Abstract
Purpose.
To investigate the safety and effects of intravitreal sirolimus for the potential treatment of geographic atrophy (GA).
Methods.
The study was a single-center, open-label, phase I/II trial enrolling six participants with bilateral GA treated with intravitreal sirolimus in only one randomly assigned eye, with the fellow eye as control. The primary efficacy outcome measure was the change in total GA area from baseline on color fundus photography (CFP); secondary outcomes included changes in GA area on fundus autofluorescence (FAF), visual acuity, central retinal thickness (CRT), and macular sensitivity from baseline.
Results.
Although no systemic adverse events were attributed to treatment, two of six participants had ocular adverse events that were possibly associated. The treated eye of one participant developed abnormal paralesional changes on FAF that were associated with accelerated retinal thinning. This accelerated retinal thinning was also seen in the treated eye of a second participant. Because of concern that these events were associated with treatment, treatment was suspended. Comparisons of treated and fellow eyes for change in visual acuity, change in GA area, and change in CRT showed no evidence of treatment benefit and generally favored the untreated fellow eye.
Conclusions.
While paralesional FAF changes and rapid retinal thinning observed are potentially part of the natural course of GA, they may possibly be related to treatment. No general evidence of anatomical or functional benefit was detected in treated eyes. Further data on intravitreal sirolimus for GA treatment will be available from a larger phase II trial. (ClinicalTrials.gov number, NCT01445548.)
Keywords: age-related macular degeneration, geographic atrophy, sirolimus, clinical trial
Intravitreal sirolimus was not associated with any general evidence of anatomical or functional benefit and may possibly be associated with RPE and retinal atrophy.
Introduction
Age-related macular degeneration (AMD) is the leading cause of late-onset visual impairment and legal blindness in people 65 years of age or older in the United States.1,2 Atrophic AMD or geographic atrophy (GA) is the form of late AMD characterized by the gradual expansion of atrophic macular changes, which results in progressive central vision loss. There are currently no treatments effective in preventing the onset of GA3 or slowing lesion growth.4,5
While the pathogenesis of GA remains incompletely understood, several lines of evidence have indicated that dysregulated immune responses may drive lesion expansion.6 Genetic studies have identified single nucleotide polymorphisms (SNPs) in complement factor genes that confer risk for advancement to late forms of AMD, including GA,7–9 implicating the alternative pathway of complement activation as a contributing immune mechanism. Activated microglia/macrophages have been documented to accumulate at GA lesions in AMD histopathologic specimens.10,11 Large drusen, a phenotypic risk factor for GA, contain immunologically active molecules such as immunoglobulins, activated microglia, and activated complement factors.12–14 These data provide rationale for GA interventions that downregulate immune responses as a means to slow lesion expansion.
In the current study, we investigated intravitreal sirolimus as a potential therapy for GA. Sirolimus is an immunosuppressive agent currently approved as an oral immunosuppressive for use following renal transplantation15 and as a coated intra-arterial stent placed after balloon angioplasty.16 Sirolimus acts by forming a complex with immunophilin FK binding protein 12 (FKBP-12), which then inhibits a multifunctional serine-threonine kinase called mammalian target of rapamycin (mTOR).17 The inhibition of mTOR results in a wide range of changes in cellular function including metabolism, growth, proliferation, and survival18; mTOR inhibition also suppresses T- and B-cell proliferation and antibody production, enabling its immunosuppressive effect.19,20 Recent studies have employed sirolimus as a local agent in the treatment of ocular disorders that include uveitis21,22 and diabetic retinopathy,23,24 as well as GA.25
Here, we used a proprietary, depot-forming formulation of sirolimus that can be delivered via the subconjunctival or intravitreal route.26 We previously evaluated the safety and effects of sirolimus delivered subconjunctivally (a 440-μg subconjunctival injection every 3 months) in a pilot study for the treatment of GA.25 While we found that repeated subconjunctival sirolimus administrations were well tolerated, a positive anatomic or functional effect on GA enlargement was not detected. As this may be due to insufficient drug delivery to the retina, the current study investigated sirolimus delivered via intravitreal injection, which can increase retinal drug concentrations. When injected into the vitreous humor, the sirolimus component of the investigational agent aggregates to form a depot due to the hydrophobicity of sirolimus and the relatively high viscosity of the excipient polyethylene glycol (PEG) 400 (Investigators' Brochure, DE-109, unpublished data, 2012; Santen Pharmaceutical Co., Ltd., Osaka, Japan). Pharmacokinetics and tolerability of this sirolimus formation were previously assessed in a preclinical rabbit model and in phase I/II clinical studies of diabetic macular edema (NCT00401115) and neovascular AMD (NCT00712491).26 The purpose of this phase I/II, open-label, prospective study was to investigate the safety and effects of intravitreal sirolimus delivered as the investigational product in eyes with GA.
Methods
This was a single-center, prospective, open-label, phase I/II study conducted at the National Institutes of Health (NIH) Clinical Center, Bethesda, Maryland, and supported by the National Eye Institute Intramural Research Program. This project has been funded in whole or in part with federal funds from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, under contract HHSN263201200001C. The study protocol and Health Insurance Portability and Accountability Act (HIPAA)-compliant informed consent forms were approved by the NIH CNS Institutional Review Board, with an external data and safety monitoring committee (DSMC) providing oversight. An investigational new drug application (IND) (102,516) was obtained from the U.S. Food and Drug Administration (FDA). The study was registered at www.clinicaltrials.gov under the identifier NCT01445548 (registration date, September 30, 2011). The study adhered to the tenets of the Declaration of Helsinki.
Study Population
Eligible participants were at least 56 years of age and had a diagnosis of bilateral GA related to AMD. The primary eligibility criteria included (1) the presence of GA in each eye of area ≥ one-half disc area (approximately 1 mm2), (2) the presence of at least one large druse (≥125 μm) in each eye, (3) best-corrected visual acuity (BCVA) between 20/20 and 20/400 in each eye, and (4) the absence of evidence or history of exudative AMD (Supplementary Table S1). The study had planned to enroll a total of 10 participants for analysis. See further explanation in the Results section below.
Study Medication
The investigational product (MS-R001), manufactured and donated by Santen Pharmaceutical Co., Ltd., comprised a proprietary formulation of a 22 μg/μL (2%) solution of sirolimus in a vehicle composed of PEG 400 and 4% ethanol. The study drug was supplied frozen in a 0.5-mL sterile injectable solution, thawed immediately prior to use, and drawn slowly into a sterile 0.3-mL Becton Dickinson (Frankling Lakes, NJ, USA) syringe with a 20-gauge needle. The study drug was protected from light before delivery and administered within 2 hours of being drawn up. The investigational product was delivered as a 440-μg intravitreous injection in a 20-μL volume. Briefly, an area 3.5 to 4.0 mm from the limbus of the treated eye was anesthetized by holding cotton-tipped applicators soaked in 0.5% proparacaine at the injection site for approximately 10 seconds, after which the study drug was slowly injected into the intravitreal space at the anesthetized location.
Study Design
After informed consent was obtained from participants following explanation about the nature and consequences of the study, one eye in each participant was randomly assigned the investigational product (i.e., the study eye). The fellow eye was assigned to observation without treatment. Investigational product was administered at the baseline visit and every 2 months thereafter for a planned duration of 24 months. The rationale for this monocular study design was based on observed correlations between the rates of GA enlargement in eyes of patients with bilateral GA.27
Study Assessments
Study visits were scheduled at baseline and at every 2 months, with one additional safety visit scheduled at month 1. Best-corrected visual acuity measurements using Early Treatment Diabetic Retinopathy Study (ETDRS) charts and protocols, slit-lamp and dilated fundus examinations, measurement of intraocular pressure, and assessment of adverse events and concomitant medications were performed at every study visit. Laboratory assessments consisting of complete blood count, serum electrolytes, serum lipid profiles, and urine analysis were performed every 4 months, with measurement of serum sirolimus levels performed every 2 months. A physical examination was performed at baseline and at the final study visit. Fundus imaging consisted of (1) stereoscopic color fundus photography (CFP); (2) fundus autofluorescence (FAF) imaging using both a confocal scanning ophthalmoscope (HRA FAF, HRA2; Heidelberg Engineering, Vista, CA, USA) and a modified Topcon 50-EX fundus camera (mFC FAF; Topcon Medical Systems, Oakland, NJ, USA) using band-pass filters for excitation (550–600 nm) and emission (660–800 nm) as previously described28; (3) spectral-domain optical coherence tomography (SD-OCT) imaging (Cirrus HD-OCT, software version 6.0; Carl Zeiss Meditec, Inc., Jena, Germany), and (4) macular sensitivity assessment using microperimetry (MP-1 microperimeter; Nidek Technologies, Padova, Italy).
The CFPs, Topcon FAF, and HRA FAF images were centrally graded manually by masked graders at the Doheny Image Reading Center (Los Angeles, CA, USA). The area of GA in field 2 (30° photographic field centered on the fovea) was determined by planimetry from color stereoscopic fundus images and FAF images according to a standardized protocol. Changes in GA area from baseline were calculated as absolute change in GA area (in mm2) and change in the square root transformation (calculated as the square root of the lesion area, in mm).29 Spectral-domain OCT imaging was performed with the 512 × 128 scan pattern (covering a 6- × 6-mm area) centered over the anatomical fovea. A circular grid as defined in the Age-Related Eye Disease Study (AREDS)30 was superimposed on the scan field and centered on the fovea. Retinal thickness in the central subfield (1-mm-diameter circle) and total macular volume in the central and inner subfields (covering the central 3-mm-diameter circle) were computed using the device software, following manual alignment if necessary. Microperimetry testing was performed using the MP-1 microperimeter (NAVIS software version 1.7.3; Nidek Technologies). Assessments were performed as previously described.31 Retinal sensitivity was calculated with a background luminance of four apostilibs (1.27 cd/m2) using a grid of 49 testing loci or points that were spaced 4° apart within a 24° × 24° square centered on the center of the macula. Scotomatous (or nonresponding) points were defined as testing loci that elicited no participant response even at the highest-intensity stimulus. Responding points were defined as all other testing loci for which a response was recorded after stimulus presentation (i.e., loci for which a response was elicited within the entire range of stimuli intensities used by the testing algorithm). The following test parameters were analyzed: number of scotomatous loci (loci with sensitivity of <0 dB) and mean retinal sensitivity (dB) of all responding (i.e., nonscotomatous) points.
Primary Outcomes
Primary outcomes were defined as overall safety assessment and change in total GA area on CFP from baseline to month 12.
Statistical Analysis
Commercial software (Prism, ver. 5.0; GraphPad, La Jolla, CA, USA) was used to calculate summary statistics. Paired t-tests were used to compare these parameters between study and fellow eyes. Error bars in graphical representations of data and reports of variation in the text indicate standard error of the mean (SEM). The study was designed to assess safety and possible proof of principle trends for benefit.
Results
Baseline Patient Demographics and Ocular Characteristics
Six participants were enrolled between December 2011 and February 2012; patient demographics and ocular characteristics at study baseline are listed in the Table and Supplementary Table S2. In the study and fellow eyes, respectively, mean GA area (on CFP) was 13.95 and 13.45 mm2, while BCVA was 52.7 letters (≈20/80) and 39.2 letters (≈20/160). Statistically significant differences were not found between the study and fellow eyes for all baseline ocular characteristics (P > 0.05 for all comparisons, paired t-test).
Table.
Baseline Demographic and Ocular Data for Participants, n = 6
| Number of participants | 6 |
| Age, y, mean ± SD (range) | 74.33 ± 8.45 (60–84) |
| Sex, female, n (%) | 2 (33.33) |
| Race, white, n (%) | 5 (83.33) |
| Lens status of study eye, pseudophakic, n (%) | |
| Study eye | 1 (8.33) |
| Fellow eye | 2 (16.66) |
| Baseline best-corrected visual acuity, letters, mean ± SD (range) | |
| Study eye | 52.7 ± 14.5 (35–73) |
| Fellow eye | 39.2 ± 20.0 (19–68) |
| Location of geographic atrophy lesion | |
| Subfoveal, involving center of fovea | 11 eyes of 6 participants |
| Nonsubfoveal, not involving center of fovea | 1 eye of 1 participant |
| Total area of GA, mm2, mean ± SD (range) | |
| Color fundus photography | |
| Study eye | 13.95 ± 3.74 (7.55–18.86) |
| Fellow eye | 13.45 ± 3.92 (9.57–19.75) |
| Fundus autofluorescence on modified fundus camera | |
| Study eye | 14.06 ± 3.83 (7.61–18.84) |
| Fellow eye | 13.99 ± 3.93 (9.78–20.21) |
| Fundus autofluorescence on confocal scanning ophthalmoscope | |
| Study eye | 13.88 ± 4.24 (6.39–18.83) |
| Fellow eye | 13.37 ± 4.37 (8.12–19.97) |
| Central subfield retinal thickness as measured on SD-OCT, μm, mean, ± SD (range) | |
| Study eye | 181 ± 41 (137–258) |
| Fellow eye | 174 ± 33 (136–238) |
| Microperimetry measurements, number of scotomatous points, mean ± SD (range) | |
| Study eye | 13.40 ± 10.95 (3–28) |
| Fellow eye | 11.80 ± 9.09 (7–28) |
| Mean overall sensitivity of nonscotomatous points, dB, mean ± SD (range) | |
| Study eye | 7.71 ± 5.06 (2.35–12.77) |
| Fellow eye | 8.20 ± 4.58 (2.53–14.05) |
| Mean overall sensitivity of all points, dB, mean ± SD (range) | |
| Study eye | 6.35 ± 4.77 (1.12–11.20) |
| Fellow eye | 6.55 ± 4.09 (0.88–11.30) |
SD, standard deviation.
Ocular and Systemic Safety of Investigational Product
A total of 49 adverse events (AEs) (ocular and systemic) were recorded (Supplementary Table S3). Serious AEs consisted of the following: portal hypertension secondary to liver cirrhosis (participant 2), hip fracture (participant 6), and cardiac valve replacement (participant 6). Serum sirolimus levels were below the level of detection (<2.0 ng/mL) in all participants and at all time points. All systemic AEs (n = 45) were judged as unrelated to the investigational product. Four ocular AEs, all involving the study eye, were reported. Two out of four ocular AEs were judged as possibly related to the investigational product preparation; these consisted of: (1) RPE atrophy at the edge of the central GA lesion with increased central retinal thinning (participant 6) and (2) increased central retinal thinning (participant 5). The remaining two ocular AEs (decrease in visual acuity, ocular surface irritation) were judged as related to the injection procedure and not to investigational product.
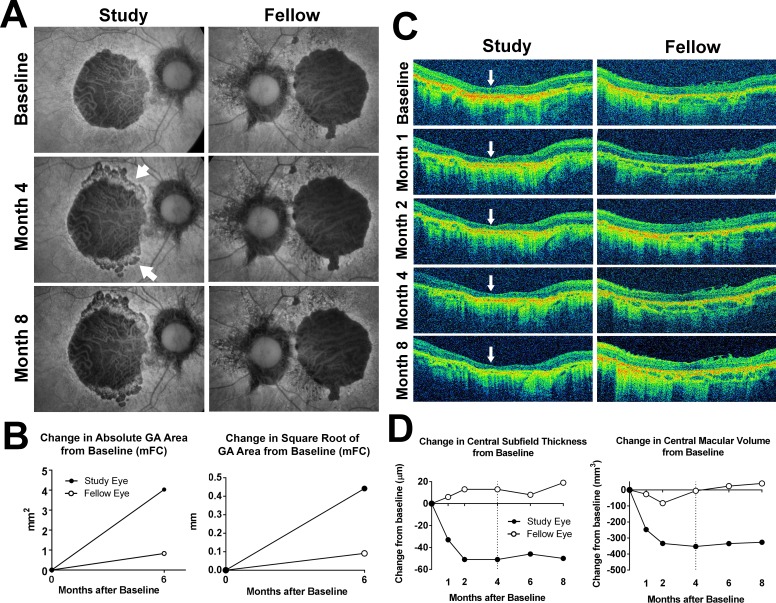
The anatomic changes for participant 6 are seen in Figure 1. The participant received two injections of investigational product in the study (right) eye at the baseline and month 2. At month 4, new areas of hypoautofluorescence on FAF imaging emerged at the superior and inferior borders of the GA lesion (Fig. 1A, arrows). These new areas of decreased AF corresponded to areas of hypopigmentation on CFP (data not shown) and to zones of RPE disruption and irregularity on SD-OCT imaging (Supplementary Fig. S1). Similar changes were not observed in the fellow eye. Investigational product administration was suspended at month 4, and follow-up visits continued every 2 months until the participant withdrew from the study at month 8 following a hip fracture. The newly emerged areas of hypoautofluorescence at month 4 maintained relative stability in size and appearance. Quantitation of square root transform of GA area from modified fundus camera (mFC) FAF images from baseline and month 6 showed a 0.44-mm increase in the study eye, compared with a 0.09-mm increase in the fellow eye (Fig. 1B). Thinning of the central retina in the study eye on SD-OCT was observed at month 1 (Fig. 1C, arrows); it decreased by approximately 40 μm by month 2 (Fig. 1D, left) and stabilized thereafter. Measurements of central macular volume demonstrated a parallel trend (Fig. 1D, right). These changes in central thickness were not seen in the fellow eye. These anatomical changes did not correspond to changes in visual acuity. Over the 8-month follow-up period, the study eye lost one ETDRS letter compared with a loss of 17 ETDRS letters in the fellow eye. Full-field ERG testing at month 4 did not indicate widespread functional loss in the study eye compared with the fellow eye (data not shown).
Figure 1.
Fundus changes in participant 6 during study treatment and follow-up. (A) Fundus autofluorescence images were obtained using a modified fundus camera (mFC). The right eye was randomized to receive investigational product (Study) while the left (Fellow) eye was observed without treatment. At month 4, after receiving two intravitreal injections of investigational product, the central GA lesion in the study eye demonstrated an expansion in the area of hypoautofluorescence at the superior and inferior margins (arrowheads). Investigational product administration was suspended at this month 4 visit and withheld thereafter. At month 8, the size and appearance of the GA lesion in the study eye appeared relatively stable. The fellow eye demonstrated no marked changes. (B) Changes in absolute (left) and square root transform (right) of GA area in the study and fellow eye at month 6 from baseline were measured from mFC FAF images. (C) Horizontal B-scans aligned through the center of the macula for the study and fellow eyes from baseline to month 8 were obtained. Marked central retinal thinning was observed at month 1 (indicated by arrows) in the study eye but not in the fellow eye. (D) Change in central retinal thickness (left) and central macular volume (right) from baseline to month 8 documented a rapid decline in the first 2 months in the study eye, which stabilized following drug suspension at month 4 (dashed line).
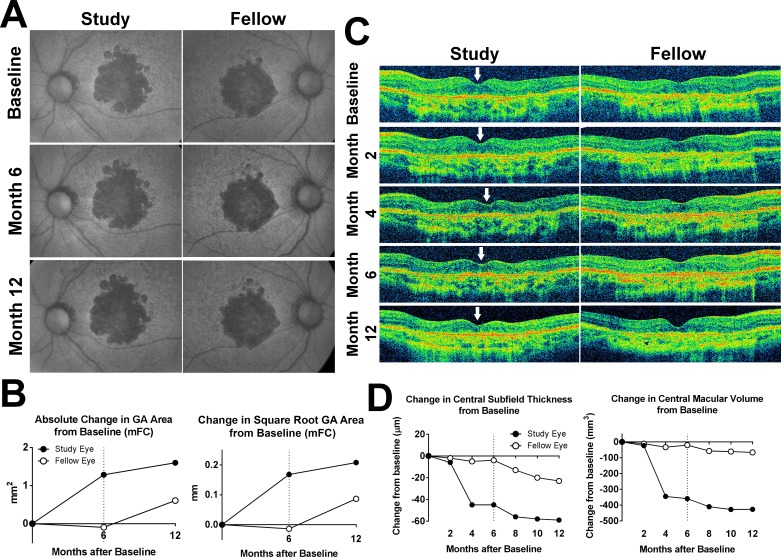
Optical coherence tomographs of the second participant demonstrating considerable central retinal thinning in the study eye following treatment (participant 5) are seen in Figure 2. Investigational product was discontinued at month 6, following three consecutive injections, when the participant reported visual symptoms of blurry vision and photopsias in the study eye. Best-corrected visual acuity in the study eye had decreased by 12 letters in this eye compared with a 14-letter improvement in the fellow eye. Change in square root GA area from baseline at month 12 was 0.21 mm in the study eye and 0.087 mm in the fellow eye. Full-field ERG testing at month 4 did not indicate additional functional loss (data not shown).
Figure 2.
Fundus changes in participant 5 during study treatment and follow-up. (A) mFC FAF images demonstrated bilateral central GA lesions at baseline that gradually expanded in both study and fellow eye in a manner not inconsistent with the natural history of GA. (B) Absolute (left) and square root transform (right) increase in GA area in the study and fellow eye at month 6 and month 12 were plotted, demonstrating a greater increase in GA area in the study eye at month 12 compared with that in the fellow eye. (C) Horizontal aligned B-scans through the center of the macula from baseline to month 12 were obtained. Longitudinal analysis demonstrated noticeable thinning of the central macula beginning at month 4 in the study eye (loci indicated by arrows). Investigational product administration was suspended for the month 6 visit and withheld thereafter. (D) Change in central retinal thickness (left) and central macular volume (right) from baseline to month 12 in the study and fellow eyes documented a rapid decline over the first 4 months in the study eye, which stabilized following drug suspension at month 6 (dashed line), while measures in the fellow eye remained comparatively stable over time.
Investigator concern that these AEs were associated with the investigational product led to the suspension of treatment and study enrollment, with concurrence from the DSMC. At the time of suspension, all six participants had completed 4 to 6 months of follow-up and had received two or three doses of investigational product (two doses for participants 1, 2, and 6; three doses for participants 3, 4, and 5). Following suspension, participants were scheduled to be followed every 2 months for a total duration of 1 year.
Area of Geographic Atrophy
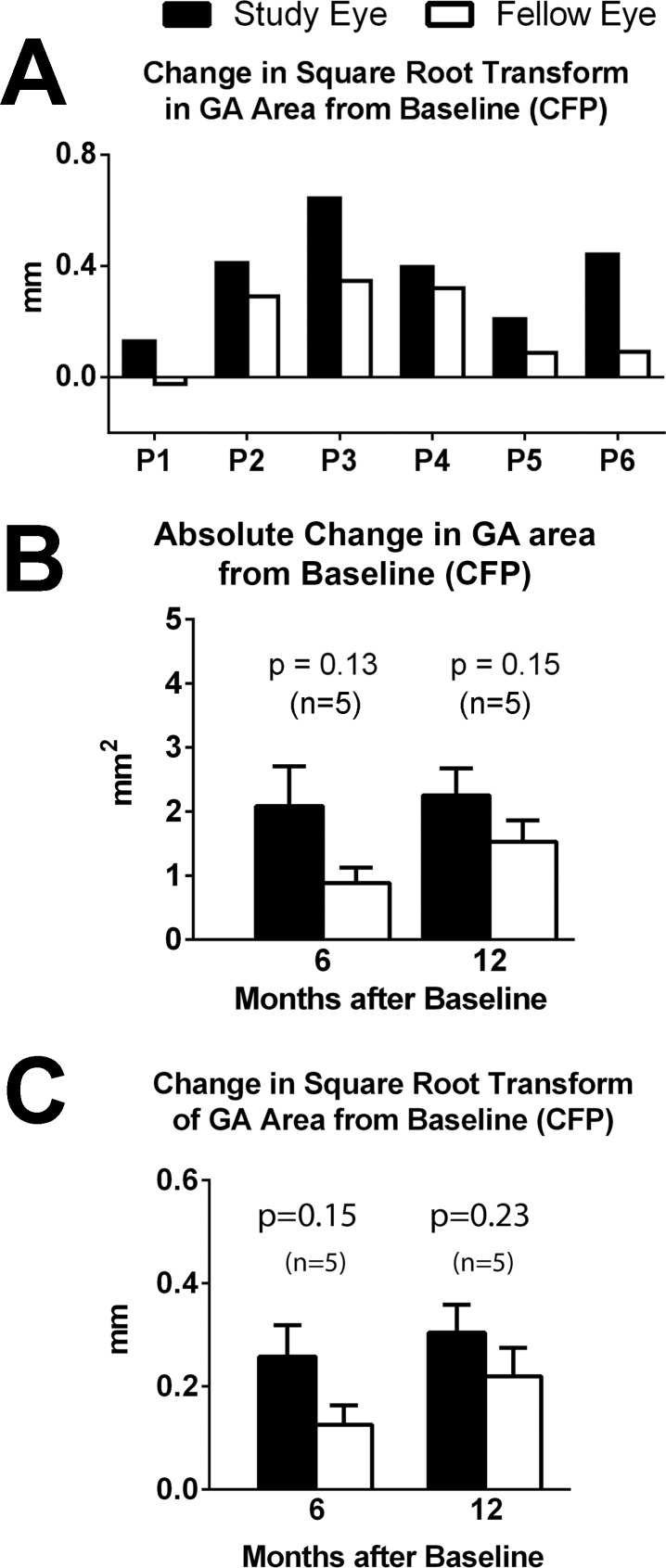
No study or fellow eye developed neovascular changes during the study. In five of the six participants, the increase in the square root of GA area from baseline was greater in the study eye than in the fellow eye (Fig. 3A). The mean absolute increase and square root increase in GA area from baseline, as graded from CFP images, were greater in the study eye at month 6 and month 12, but these differences between the eyes were not found to reach statistical significance (Figs. 3B, 3C) (P > 0.05, two-tailed paired t-test). Similar trends favoring the untreated fellow eye were found for GA areas measured from FAF images (mFC and SLO modalities) (Supplementary Fig. S2).
Figure 3.
Change in GA area measurement on color fundus photography (CFP) in the study and fellow eyes from baseline. (A) Changes in the square root transform of GA area from baseline to the month of last available follow-up (visits are plotted for each participant as indicated by P1, P2, and so on) for study (black bars) and fellow (white bars) eyes. The latest month for image grading was 12 months for participants P1 through P5 and 6 months for P6. (B) Mean increase in absolute GA area from baseline at month 6 (n = 5 participants, data for P1 unavailable) and month 12 (n = 5, data for P6 unavailable). (C) Mean increase in square root transform of GA area from baseline at month 6 and month 12 (n = 5).
Central Retinal Subfield Thickness and Macular Volume
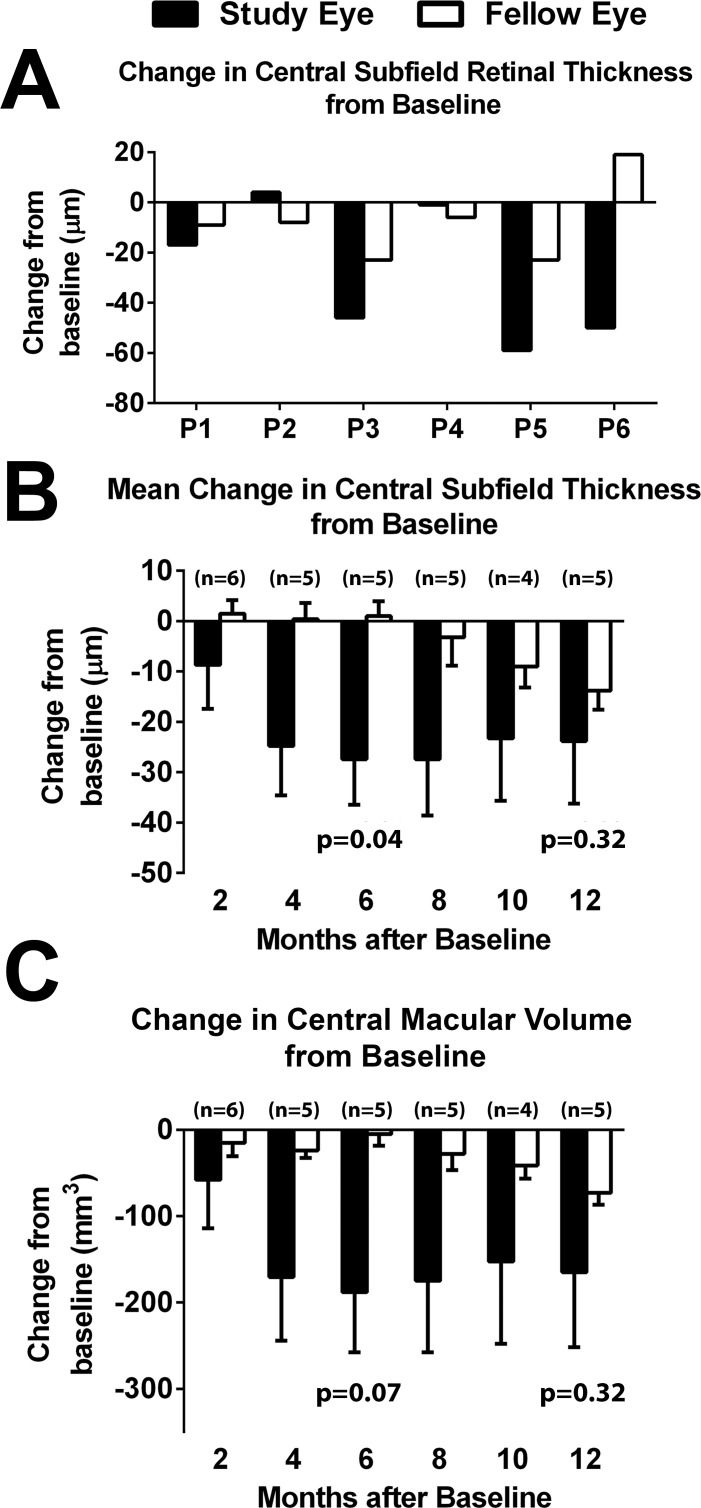
Comparisons of changes in central retinal thickness from baseline to last available study visit show that four of six participants had a greater decrease in study eye compared with fellow eye (Fig. 4A). Mean changes in central retinal subfield thickness and macular volume demonstrated greater retinal thinning for study compared with fellow eyes for all time points (Figs. 4B, 4C).
Figure 4.
Change in central retinal subfield thickness as measured by SD-OCT in the study and fellow eyes from baseline. (A) Change in central retinal subfield thickness from baseline to the latest month in study (black bars) and fellow (white bars) eyes. The latest month for image grading was 12 months for participants P1 through P5 and 8 months for P6. In four of six participants, the decrease in central subfield thickness was greater in the study compared with the fellow eye. (B) Mean change in central retinal subfield thickness from baseline at 2, 4, 6, 8, 10, and 12 months. Number of participants (n) available for analysis is indicated at each time point. (C) Mean change in central macular volume from baseline at 2, 4, 6, 8, 10, and 12 months.
Visual Acuity
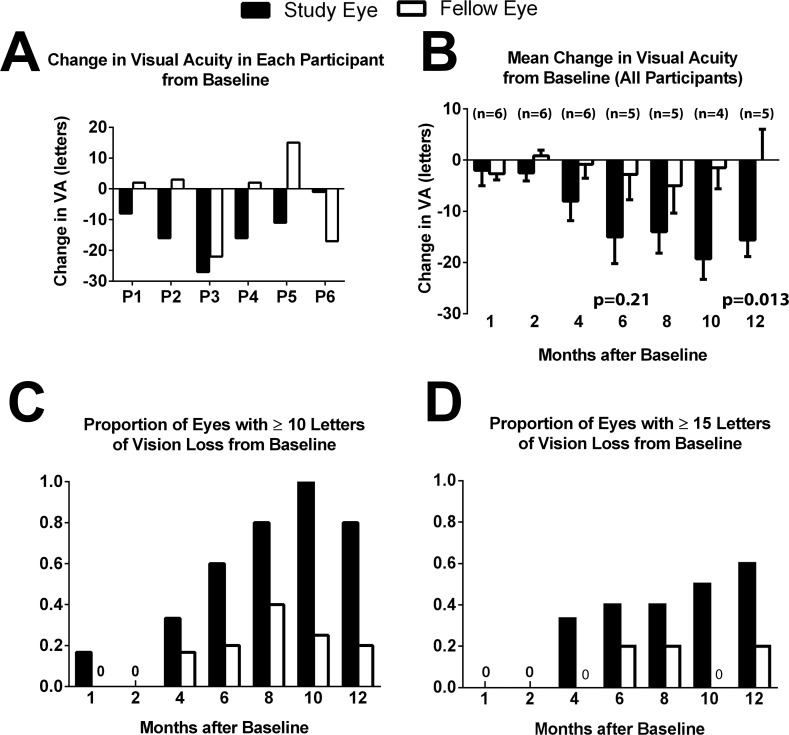
Comparisons of changes in BCVA from baseline to last available study visit show that five of six participants experienced a greater loss in BCVA in the study eye compared with the fellow eye (Fig. 5A). Mean change in visual acuity from baseline to 12 months showed a progressively greater BCVA loss in study eyes compared with fellow eyes (Fig. 5B). The proportion of participants losing ≥10 (Fig. 5C) or ≥15 ETDRS letters (Fig. 5D) in BCVA compared with baseline was also higher among study eyes than fellow eyes.
Figure 5.
Change in visual acuity from baseline in the study and fellow eyes. (A) Change in visual acuity (in ETDRS letters) from baseline to the latest month for each participant (indicated by number) for the study eye (black bars) and fellow (white bars) eye. The latest month for which data were available was 12 months for participants P1 through P5 and 8 months for P6. In five of six participants, a greater loss in visual acuity occurred in study eye as compared with fellow eye. (B) Mean change in visual acuity in study and fellow eyes from baseline at 1, 2, 4, 6, 8, 10, and 12 months. Number of participants (n) is indicated at each time point. (C) Proportions of the study and fellow eyes experiencing a ≥10 ETDRS letter loss in visual acuity from baseline at 1, 2, 4, 6, 8, 10, and 12 months. (D) Proportions of the study and fellow eyes experiencing a ≥15 ETDRS letter loss in visual acuity from baseline at 1, 2, 4, 6, 8, 10, and 12 months. P1 was absent between month 6 and month 10, while P6 completed 8 months of follow-up.
Retinal Sensitivity as Measured by Microperimetry
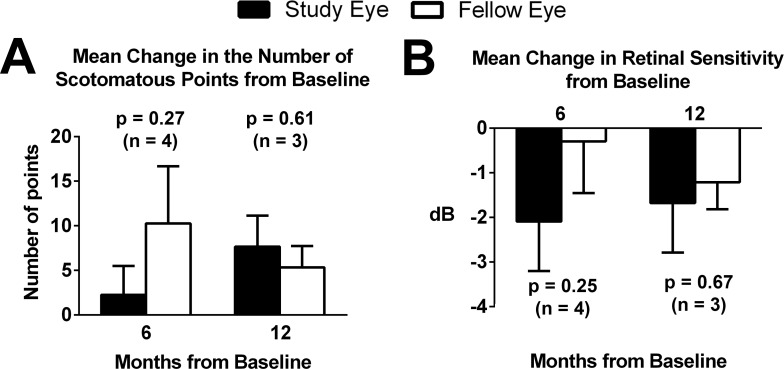
Microperimetry data were analyzed for four of six participants. Two participants were unable to maintain steady fixation for testing. Figure 6A demonstrates the mean change in the number of scotomatous points from baseline to 6 and 12 months. Greater decreases in retinal sensitivity were observed in study eyes compared with fellow eyes, but a statistically significant difference between the eyes was not found (Fig. 6B).
Figure 6.
Change in microperimetry test parameters in the study and fellow eyes from baseline. (A) Mean change in the number of scotomatous points from baseline at 6 and 12 months for the study eye (black bars) and fellow eye (white bars). Number of participants (n) for which data were available is indicated at each time point. (B) Mean change in the average retinal sensitivity of nonscotomatous points from baseline at 6 and 12 months. All comparisons between study and fellow eyes at each time point were not found to be significant.
Discussion
We found that intravitreal sirolimus was not associated with systemic safety issues in participants with GA. This replicates the favorable systemic safety profile observed previously in clinical studies involving subconjunctival or intravitreal sirolimus for GA25 and other ocular indications (diabetic macular edema and uveitis).21–24 In prior preclinical26 and phase I23 studies, systemic exposure to locally delivered sirolimus was below 2 ng/mL, and well below the therapeutic range for systemic immunosuppression.32 In addition, as no study or fellow eye developed neovascular changes during the study, the effect of the investigational agent on the development of choroidal neovascularization cannot be discerned; sirolimus has been previously found to exert antiangiogenic effects in animal models of ocular neovascularization.33,34 Whether the two worrisome ocular AEs (central retinal thinning in two study eyes and para-GA FAF change in one study eye) were related to investigational product preparation is uncertain. It is possible that the development of retinal changes in areas bordering the GA lesion in participant 6 represents an acute onset of local RPE disorganization and atrophy not typically associated with GA. The other possible investigational product-related ocular change, observed in two out of six study eyes, involved an accelerated thinning of the central retina in the treated eye. The rates of retinal thinning here, corresponding to decreases in central retinal thickness of −45 and −51 μm in participants 5 and 6, respectively, over 4 months, were considerably greater than that previously observed in the natural history of GA (a decrease of −7.0 ± 2.7 μm/year, mean ± SEM, n = 8 eyes25).
Adverse events involving RPE atrophy and retinal thinning of the nature described here were not previously encountered in GA patients treated with subconjunctival sirolimus,25 nor in clinical trials involving the use of intravitreal sirolimus for other ocular diseases, including a phase I/II trial of repeat dosing of intravitreal sirolimus (352 μg every 2 months for three injections) for subfoveal choroidal neovascularization in AMD (NCT00712491),26 a dose-escalating phase I trial involving a single injection of intravitreal sirolimus (up to 352 μg) for diabetic macula edema,23 and a phase II trial involving intravitreal sirolimus (352 μg every 2 months) for noninfectious uveitis.21 Preclinical studies in rabbits involving a single injection of intravitreal sirolimus (220 μg, equivalent to a 660-μg dose in a human eye) also did not induce marked anatomical changes.26 Also, studies conducted with only vehicle (PEG 400 and ethanol) components of varying grades of PEG 400 and amounts of ethanol did not reveal an influence of PEG 400 grade or ethanol content. Little or no effects were observed at the commensurate injection volume (10 μL in a rabbit eye), although focal retinal lesions were observed at 4× to 10× larger volumes (Investigators' Brochure, DE-109, unpublished data, 2012; Santen Pharmaceutical Co., Ltd., Osaka, Japan). Trials are currently ongoing that involve higher levels of chronic intravitreal sirolimus exposure, including phase II and III trials for uveitis involving 440- and 880-μg doses at different administration frequencies (NCT01280669, NCT01358266).35 A larger phase II multicenter trial for GA involving 440-μg intravitreal doses delivered monthly was recently terminated (NCT01675947). These trials will be a source of safety data on sirolimus in eyes with and without GA.
How sirolimus may be related to the observed effects in treated eyes with GA is unclear. The mTOR signaling pathway integrates inputs from multiple stimuli,36 regulating cellular processes via diverse cell-specific molecular pathways. As such, sirolimus may exert complex and dose-dependent influences in different cell types. In RPE cells, sirolimus has been reported to reduce RPE loss and/or dedifferentiation in vivo37 and suppress RPE senescence both in vitro38 and in vivo,39 leading to a positive influence on survival. On the other hand, it has been reported to negate the protective effect of nerve growth factor on RPE cells undergoing oxidative stress, suggesting a negative survival influence.40 Sirolimus appears to exert positive effects on photoreceptor39 and ganglion cell41 survival in animal models of degeneration and injury, but can also induce degeneration-like alterations in photoreceptor cones.42 Finally, the ability of systemic sirolimus to influence uveitis in animal models has been reported to show a dose dependence, attenuating inflammation at higher doses but paradoxically exacerbating it at lower doses.43 These data underscore the potentially context-specific, disease-specific, and complex therapeutic effects of sirolimus.
In conclusion, in this small phase I/II study in patients with GA, we did not find evidence for anatomical or functional benefit associated with intravitreal sirolimus. Also, the investigational product preparation may be associated with an increase in retinal and/or RPE atrophy in a subset of GA patients, but an assessment of the prevalence of these effects and associated risk factors will require further data from other upcoming studies.
Acknowledgments
Supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health. PAP is supported by the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer, Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors.
Disclosure: P.A. Petrou, None; D. Cunningham, None; K. Shimel, None; M. Harrington, None; K. Hammel, None; C.A. Cukras, None; F.L. Ferris, None; E.Y. Chew, None; W.T. Wong, None
References
- 1. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104: 7–21. [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992; 99: 933–943. [DOI] [PubMed] [Google Scholar]
- 3. Chew EY, Clemons TE, Agron E, et al. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013; 120: 1604–1611, e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung E, Landa G. Update on current and future novel therapies for dry age-related macular degeneration. Expert Rev Clin Pharmacol. 2013; 6: 565–579. [DOI] [PubMed] [Google Scholar]
- 5. Meleth AD, Wong WT, Chew EY. Treatment for atrophic macular degeneration. Curr Opin Ophthalmol. 2011; 22: 190–193. [DOI] [PubMed] [Google Scholar]
- 6. Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013; 13: 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424. [DOI] [PubMed] [Google Scholar]
- 8. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421. [DOI] [PubMed] [Google Scholar]
- 9. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003; 76: 463–471. [DOI] [PubMed] [Google Scholar]
- 11. Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010; 94: 918–925. [DOI] [PubMed] [Google Scholar]
- 12. Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134: 411–431. [DOI] [PubMed] [Google Scholar]
- 13. Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001; 73: 887–896. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues EB. Inflammation in dry age-related macular degeneration. Ophthalmologica. 2007; 221: 143–152. [DOI] [PubMed] [Google Scholar]
- 15. Camardo J. The Rapamune era of immunosuppression 2003: the journey from the laboratory to clinical transplantation. Transplant Proc. 2003; 35 (3 suppl): 18S–24S. [DOI] [PubMed] [Google Scholar]
- 16. Vishnevetsky D, Patel P, Tijerino H, Gandhi PJ. Sirolimus-eluting coronary stent. Am J Health Syst Pharm. 2004; 61: 449–456. [DOI] [PubMed] [Google Scholar]
- 17. Napoli KL, Taylor PJ. From beach to bedside: history of the development of sirolimus. Ther Drug Monit. 2001; 23: 559–586. [DOI] [PubMed] [Google Scholar]
- 18. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998; 31: 335–340. [DOI] [PubMed] [Google Scholar]
- 20. Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003; 35 (3 suppl): 7S–14S. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013; 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sen HN, Larson TA, Meleth AD, Smith WM, Nussenblatt RB. Subconjunctival sirolimus for the treatment of chronic active anterior uveitis: results of a pilot trial. Am J Ophthalmol. 2012; 153: 1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dugel PU, Blumenkranz MS, Haller JA, et al. A randomized, dose-escalation study of subconjunctival and intravitreal injections of sirolimus in patients with diabetic macular edema. Ophthalmology. 2012; 119: 124–131. [DOI] [PubMed] [Google Scholar]
- 24. Krishnadev N, Forooghian F, Cukras C, et al. Subconjunctival sirolimus in the treatment of diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong WT, Dresner S, Forooghian F, et al. Treatment of geographic atrophy with subconjunctival sirolimus: results of a phase I/II clinical trial. Invest Ophthalmol Vis Sci. 2013; 54: 2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mudumba S, Bezwada P, Takanaga H, et al. Tolerability and pharmacokinetics of intravitreal sirolimus. J Ocul Pharmacol Ther. 2012; 28: 507–514. [DOI] [PubMed] [Google Scholar]
- 27. Fleckenstein M, Adrion C, Schmitz-Valckenberg S, et al. Concordance of disease progression in bilateral geographic atrophy due to AMD. Invest Ophthalmol Vis Sci. 2010; 51: 637–642. [DOI] [PubMed] [Google Scholar]
- 28. Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003; 110: 392–399. [DOI] [PubMed] [Google Scholar]
- 29. Feuer WJ, Yehoshua Z, Gregori G, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no. 26. JAMA Ophthalmol. 2013; 131: 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005; 123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meleth AD, Mettu P, Agron E, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011; 52: 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001; 40: 573–585. [DOI] [PubMed] [Google Scholar]
- 33. Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004; 10: 964–972. [PubMed] [Google Scholar]
- 34. Yagasaki R, Nakahara T, Ushikubo H, Mori A, Sakamoto K, Ishii K. Anti-angiogenic effects of mammalian target of rapamycin inhibitors in a mouse model of oxygen-induced retinopathy. Biol Pharm Bull. 2014; 37: 1838–1842. [DOI] [PubMed] [Google Scholar]
- 35. Maya JR, Sadiq MA, Zapata LJ, et al. Emerging therapies for noninfectious uveitis: what may be coming to the clinics. J Ophthalmol. 2014; 2014: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santulli G, Totary-Jain H. Tailoring mTOR-based therapy: molecular evidence and clinical challenges. Pharmacogenomics. 2013; 14: 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao C, Yasumura D, Li X, et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest. 2011; 121: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, Wang J, Cai J, Sternberg P. Altered mTOR signaling in senescent retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2010; 51: 5314–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol. 2012; 181: 472–477. [DOI] [PubMed] [Google Scholar]
- 40. Cao GF, Liu Y, Yang W, et al. Rapamycin sensitive mTOR activation mediates nerve growth factor (NGF) induced cell migration and pro-survival effects against hydrogen peroxide in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2011; 414: 499–505. [DOI] [PubMed] [Google Scholar]
- 41. del Olmo-Aguado S, Nunez-Alvarez C, Ji D, Manso AG, Osborne NN. RTP801 immunoreactivity in retinal ganglion cells and its down-regulation in cultured cells protect them from light and cobalt chloride. Brain Res Bull. 2013; 98: 132–144. [DOI] [PubMed] [Google Scholar]
- 42. Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009; 12: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Z, Wu X, Duan J, et al. Low dose rapamycin exacerbates autoimmune experimental uveitis. PLoS One. 2012; 7: e36589. [DOI] [PMC free article] [PubMed] [Google Scholar]