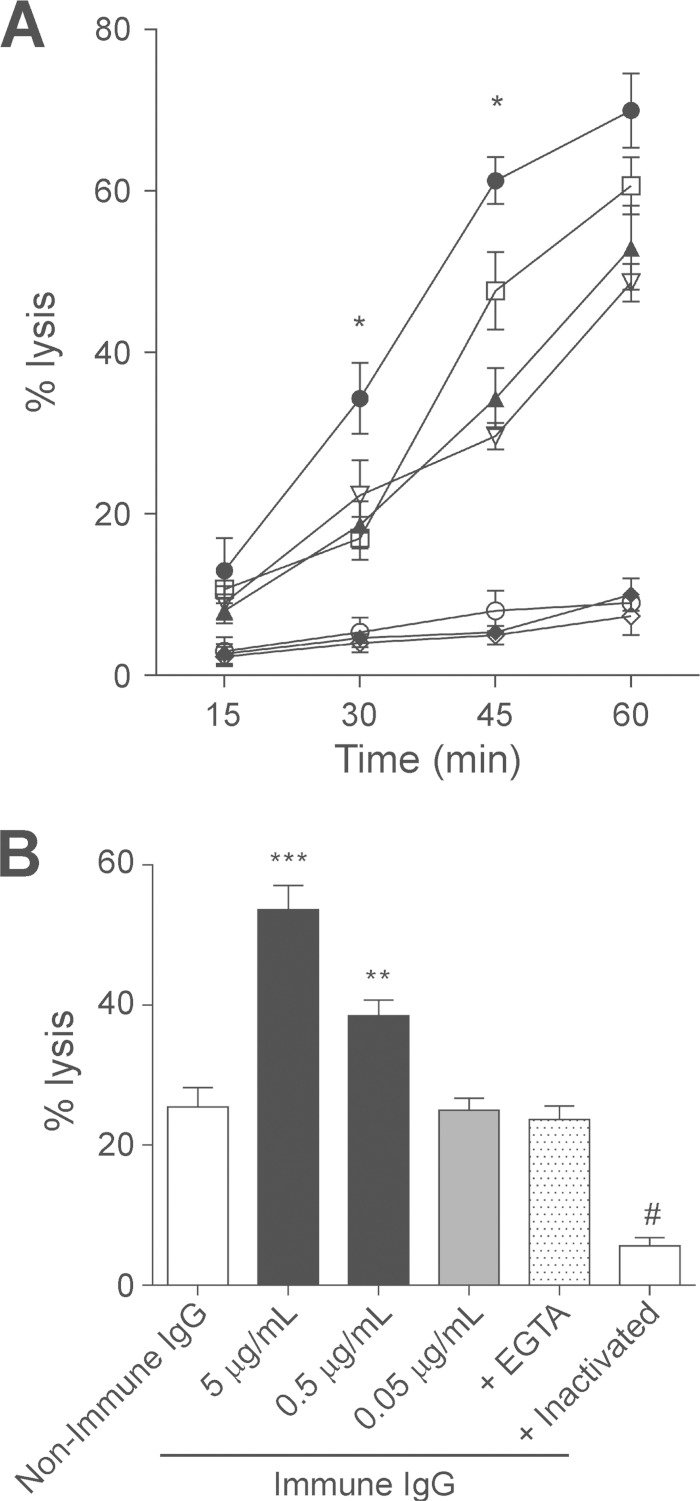
Resistance to serum complement correlates with E. histolytica virulence, but antibodies directed to surface antigens may activate the classical complement pathway and facilitate cell lysis (32, 33). Therefore, we tested the ability of IgG from EhMSP-1-immunized animals to stimulate cell lysis via activation of the classical complement cascade. Entamoeba histolytica trophozoites exposed to serum (as a source of complement) and IgG from immunized animals lysed more rapidly than trophozoites exposed to IgG from control animals (Fig. 5A). This effect was dose dependent (Fig. 5B) and due to specific activation of the classical complement cascade, since it was abolished by addition of Mg2+-EGTA. Nevertheless, cell lysis proceeded in the presence of EGTA, indicating slower, antibody-independent activation of the alternative complement pathway by E. histolytica trophozoites. This was consistent with previously published data (33). Spontaneous cell lysis in all assays was minimal. Collectively, the data indicated that immunization with EhMSP-1 fragments stimulates production of complement-fixing IgG antibodies that accelerate amebic lysis.
FIG 5.
EhMSP-1 vaccination facilitates lysis of E. histolytica trophozoites through activation of the classical complement cascade. (A) Kinetics of amebic lysis via the classical complement cascade following in vitro exposure to polyclonal IgG from immunized and nonimmunized animals. Amebae were preincubated with purified IgG (5 μg/ml) from either immune or nonimmune animals, followed by addition of normal hamster serum and evaluation of cell lysis at the indicated times. Heat-inactivated serum and EGTA, which inhibits the classical complement cascade, served as additional controls for specificity. Conditions: [circf], immune IgG plus normal human serum; [squlo], nonimmune IgG plus normal human serum; [trif], immune IgG plus normal human serum plus EGTA; [itrio], nonimmune IgG plus normal human serum plus EGTA; [diaf], immune IgG plus inactivated serum; [circo], nonimmune IgG plus inactivated serum; [diao], only amebae plus inactivated serum. *, P < 0.05 versus nonimmune IgG plus normal serum (n = 3, mean and SEM). (B) Dose effect of purified IgG from EhMSP-1-vaccinated animals on amebic lysis. Amebic trophozoites were preincubated with purified IgG from immunized (immune) and control (nonimmune) animals at either 5 μg/ml or the indicated concentration. Cell lysis was measured 30 min following addition of normal human serum. Incubation in the presence of EGTA or heat-inactivated serum served as an additional control for specificity. P was <0.001 for *** and #, and P was <0.05 for ** versus nonimmune IgG control in each case (n = 3, mean and SEM).
DISCUSSION
Entamoeba histolytica has no known animal reservoir. Thus, an effective vaccine to prevent amebiasis could conceivably enable global eradication of Entamoeba histolytica (7, 34). Here, we showed that vaccination of animals with recombinant fragments of the recently described amebic metallosurface proteinase EhMSP-1 significantly reduces the occurrence of severe amebic liver abscess and, as evidenced by measurement of serum transaminase levels, liver injury following direct hepatic challenge with E. histolytica. EhMSP-1 is also immunogenic in the majority of naturally occurring human E. histolytica infections. IgG antibodies produced in response to EhMSP-1 vaccination bind the surface of amebic trophozoites and facilitate amebic lysis via activation of the classical complement cascade, providing a potential mechanism of protection. These data serve as proof of principle establishing EhMSP-1 as a candidate antigen for inclusion in a multivalent E. histolytica vaccine.
Our study is significant because identification of additional protective antigens such as EhMSP-1 may aid in developing an effective multivalent vaccine. As noted above, published data demonstrate that natural immunity to amebiasis correlates with lectin-specific secretory IgA but is limited to approximately 20% of infected children (8–10). Thus, previously published data simultaneously provide hope that a vaccine can be developed but also indicate that it may be necessary to include antigens in addition to the GalNAc lectin to achieve protection in the majority of children. Vaccination with EhMSP-1 fragments was 68% effective at preventing severe liver abscesses following direct inoculation of trophozoites in two independent animal experiments. Thus, protection from liver abscess by EhMSP-1 vaccination is comparable to the level of protection observed with other amebic antigens, including the GalNAc-specific adherence lectin (67% protective in initial trials) (14), a combination of a cysteine proteinase (EhCP112) and an adhesin (EhADH112) (72% effective) (30), and the SREHP (64 to 100% effective) (16).
These initial studies support the potential of EhMSP-1 as a protective antigen, but additional work is required. We chose to test the ability of EhMSP-1 vaccination in a model of liver abscess, because the model and method for delivery of the antigen to stimulate production of IgG are well established (30, 31). However, an essential next step for assessing the potential of vaccination with EhMSP-1 will be to determine if it also protects from intestinal disease, which is the main cause of morbidity due to amebiasis. Stimulating production of anti-EhMSP-1 secretory IgA (sIgA) may require alternative vaccination regimens, possibly including alternative adjuvants. GalNAc-specific lectin-based vaccines are currently the only amebic antigens that have demonstrated efficacy in a colitis model (15). In addition, our data show that humans infected with Entamoeba histolytica produce serum IgG antibodies, but stop short of determining if sIgA antibodies specific for EhMSP-1 are produced during naturally occurring amebic colitis. Furthermore, it is unknown if the presence of anti-EhMSP-1 sIgA is correlated with natural protection. A larger study of children with amebic colitis would be necessary to examine this question. Finally, while our data suggest that EhMSP-1 vaccination protects from liver abscess formation by stimulating production of complement-fixing IgG antibodies, the previously demonstrated role of EhMSP-1 in control of E. histolytica adherence and motility raises the possibility that EhMSP-1 vaccination is protective because the antibodies generated interfere with its function (18). This possibility remains to be investigated. Such follow-up work should include studies to map the protective epitopes and also to determine if IgG antibodies generated in response to EhMSP-1 vaccination inhibit EhMSP-1 proteolytic activity. Additional studies using in vivo depletion of complement could also determine the relative contributions of complement activation and/or interference with EhMSP-1 function to protection following vaccination. These studies are planned and may help to determine the potential of EhMSP-1 for producing a durable vaccine versus selection of E. histolytica EhMSP-1 mutants that enable immune evasion as a result of vaccination-induced immune pressure.
In summary, the results presented here provide support for further development of EhMSP-1 as a target for vaccination to prevent invasive amebiasis. Further studies are under way to extend these results and determine the precise mechanism of protection following EhMSP-1 vaccination.
ACKNOWLEDGMENTS
We thank members of the Huston and Barbosa laboratories for helpful discussions.
This work was supported by NIAID R01 AI072021 and NIGMS P20 GM103496 to C.D.H.
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Korpe PS, Liu Y, Siddique A, Kabir M, Ralston K, Ma JZ, Haque R, Petri WA Jr. 2013. Breast milk parasite-specific antibodies and protection from amebiasis and cryptosporidiosis in Bangladeshi infants: a prospective cohort study. Clin Infect Dis 56:988–992. doi: 10.1093/cid/cis1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque R, Ali IM, Petri WA Jr. 1999. Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. Am J Trop Med Hyg 60:1031–1034. [DOI] [PubMed] [Google Scholar]
- 5.Taniuchi M, Sobuz SU, Begum S, Platts-Mills JA, Liu J, Yang Z, Wang XQ, Petri WA Jr, Haque R, Houpt ER. 2013. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J Infect Dis 208:1794–1802. doi: 10.1093/infdis/jit507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haque R, Huston CD, Hughes M, Houpt E, Petri WA. 2003. Amebiasis. N Engl J Med 348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 7.Stanley SL., Jr 1997. Progress towards development of a vaccine for amebiasis. Clin Microbiol Rev 10:637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA. 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis 183:1787–1793. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 9.Haque R, Mondal D, Duggal P, Kabir M, Roy S, Farr BM, Sack RB, Petri WA. 2006. Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect Immun 74:904–909. doi: 10.1128/IAI.74.2.904-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque R, Duggal P, Ali IM, Hossain MB, Mondal D, Sack RB, Farr BM, Beaty TH, Petri WA. 2002. Innate and acquired resistance to amebiasis in Bangladeshi children. J Infect Dis 186:547–552. doi: 10.1086/341566. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Stanley SLJ. 1994. Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect Immun 62:2605–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinets A, Zhang T, Guillen N, Gounon P, Bohle B, Vollmann U, Scheiner O, Wiedermann G, Stanley SL, Duchene M. 1997. Protection against invasive amebiasis by a single monoclonal antibody directed against a lipophosphoglycan antigen localized on the surface of Entamoeba histolytica. J Exp Med 186:1557–1565. doi: 10.1084/jem.186.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soong CJ, Torian BE, Abd-Alla MD, Jackson TF, Gatharim V, Ravdin JI. 1995. Protection of gerbils from amebic liver abscess by immunization with recombinant Entamoeba histolytica 29-kilodalton antigen. Infect Immun 63:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri WA Jr, Ravdin JI. 1991. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun 59:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houpt E, Barroso L, Lockhart L, Wright R, Cramer C, Lyerly D, Petri WA. 2004. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22:611–617. doi: 10.1016/j.vaccine.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Cieslak PR, Stanley SL. 1994. Protection of gerbils from amebic liver abscess by immunization with a recombinant Entamoeba histolytica antigen. Infect Immun 62:1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd Alla MD, Wolf R, White GL, Kosanke SD, Cary D, Verweij JJ, Zhang MJ, Ravdin JI. 2012. Efficacy of a Gal-lectin subunit vaccine against experimental Entamoeba histolytica infection and colitis in baboons (Papio sp.). Vaccine 30:3068–3075. doi: 10.1016/j.vaccine.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira JE, Sateriale A, Bessoff KE, Huston CD. 2012. Control of Entamoeba histolytica adherence involves metallosurface protease 1, an M8 family surface metalloprotease with homology to leishmanolysin. Infect Immun 80:2165–2176. doi: 10.1128/IAI.06389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao C, Donelson JE, Wilson ME. 2003. The major surface protease (MSP or GP63) of Leishmania sp. biosynthesis, regulation of expression, and function. Mol Biochem Parasitol 132:1–16. doi: 10.1016/S0166-6851(03)00211-1. [DOI] [PubMed] [Google Scholar]
- 20.Singh B, Sundar S. 2012. Leishmaniasis: vaccine candidates and perspectives. Vaccine 30:3834–3842. doi: 10.1016/j.vaccine.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 21.Aurrecoechea C, Brestelli J, Brunk BP, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer ET, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Srinivasamoorthy G, Stoeckert CJ, Thibodeau R, Treatman C, Wang H. 2010. EuPathDB: a portal to eukaryotic pathogen databases. Nucleic Acids Res 38:D415–D419. doi: 10.1093/nar/gkp941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perosa F, Carbone R, Ferrone S, Dammacco F. 1990. Purification of human immunoglobulins by sequential precipitation with caprylic acid and ammonium sulphate. J Immunol Methods 128:9–16. doi: 10.1016/0022-1759(90)90458-8. [DOI] [PubMed] [Google Scholar]
- 23.Diamond LS, Harlow DR, Cunnick CC. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72:431–432. doi: 10.1016/0035-9203(78)90144-X. [DOI] [PubMed] [Google Scholar]
- 24.Bhol KC, Mukherjee RM, Banerjee M, Maitra TK, Jalan KN. 1991. Enhancement of virulence of Entamoeba histolytica by in vitro liver treatment. Ann Trop Med Parasitol 85:341–344. [DOI] [PubMed] [Google Scholar]
- 25.Qiagen. June 2003. The QIAexpressionist. A handbook for high-level expression and purification of 6xHis-tagged proteins, 5th ed. Qiagen, Valencia, CA. [Google Scholar]
- 26.Rehm BH, Qi Q, Beermann BB, Hinz HJ, Steinbuchel A. 2001. Matrix-assisted in vitro refolding of Pseudomonas aeruginosa class II polyhydroxyalkanoate synthase from inclusion bodies produced in recombinant Escherichia coli. Biochem J 358:263–268. doi: 10.1042/0264-6021:3580263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malyala P, Singh M. 2008. Endotoxin limits in formulations for preclinical research. J Pharm Sci 97:2041–2044. doi: 10.1002/jps.21152. [DOI] [PubMed] [Google Scholar]
- 28.Yan W, Faisal SM, McDonough SP, Divers TJ, Barr SC, Chang CF, Pan MJ, Chang YF. 2009. Immunogenicity and protective efficacy of recombinant Leptospira immunoglobulin-like protein B (rLigB) in a hamster challenge model. Microbes Infect 11:230–237. doi: 10.1016/j.micinf.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Schifferli J, Woo P, Peters D. 1982. Complement-mediated inhibition of immune precipitation. I. Role of the classical and alternative pathways. Clin Exp Immunol 47:555–562. [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Lopez C, Orozco E, Sanchez T, Garcia-Perez RM, Hernandez-Hernandez F, Rodriguez MA. 2004. The EhADH112 recombinant polypeptide inhibits cell destruction and liver abscess formation by Entamoeba histolytica trophozoites. Cell Microbiol 6:367–376. doi: 10.1111/j.1462-5822.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheng XJ, Tachibana H. 2001. Protection of hamsters from amebic liver abscess formation by immunization with the 150- and 170-kDa surface antigens of Entamoeba histolytica. Parasitol Res 87:126–130. doi: 10.1007/s004360000323. [DOI] [PubMed] [Google Scholar]
- 32.Reed SL, Sargeaunt PG, Braude AI. 1983. Resistance to lysis by human serum of pathogenic Entamoeba histolytica. Trans R Soc Trop Med Hyg 77:248–253. doi: 10.1016/0035-9203(83)90083-4. [DOI] [PubMed] [Google Scholar]
- 33.Reed SL, Curd JG, Gigli I, Gillin FD, Braude AI. 1986. Activation of complement by pathogenic and nonpathogenic Entamoeba histolytica. J Immunol 136:2265–2270. [PubMed] [Google Scholar]
- 34.Huston CD, Petri WA Jr. 1998. Host-pathogen interaction in amebiasis and progress in vaccine development. Eur J Clin Microbiol Infect Dis 17:601–614. doi: 10.1007/s100960050143. [DOI] [PubMed] [Google Scholar]