Abstract
Purpose.
To quantify and compare phase retardation amplitude and regularity associated with the Henle fiber layer (HFL) between nonexudative AMD patients and age-matched controls using scanning laser polarimetry (SLP) imaging.
Methods.
A scanning laser polarimeter was used to collect 15 × 15° macular-centered images in 25 patients with nonexudative AMD and 25 age-matched controls. Raw image data were used to compute macular phase retardation maps associated with the HFL. Consecutive, annular regions of interest from 0.5 to 3.0° eccentricity, centered on the fovea, were used to generate intensity profiles from phase retardation data and analyzed with two complementary techniques: a normalized second harmonic frequency (2f) of the fast Fourier Transform (FFT) analysis and a curve fitting analysis using a 2f sine function. Paired t-tests were used to compare the normalized 2f FFT magnitude at each eccentricity between the two groups, the eccentricity that yielded the maximum normalized 2f FFT between paired individuals across the two groups, and curve fitting RMS error at each eccentricity between the two groups.
Results.
Normalized 2f FFT components were lower in the AMD group at each eccentricity, with no difference between the two groups in the maximum normalized 2f FFT component eccentricity. The root-mean-square (RMS) error from curve fitting was significantly higher in the AMD group.
Conclusions.
Phase retardation changes in the central macula indicate loss and/or structural alterations to central cone photoreceptors in nonexudative AMD patients. Scanning laser polarimetry imaging is a noninvasive method for quantifying cone photoreceptor changes associated with central macular disease.
Keywords: Henle fiber layer, scanning laser polarimetry, photoreceptors, age-related macular degeneration
Polarization sensitive imaging can be used to quantify changes to specific cellular structures in the retina, including the Henle fiber layer. The phase retardation signal originating from the Henle fiber layer is altered in patients with non-exudative AMD with good visual acuity.
Introduction
The photoreceptors in the central macula are essential to normal visual function, including our highest spatial acuity and color vision. These photoreceptors are prone to damage in a number of progressive, sight-threatening retinal diseases including AMD,1–6 the leading cause of blindness in individuals aged older than 65 years in the United States.7–12 Because of their small size and high packing density, compounded by the degradation of optical media in the human eye with age, the photoreceptors in and around the fovea are difficult to assess directly, even with the most sophisticated high-resolution imaging techniques.13–17 This becomes even more difficult in patients with AMD, where considerable variation in image quality often precludes the visualization of even the larger nonfoveal cones, influenced by poor fixation; higher rates of tear film abnormalities; and media opacities, such as cataracts.18 As new modalities become available for the treatment of retinal disease, assessment of these photoreceptors becomes increasingly important, making indirect measures of density and regularity a valuable complementary tool.
The central macula is highly organized, with structural characteristics unique to that location. Unlike the more peripheral retina, inner cells of the central macula are displaced laterally, giving rise to the characteristic topographical depression known as the foveal pit. These displaced photoreceptor axons constitute the Henle fiber layer (HFL), with some individual axons extending more than 700 μm.18 Within a few degrees of the foveal center, these axons splay out in a predominantly radially symmetric pattern with respect to the central macula,18 where the cone axons are obliquely oriented. At eccentricities greater than a few degrees from the fovea, all retinal layers are present in a laminar arrangement, and orientation of these axons changes to become more parallel to incident light, with directional OCT studies indicating that photoreceptor axons do not become parallel to incident light until eccentricities beyond 10 degrees from fixation.19
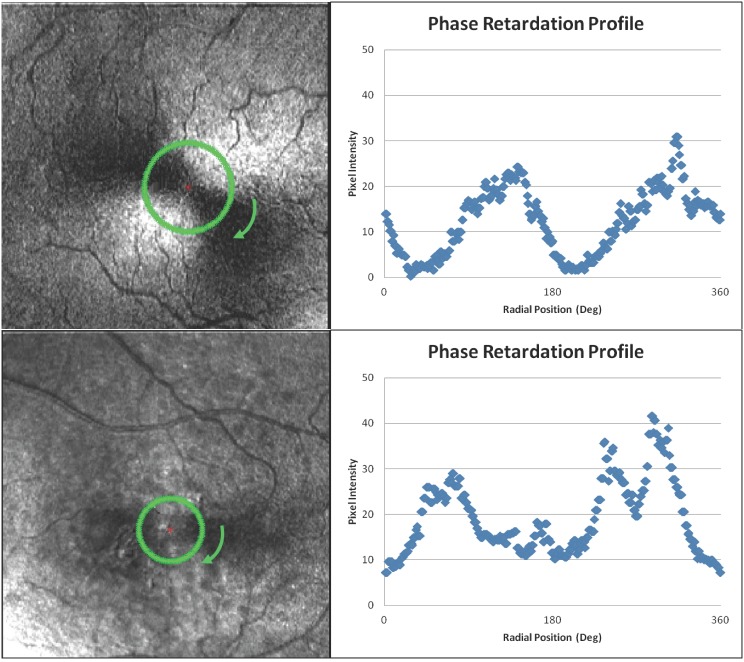
The unique orientation and packing geometry, together with the packing arrangement of subcellular components, cause the photoreceptor axons of the HFL to exhibit the optical property known as birefringence, a measure of phase retardation per unit distance. Analogous to a uniaxial crystal, axons in the HFL retard light in one orientation of polarization more than another.20–22 The interaction of the birefringent cornea and the HFL creates a characteristic macular cross pattern when imaged using scanning laser polarimetry (SLP) techniques (Fig. 1, top- and bottom-left), isolating the HFL based on its intrinsic optical properties.
Figure 1.
Top-left: Macular cross pattern from a 77-year-old clinically normal subject with annular region of interest at the eccentricity of the maximum normalized 2f and 4f FFT (1.9°). Top-right: Phase retardation profile at that eccentricity demonstrating a clear 2f function. Bottom-left: Macular cross pattern from a 68-year-old nonexudative AMD subject with annular region of interest at the eccentricity of the maximum normalized 2f and 4f FFT (1.4°). Bottom-right: Phase retardation profile at that eccentricity demonstrating a disrupted 2f function. It is worth noting that the images in the raw datasets have low intensity, making retinal features and the macular cross pattern itself difficult to visualize. The contrast of the images presented in this figure has been stretched for improved visualization. The data presented in the graphs and the data used for analysis were derived from the raw data and not the contrast-enhanced images.
The macular cross pattern has a number of attributes, consistent across individuals, that can be used to model and quantify the foveal architecture using phase retardation as a surrogate marker for photoreceptor regularity and density. Within 0.5° of the fovea center, there is little phase retardation due to the displacement of nearly all retinal structures, including photoreceptor cell bodies. At eccentricities between 0.5 and 2.5°, the macular cross pattern is radially symmetric with respect to the center of the fovea.23 Systematic variation in phase retardation along concentric rings can be described by a sine function with twice the frequency (2f) or four times the frequency (4f) of the ring (Fig. 1, top- and bottom-right). In normal subjects, the phase retardation signal in SLP imaging increases monotonically between 0.5 and approximately 1.25°,23,24 consistent with contributions of a larger cumulative number of cone photoreceptor axons and consistent with polarization sensitive OCT imaging.25 This radial thickness- and orientation-dependent signal reaches its maximum at approximately 1.25 to 1.5°, plateaus, and decreases at eccentricities beyond 2.5° as photoreceptor axons take on an increasingly parallel orientation relative to incident light. At greater eccentricities, the dominant phase retardation signal originates from other structures, such as the retinal nerve fiber layer, and at these eccentricities, there is little or no phase retardation signal associated with the HFL.23–25
Fast Fourier transform (FFT) and curve fitting analysis can be used to quantify the structural regularity of the HFL.24 Amplitude of curve fitting using a sine function and normalized components of the FFT provide information regarding photoreceptor axon density, while curve fitting RMS error can be used to quantify regularity.23,24 Changes in both maximum eccentricity and phase retardation amplitude occur as part of normal aging,23,24 suggesting a loss or reorganization of the photoreceptor mosaic in the central macula. This is consistent with complementary methods, such as cone photopigment mapping26 or cone photoreceptor counting.17
Diseases that affect the central macula result in disruption to the HFL and the normally well-ordered macular cross pattern. Changes in the phase retardation associated with the HFL have been demonstrated in central serous chorioretinopathy27 and AMD.28–30 The macular cross pattern is robust and persists even in patients with extensive pathology such as exudative AMD, with irregularity correlating with reduced visual acuity.30 This is consistent with histology, where foveal cones are present, albeit severely disrupted and reduced in density, in locations overlying pathological features in nonexudative and exudative AMD lesions.31 In this study, we characterized the macular cross pattern in patients with nonexudative AMD who have good visual acuity and age-matched control subjects, examining the radial pattern of phase retardation as a surrogate marker for foveal cones. To provide insight into the pathological mechanisms underlying AMD, we compared the amplitude of phase retardation, the regularity of the macular cross pattern, and the eccentricity that yields the maximum phase retardation signal associated with the HFL.
Methods
2.1 Subjects
We included 25 subjects with nonexudative AMD and 25 age-matched controls. All subjects had a comprehensive eye examination within the previous year to confirm their ocular health status. Individuals in the AMD group had clinical findings of intermediate nonexudative AMD with visual acuity better than 20/40. None of the subjects exhibited geographic atrophy or choroidal neovascularization, both on clinical examination and imaging. Several subjects did have higher risk characteristics, including large drusen in 100% of the subjects and pigment clumping in 40%. Subjects in this group, 8 males, 17 females, were aged 56 to 80 years (average 70.2 ± 7.0 years). Individuals in the age-matched control group had no clinical findings of retinal pathology, other than changes considered to be related to normal aging. Subjects in the control group, 7 males, 18 females, were aged 59 to 81 years (average 69.8 ± 6.5 years). Control subjects were required to have visual acuity of 20/20 or better, unless reduced acuity could be attributed to normal aging lens opacification. Patients with systemic diseases that carry a high likelihood of ocular manifestations were excluded from both groups. Refractive errors in both groups were restricted to ± 6 diopters (D) of ammetropia.
Subjects were recruited from the Indiana University School of Optometry (Bloomington, IN, USA), the Department of Ophthalmology, University Hospital (Aachen, Germany), and the Schepens Eye Institute (Boston, MA, USA) following the human subjects protocol procedures at the respective institutions. Informed consent was obtained from all subjects after explanation of the nature and possible consequences of the study. The research followed the tenets of the Declaration of Helsinki.
2.2 Instrumentation and Imaging
Instrumentation and image acquisition techniques used for this study have been described previously in detail.24,28,30,32–36 In summary, a GDx confocal scanning laser polarimeter was used to collect 15 × 15° macular-centered images in each subject by raster scanning a 780-nm linearly polarized light source on the retina. The instrument used in this study has a fixed birefringent element with a magnitude of 60 nm (single pass retardance) and a fast axis oriented at 15° nasally downward to compensate for corneal birefringence.37 Corneal compensation is incomplete with this technique, resulting in a macular cross pattern in each subject. Light returning from the eye is collected by two polarization sensitive detectors: a parallel detector collecting light with the same polarization as the input light and a crossed detector collecting light with polarization that is orthogonal to the input polarization. Each detector produces images, collected in pairs, at 20 different input polarization conditions with 256 × 256 pixel resolution and 8-bit gray scale.
Images were randomly acquired in one eye of each subject (12 right eyes, 13 left eyes for each group) noninvasively and without mydriasis. Three datasets were acquired for each subject, and the best was chosen for analysis to ensure images were well-focused, adequately and evenly illuminated, and without motion artifacts.
2.3 Image Processing
Data from the crossed detector of the GDx were used for analysis. Raw image data were exported and processed using custom computing routines (MATLAB; MathWorks, Natick, MA, USA).23,24,27,32,33 At each pixel location, the modulated intensity across the 20-input polarizations was used to generate local phase retardation values. Combined, these values provide a representation of phase retardation over the 15 × 15° retinal area with 256 × 256 pixel resolution. There is an inherent central reflection artifact with the GDx. Prior to data analysis, this artifact was manually removed and replaced column-by-column with the average of the first pixel above and the first pixel below the reflection artifact.
2.4 Data Analysis and Modeling
Regions of interest for phase retardation profiles were generated from concentric rings ranging continuously in 1-pixel radius increments from 0.5 to 3.0° eccentricity centered on the fovea (Fig. 1, top- and bottom-left). To reduce noise, each profile was averaged with the immediately adjoining inner and outer profile. In normal subjects, these phase retardation profiles from circular regions of interest generate sine functions with frequencies that are primarily twice that of the ring (a 2f component) or four times that of the ring (a 4f component). Two complementary techniques were used to analyze these characteristic profiles: a normalized FFT component analysis and a curve fitting analysis using a 2f sine function. For the FFT analysis, we combined the 2f and 4f component of each phase retardation profile from the circular regions of interest and normalized by the remaining components, since some normal subjects have double the number of arms in their macular cross patterns. Assuming radial symmetry of the HFL, this normalized 2f FFT component gives the relative contribution of the HFL to the overall phase retardation profile, an indirect measure of photoreceptor density. The eccentricity that yields the maximum normalized 2f FFT component acts to identify alterations to the photoreceptor distribution density in the central macula. For the curve fitting analysis, each phase retardation profile from a circular region of interest was fit to a 2f sine curve. The resulting RMS error gives an indication of goodness of fit, used to assess photoreceptor distribution regularity.
2.4.1 Phase Retardation Amplitude.
The normalized 2f FFT, a measure related to the phase retardation amplitude, was used as a surrogate marker for photoreceptor density in the central macula. Within each of the two groups, the normalized 2f FFT component was averaged at each eccentricity between 0.5 and 3.0 deg. This averaged normalized 2f FFT value at each eccentricity was compared between the AMD subjects and matched controls using a paired t-test. To quantify variability in the normalized 2f FFT in each group, the coefficient of variation (COV, standard deviation/mean) was calculated at each eccentricity and compared between the 2 groups using a paired t-test.
2.4.2 Maximum Phase Retardation Eccentricity.
The eccentricity that yielded the maximum normalized 2f FFT component was used to examine differential effects in photoreceptor density across the central macula. The maximum normalized 2f FFT eccentricity was compared between AMD subjects and matched controls using a paired t-test.
2.4.3 Curve Fitting RMS Error.
The root-mean-square error from curve fitting, an estimate of the goodness of fit for a 2f sine function to the phase retardation profiles, was used as a measure of photoreceptor axon regularity in the central macula. Within each of the two groups, the RMS error was averaged at each eccentricity between 0.5 and 3.0°. This averaged RMS error value at each eccentricity was compared between the AMD subjects and matched controls using a paired t-test.
Results
3.1 Phase Retardation Amplitude
An intact macular cross pattern was present in all subjects in both groups. Subjects with AMD had a lower normalized 2f FFT component compared with age-matched controls (paired t-test, P < 0.001). Figure 2 (top) shows the average value of phase retardation across the different eccentricities for both the AMD and matched control groups. We found considerable variability in HFL phase retardation, even in the control group, consistent with previous findings obtained with different subjects and a different instrument. Average COV of the normalized 2f FFT was higher in the AMD group than in matched controls (paired t-test, P < 0.001) and average COV between 0.5 and 3.0° was 0.295 (range, 0.244 to 0.355) and 0.344 (range, 0.273 to 0.443) in the control and AMD groups, respectively. Despite this variability, the average normalized 2f FFT value remained lower in the AMD group at all eccentricities.
Figure 2.
Top: The averaged normalized 2f FFT for each of the two subject groups for eccentricities between 0.5 and 3.0° demonstrating a smaller normalized 2f FFT for the AMD group at each eccentricity. Bottom: The averaged RMS error from 2f curve fitting for each of the two subject groups for each eccentricity between 0.5 and 3.0° demonstrating higher RMS at each eccentricity resulting from poorer curve fitting in the AMD group.
3.2 Maximum Phase Retardation Eccentricity
The eccentricity that yielded the maximum normalized 2f FFT component did not differ between age-matched individuals in the two groups (paired t-test, P = 0.234). The eccentricity that yielded the maximum normalized 2f FFT component, averaged at each eccentricity within groups, was 1.364° for the AMD subjects and 1.648° for the age-matched control subjects (Fig. 2, top). Phase retardation profiles followed a pattern consistent with previous findings, with little phase retardation close to the foveal center, increasing to a maximum within the central 2°, and leveling off or decreasing at greater eccentricities. The standard deviation for the eccentricity of the maximum normalized 2f FFT component was 0.516° for individuals in the AMD group and 0.303° for individuals in the age-matched control group. This variability resulted in considerable overlap between the two groups and the 95% confidence interval around the control data for the eccentricity of the maximum normalized 2f FFT component contained 21 of the 25 AMD subjects.
3.3 Curve Fitting RMS Error
AMD subjects had a higher RMS error in 2f sine curve fitting compared to age-matched controls (paired t-test, P < 0.001). Figure 2 (bottom) shows the average value for RMS error at different eccentricities for both the AMD and matched control groups. The root-mean-square error remained higher in the AMD group at all eccentricities.
Discussion
Photoreceptors in the central macula are prone to damage in many progressive sight threatening diseases, including AMD. Direct assessment of these photoreceptors is difficult in the aging eye, making indirect techniques particularly valuable. Indirect techniques must utilize unique optical signatures to isolate structures of interest and permit quantitative metrics for comparison. As a complementary technique for assessing the central macula, SLP imaging can utilize the phase retardation signal generated by the photoreceptor axons of the HFL, the only known birefringent structure in the central macula, to selectively study the central macular photoreceptor density and regularity. Similar to the nerve fiber layer (NFL), the magnitude of the phase retardation signal in the central macula would be proportional to the number of axons that make up the Henle fiber layer at any given eccentricity. These central photoreceptor axons splay out in a well-organized, radially symmetric pattern,18 suggesting that the phase retardation profiles would also demonstrate some form of radial symmetry. This is indeed the case, and normal individuals show consistent radial symmetry. The analysis using this technique is not straightforward, complicated by the fact that the birefringence of the cornea is incompletely compensated, creating a macular cross pattern instead of an annulus centered on the fovea. For that reason, our analysis uses specific FFT and curve fitting that accounts for this radial modulation, allowing us to assess the decrease in intensity and deviations from a smooth 2f or 4f curve fitting that would occur if the photoreceptor axon density was reduced or if there were focal alterations resulting from regularity changes to the photoreceptor axons. The intrinsic birefringent property of specific cells in the retina is extremely sensitive to neurodegenerative changes and primate models of glaucoma demonstrate phase retardation changes to the NFL in SLP imaging in advance of thickness changes using OCT.38,39 The reduced phase retardation signal in the NFL correlates with degradation of critical components in axonal walls, the fundamental source of its birefringent signal. We anticipate similar changes to the axonal walls are occurring in the Henle fiber layer, making polarization sensitive signals good candidates for early detection of neurodegenerative changes in the macula in advance of thickness alterations where cells are present, but in a degenerating or compromised state. We considered the possibility of Müller cell contributions to the phase retardation signal in the central macula, but believe that Müller cells do not contribute, or contribute negligibly, to the phase retardation signal associated with the Henle fiber layer. This is consistent with recent studies showing an increase in thickness of the outer plexiform and Henle fiber layers associated with increasing age, attributed to an increase in Müller cell volume,40 while macular phase retardation continues to decrease as a function of normal aging.24 If Müller cells were contributing significantly to the phase retardation signal in the central macula, we do not believe phase retardation would decrease in a manner consistent with in vivo photoreceptor density changes that are also associated with normal aging.15
The persistent nature of the macular cross pattern in AMD patients suggests some sparing of cone photoreceptor axons in the presence of nonexudative changes (see Supplementary Fig. S1), consistent with the origination of pathology at the deeper retinal layers, specifically the RPE/Bruch's membrane interface. An intact macular cross provides evidence that the more superficial retinal structures, including the HFL, remain less affected in the earliest stages of AMD. Despite an intact macular cross and good visual acuity, the AMD group in this study demonstrated reduced normalized 2f FFT values with higher curve fitting RMS error at all eccentricities. These changes can be explained primarily through 2 mechanisms: morphological alterations to the foveal architecture caused by disruptions to normal photoreceptor packing regularity from deeper AMD pathology; a decrease in the photoreceptor density in the AMD group; or some combination of the two. Foveal phase retardation changes associated with AMD likely occur through a combination of these mechanisms, and it is difficult to assign a relative contribution to either, as the metrics used in this study, amplitude of the normalized 2f FFT and curve fitting RMS error, are linked. For example, focal alterations to the deep retina, occurring with the development of drusen and a resultant alteration to the geometry of the phase retardation signal, may cause the overlying photoreceptor axons to take on a more parallel alignment with respect to the imaging light or a more irregular orientation that differs from neighboring axons, resulting in a local reduction in normalized 2f FFT amplitude at that location combined with higher curve fitting RMS error. The expected result of misalignment of the photoreceptor outer segments due to the irregular thickening beneath them from drusen and RPE changes may contribute little to these changes, since the main polarization content measured is thought to be related to the axons, not the outer or inner segments. Changes in any portion of the photoreceptors may be related to the increased RMS error in the measurements, however. Alternatively, reduced phase retardation could occur through a decrease in photoreceptor density, either through photoreceptor loss or migration to a more eccentric location. An increase in curve fitting RMS error would also result if photoreceptor density changes occurred nonuniformly. Photoreceptor loss instead of migration becomes more likely, as the eccentricity that yielded the maximum normalized 2f FFT eccentricity did not differ between the two groups. We believe that loss of photoreceptors is the dominant mechanism for the amplitude changes observed in this study, given the degree to which the photoreceptor orientation would have to be altered over the central 3° to meaningfully impact phase retardation globally. The macular cross pattern persists in patients with nonexudative AMD, even in the presence of large coalesced drusen throughout the central macula.35 This is consistent with both histological31 and high resolution imaging studies evaluating drusen at multiple stages,41 where photoreceptor density is reduced over drusen and other pathological features associated with AMD.
For pathology assessment of AMD, SLP imaging has several practical advantages and provides a robust dataset beyond phase retardation. The near infrared source and confocal nature of SLP imaging reduces unwanted light scatter and produces clear images with high contrast through aging media. This can be done at safe, comfortable light levels, noninvasively and without mydriasis. The datasets collected from the instrument used in this study cover a 15 × 15° retinal area, which has significant advantages when compared to the small field of view over which high-resolution imaging can be achieved currently, and SLP can be done at a much lower cost. The raw datasets from SLP imaging also contain information regarding multiple light/tissue interactions that can be examined independently, providing information about pathological changes occurring to separate cellular structures. In this study, the primary focus was phase retardation as an intrinsic marker of the HFL, but additional markers such as depolarization can be used to highlight compromises to the structural integrity of the RPE in SLP imaging.27,32 Because these light/tissue interactions are derived from the same raw dataset, locations on the different images are corresponding and comparative analysis of different light/tissue interactions becomes straightforward.
Despite its many advantages, phase retardation measurements derived from SLP have limitations. Corneal compensation, even the partial compensation in this study, is not ideal for FFT amplitude phase retardation analysis because the retardation signal is reduced or altered by a constant value for each subject. Nevertheless, all subjects in our study demonstrated an intact macular cross pattern with a strong 2f or 4f component, indicating that corneal compensation was only partial in all subjects, but the influence of residual corneal birefringence varies widely across individuals in both magnitude and orientation.42 This corneal influence contributes to the variability of phase retardation amplitude across subjects, and as stated above, the better compensation from the device results in flatter phase retardation profiles and lower amplitudes of the 2f and 4f FFT components. Phase retardation profiles are also of lower amplitude in the odd harmonics, the denominator in our ratio measure, so effects of individual differences in corneal birefringence are lessened rather than reducing the signal-to-noise ratio of measurements. Residual corneal birefringence is unlikely to bias results in favor of either study group. An additional limitation is the lack of depth information in en face imaging that is available in cross-sectional techniques like optical coherence tomography (OCT). Polarization sensitive OCT provides information regarding the axial location of different polarization signals from the retina in AMD and has primarily studied the polarization effects at the RPE.29,43–47 Phase retardation changes in exudative AMD patients are readily measurable, but of smaller magnitude than the signal originating from the sclera.29 Exudative lesions in late-stage AMD have been shown to demonstrate birefringent properties consistent between SLP imaging and polarization sensitive OCT imaging.29 Because the subjects for this study were limited to patients with nonexudative changes and without regions of atrophy that reveal strongly birefringent sclera, the phase retardation signal, highly specific to the HFL, is not likely confounded by other cellular or structural components.
Insight into both the prognosis for individual patients and mechanisms of AMD can be provided by novel imaging techniques. Specific light/tissue interactions are likely to improve our abilities to monitor the efficacy of new and existing treatments and advance the development of earlier interventions for a number of retinal diseases in the future. However, advantages of SLP imaging are not limited to AMD. Similar analyses could easily be applied to other retinal diseases where visual complaints precede obvious clinical changes in the central macula or when photoreceptors are presumed to be damaged or dysfunctional prior to severe visual acuity loss.
Acknowledgments
Supported by Grants K23-EY017886 (DAV); RO1-EY007624 (AEE); RO1-EB002346 (AEE); and P30-EY019008 (SAB) from the National Eye Institute. The authors alone are responsible for the content and writing of the paper.
Disclosure: D.A. VanNasdale, None; A.E. Elsner, None; T.D. Peabody, None; K.D. Kohne, None; V.E. Malinovsky, None; B.P. Haggerty, None; A. Weber, None; C.A. Clark, None; S.A. Burns, None
References
- 1. Eisner A, Stoumbos VD, Klein ML, Fleming SA. Relations between fundus appearance and function. Eyes whose fellow eye has exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 1991; 32: 8–20. [PubMed] [Google Scholar]
- 2. Eisner A, Klein ML, Zilis JD, Watkins MD. Visual function and the subsequent development of exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 1992; 33: 3091–3102. [PubMed] [Google Scholar]
- 3. Midena E, Degli Angeli C, Blarzino MC, Valenti M, Segato T. Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997; 38: 469–477. [PubMed] [Google Scholar]
- 4. Elsner AE, Burns SA, Weiter JJ. Cone photopigment in older subjects: decreased optical density in early age-related macular degeneration. J Opt Soc Am A Opt Image Sci Vis. 2002; 19: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Remky A, Elsner AE. Blue on yellow perimetry with scanning laser ophthalmoscopy in patients with age related macular disease. Br J Ophthalmol. 2005; 89: 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimitrov PN, Robman LD, Varsamidis M, et al. Visual function tests as potential biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 9457–9469. [DOI] [PubMed] [Google Scholar]
- 7. Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998; 116: 653–658. [DOI] [PubMed] [Google Scholar]
- 8. Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572. [DOI] [PubMed] [Google Scholar]
- 9. Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007; 114: 253–262. [DOI] [PubMed] [Google Scholar]
- 10. Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007; 14: 184–187. [DOI] [PubMed] [Google Scholar]
- 11. Klein R, Knudtson MD, Lee KE, Gangnon RE, Klein BE. Age-period-cohort effect on the incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2008; 115: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013; 54: ORSF5–ORSF13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chui TY, Song H, Burns SA. Adaptive-optics imaging of human cone photoreceptor distribution. J Opt Soc Am A Opt Image Sci Vis. 2008; 25: 3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li KY, Tiruveedhula P, Roorda A. Intersubject variability of foveal cone photoreceptor density in relation to eye length. Invest Ophthalmol Vis Sci. 2010; 51: 6858–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song H, Chui TY, Zhong Z, Elsner AE, Burns SA. Variation of cone photoreceptor packing density with retinal eccentricity and age. Invest Ophthalmol Vis Sci. 2011; 52: 7376–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou W, Qi X, Burns SA. Woofer-tweeter adaptive optics scanning laser ophthalmoscopic imaging based on Lagrange-multiplier damped least-squares algorithm. Biomed Opt Express. 2011; 2: 1986–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chui TY, Song H, Clark CA, Papay JA, Burns SA, Elsner AE. Cone photoreceptor packing density and the outer nuclear layer thickness in healthy subjects. Invest Ophthalmol Vis Sci. 2012; 53: 3545–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007; 47: 2901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lujan BJ, Roorda A, Knighton RW, Carroll J. Revealing Henle's fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brink HB, van Blokland GJ. Birefringence of the human foveal area assessed in vivo with Mueller-matrix ellipsometry. J Opt Soc Am A. 1988; 5: 49–57. [DOI] [PubMed] [Google Scholar]
- 21. Huang XR, Knighton RW. Theoretical model of the polarization properties of the retinal nerve fiber layer in reflection. Appl Opt. 2003; 42: 5726–5736. [DOI] [PubMed] [Google Scholar]
- 22. van Blokland GJ. Ellipsometry of the human retina in vivo: preservation of polarization. J Opt Soc Am A. 1985; 2: 72–75. [DOI] [PubMed] [Google Scholar]
- 23. Elsner AE, Weber A, Cheney MC, Vannasdale DA. Spatial distribution of macular birefringence associated with the Henle fibers. Vision Res. 2008; 48: 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. VanNasdale DA, Elsner AE, Hobbs T, Burns SA. Foveal phase retardation changes associated with normal aging. Vision Res. 2011; 51: 2263–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cense B, Wang Q, Lee S, et al. Henle fiber layer phase retardation measured with polarization-sensitive optical coherence tomography. Biomed Opt Express. 2013; 4: 2296–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elsner AE, Burns SA, Beausencourt E, Weiter JJ. Foveal cone photopigment distribution: small alterations associated with macular pigment distribution. Invest Ophthalmol Vis Sci. 1998; 39: 2394–2404. [PubMed] [Google Scholar]
- 27. Miura M, Elsner AE, Weber A, et al. Imaging polarimetry in central serous chorioretinopathy. Am J Ophthalmol. 2005; 140: 1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elsner AE, Weber A, Cheney MC, VanNasdale DA, Miura M. Imaging polarimetry in patients with neovascular age-related macular degeneration. J Opt Soc Am A Opt Image Sci Vis. 2007; 24: 1468–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miura M, Yamanari M, Iwasaki T, et al. Imaging polarimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 2661–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber A, Elsner AE, Miura M, Kompa S, Cheney MC. Relationship between foveal birefringence and visual acuity in neovascular age-related macular degeneration. Eye (Lond). 2007; 21: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236–1249. [PubMed] [Google Scholar]
- 32. Burns SA, Elsner AE, Mellem-Kairala MB, Simmons RB. Improved contrast of subretinal structures using polarization analysis. Invest Ophthalmol Vis Sci. 2003; 44: 4061–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mellem-Kairala MB, Elsner AE, Weber A, Simmons RB, Burns SA. Improved contrast of peripapillary hyperpigmentation using polarization analysis. Invest Ophthalmol Vis Sci. 2005; 46: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 34. VanNasdale DA, Elsner AE, Weber A, Miura M, Haggerty BP. Determination of foveal location using scanning laser polarimetry. J Vis. 2009; 9: 21.1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. VanNasdale DA, Elsner AE, Kohne KD, et al. Foveal localization in non-exudative AMD using scanning laser polarimetry. Optom Vis Sci. 2012; 89: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weber A, Cheney M, Smithwick Q, Elsner A. Polarimetric imaging and blood vessel quantification. Opt Express. 2004; 12: 5178–5190. [DOI] [PubMed] [Google Scholar]
- 37. Weinreb RN, Bowd C, Greenfield DS, Zangwill LM. Measurement of the magnitude and axis of corneal polarization with scanning laser polarimetry. Arch Ophthalmol. 2002; 120: 901–906. [DOI] [PubMed] [Google Scholar]
- 38. Fortune B, Wang L, Cull G, Cioffi GA. Intravitreal colchicine causes decreased RNFL birefringence without altering RNFL thickness. Invest Ophthalmol Vis Sci. 2008; 49: 255–261. [DOI] [PubMed] [Google Scholar]
- 39. Fortune B, Burgoyne CF, Cull G, Reynaud J, Onset Wang L. and progression of peripapillary retinal nerve fiber layer (RNFL) retardance changes occur earlier than RNFL thickness changes in experimental glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 5653–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Curcio CA, Messinger JD, Sloan KR, Mitra A, McGwin G, Spaide RF. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Invest Ophthalmol Vis Sci. 2011; 52: 3943–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boretsky A, Khan F, Burnett G, et al. In vivo imaging of photoreceptor disruption associated with age-related macular degeneration: A pilot study. Lasers Surg Med. 2012; 44: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Knighton RW, Huang XR. Linear birefringence of the central human cornea. Invest Ophthalmol Vis Sci. 2002; 43: 82–86. [PubMed] [Google Scholar]
- 43. Ahlers C, Götzinger E, Pircher M, et al. Imaging of the retinal pigment epithelium in age-related macular degeneration using polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2010; 51: 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baumann B, Gotzinger E, Pircher M, et al. Segmentation and quantification of retinal lesions in age-related macular degeneration using polarization-sensitive optical coherence tomography. J Biomed Opt. 2010; 15: 061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schlanitz FG, Baumann B, Spalek T, et al. Performance of automated drusen detection by polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 4571–4579. [DOI] [PubMed] [Google Scholar]
- 46. Baumann B, Baumann SO, Konegger T, et al. Polarization sensitive optical coherence tomography of melanin provides intrinsic contrast based on depolarization. Biomed Opt Express. 2012; 3: 1670–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schütze C, Bolz M, Sayegh R, et al. Lesion size detection in geographic atrophy by polarization-sensitive optical coherence tomography and correlation to conventional imaging techniques. Invest Ophthalmol Vis Sci. 2013; 54: 739–745. [DOI] [PubMed] [Google Scholar]