Abstract
The coastal sabkha in Ras Gemsa, Red Sea coast with its colonizing microbial mats and biofilms was investigated. The sabkha sediments consist mainly of terrigenous siliciclastic material accompanied by the development of evaporites. Halite serves as a good conduit for light and reduces the effect of intensive harmful solar radiation, which allows microbial mats to survive and flourish. The microbial mats in the evaporite–siliciclastic environments of such sabkha display distinctive sedimentary structures (microbially induced sedimentary structures), including frozen multidirected ripple marks, salt-encrusted crinkle mats, jelly roll structure, and petee structures. Scanning electron microscopy of the sediment surface colonized by cyanobacteria revealed that sand grains of the studied samples are incorporated into the biofilm by trapping and binding processes. Filamentous cyanobacteria and their EPS found in the voids in and between the particles construct a network that effectively interweaves and stabilizes the surface sediments. In advanced stages, the whole surface is covered by a spider web-like structure of biofilm, leading to a planar surface morphology. Sabkha with its chemical precipitates is a good model for potential preservation of life signatures. It is worthy to note that the available, published works on the subject of the present work are not numerous.
Keywords: Biofilms, Coastal sabkha, Evaporites, Microbial mats, Siliciclastics
Introduction
Many studies on microbial mats, the oldest and most successful microorganisms, showed that metabolic activity of cyanobacteria and heterotrophic bacteria in carbonate marine environments induces the precipitation of carbonates, which in turn form a microbial buildup named stromatolites [1–3]. Recent studies have shown that microbial mats are also of paleoenvironmental significance in shallow siliciclastic shelf settings through much of Earth history. Increasingly, microbial communities are recognized for playing a potentially important role in defining and modifying surface sediment characteristics in various settings, ranging from terrestrial, through marginal marine to continental margins [4]. Siliciclastic microbially induced sedimentary structures MISS [5–10] is adding to our knowledge about both present and past life. Systematic studies, leading from modern to increasingly older deposits, have revealed that fossil MISS occur in tidal flat and shelf sandstones of Phanerozoic, Proterozoic, and Archean ages and appear to have shown very little changes since at least 3.2 Ga [9,11–13]. The morphologies and paleoenvironmental distribution of such structures record the former presence of photoautotrophic microbial mats.
Sabkhas or “salt flats” are among the most saline natural environments that form under arid or semiarid climate. Their level is dictated by the local level of the water table and forms an equilibrium geomorphological surface, which may be periodically inundated by water [14,15]. Capillary evaporation leads to an increase in salinity of the interstitial waters and thus favors the formation of evaporites. The study of sabkha is important for several fields. Biologists were increasingly interested in the study of hypersaline ecosystems as amazingly high primary productivities are supported by such systems. Geologists became aware of the fact that many metal–sulfide deposits are associated with paleosabkha conditions [16,17]. Moreover, sabkhas have received many recent studies as they form important permeability barriers in both aquifers and hydrocarbon reservoirs [18].
In modern tidal flat environments, e.g. sabkhas, where high salinity restricts metazoans grazing, microbial mats tend to flourish. Siliciclastic sediments are widely overgrown by a great variety of benthic microorganisms, especially cyanobacteria which are most abundant in the upper intertidal and lower supratidal zones [19]. Cyanobacteria are blossoming in wet sandy environments and secrete sufficient extracellular polysaccharides (EPS). The EPS are adhesive mucilage that enables the benthic microorganisms to attach themselves to solid substrates such as the surface of a quartz grain, to transport nutrients toward the cell, and to buffer the microbes against the changing salinities in their microhabitat [20].
A biofilm is a collection of microorganisms, and their extracellular products bound to a solid surface termed as substratum [21]. It can be also regarded as microbially stabilized water [22] that glue and fix the surface sediments in a process known as biostabilization which increases stability against erosion [23–26].
The aim of the present work is to characterize different sedimentary structures induced by the microbial activity in evaporites and siliciclastic sediments in the coastal sabkha hypersaline environment, also to discuss the fossilization potential of microbes in evaporites. It is worthy to note that these structures are documented for the first time in this particular area of Egypt. In this respect, the detailed characteristics of their formation are given, especially in the absence of many published works on the subject.
Study area
The study area is the coastal sabkha of little Gemsa on the western side of the Gulf of Suez along the Red Sea coast Fig. 1. The Gulf of Suez is a large elongated embayment which is part of the rift system dividing the African and the European Asian plates. The modern Gulf of Suez occupies the central trough of the Suez rift which is only 50–70 m in depth [27]. A relatively wide gently sloping, coastal plain exists along this Gulf. The shoreline comprises a low-angle siliciclastic carbonate ramp depositional system that passes onshore into an extensive coastal sabkha environment. The sabkha extends over a large area and displays a low slope with isolated patches of high salt tolerant vegetation. It has no surface connection with the sea but a subterranean one, fluctuating with the tide in the open sea.
Fig. 1.

Location map of the study area.
Ras Gemsa is located between 27°39′0″N and 33°34′60″E and covers an area of about 10 km2. Gemsa forms two tongues which are extending out in the sea. The eastern one is named Great Gemsa and is 6.5 km long, while the western one is named Little Gemsa and is 3.5 km long Fig. 1 [28]. They form interbeds of evaporitic sulfates and marls of Miocene age. Sand and gravel intercalations are found and marked by the occurrence of an alluvial fan. The two tongues are separated by a lagoon of about 4 km2. Numerous small wadis drain the mountains of the area and dissect adjacent plains. These are lined with scattered Acacia trees. In little Gemsa, wide flat area of extensive intertidal mud and sand flats develops. The intertidal sediment deposits are subjected to regular emergence which leaves the surficial sediment layers exposed to extreme temperatures, rain and wind erosion, subsequent drying and compaction. Perennial and shallow ephemeral water bodies exist, and the formation of salt crusts depends on the annual alternation of dry and rainy seasons. These are depositional areas of no water current velocities. The sediments often experience large fluctuations in water content, salinity, and temperature resulting in extreme conditions that limit the range of organisms able to inhabit such an environment. Lower areas are submerged for longer periods of time. The sabkha deposits consist mainly of brown sand and silt with evaporites including gypsum, and halite. Non-evaporite components, mostly of detrital origin, include quartz, feldspars, and clay minerals. The sabkha region is dominated by deposition of clastics accompanied by the development of evaporites.
Material and methods
The material used in the present study was collected during June and December 2011 and October 2012. Thirty six surface sediment samples were obtained from different zones of the sabkha using a standard Ekman grap with a sampling dimension of 231 cm2. Sediment samples obtained were subjected to granulometric and microscopic studies. The grain size analysis of the sand (0.063–2 mm) and gravel (>2 mm) fractions was carried out using dry–wet sieving techniques; silt and clay fractions (finer than 0.063 mm) were analyzed using the pipette method [29].
Areas around the saline pools with luxuriant microbial mats and biofilm-forming assemblages were selected for sampling. Selection was based on mat development and accessibility. Samples of the microbial surface were collected by inverting a Petri dish and pressing it into the mat. The dish was then removed, and the shallow mat core carefully lifted and placed right-side-up in the Petri dish. The samples were studied and investigated under the binocular microscope. For scanning electron microscopy (SEM), 10 samples were placed in small glass tubes (diameter 0.5 cm) fixed immediately in 4% glutaraldehyde solution diluted with water from the sampling site. This treatment prevents osmotic shock and artifacts. The water was then removed in an ethanol series from 10% to 95% followed by two passages through absolute ethanol. Samples were then critical-point dried, gold-sputtered, and studied under the SEM Jeol JSM 35 CF, Tokyo.
Climatic setting
The study area could be regarded as semiarid. The climate is hot and dry, and rainfall is scarce. Data from Egyptian Meteorological authority, 2012 showed that the maximum temperature recorded in the summer season (June–September) is 27.5 °C, while the minimum, recorded in the winter season (December–March), is 17.8 °C. Relative humidity varies between 43% and 55%. The annual mean rainfall is 3 cm, concentrated in a few showers in the winter season, whereas there is almost no rainfall for the rest of the year. The area is one of the intense evaporations, where annual mean evaporation rates along the coast are estimated to be 13.9 mm/month. Northerly and northwesterly winds dominate the Gulf of Suez. Stormy southern winds are much less frequent and occur mainly in February. These arid conditions keep the surface water salinity above 220 g/L and allow the formation of the sabkha along the coast. It also affects the formation of evaporites. The fresh water supply in the area is limited to the amount which could account for all the detrital material in the sabkha area. Strong onshore winds prevail in the area and could contribute some material from the Miocene outcrops and the basement rocks.
Results and discussion
Grain size distribution
The grain size distribution and related textural classification of the surface sediments in the sabkha are summarized in Table 1. The surface sediments of the investigated area consist of a wide variety of textural classes. In the majority of sites, sand and gravel fractions constitute the bulk of the sediment fractions Table 1. The increase in gravel content in some samples reflects the abundance of transported terrigenous sediments and biogenic materials. The high mud content is most probably due to the terrigenous flux of wadies.
Table 1.
Grain size distribution of the studied sediment samples (values in %).
| Site | No. of samples | Average for each 3 samples |
Textural classification | |||
|---|---|---|---|---|---|---|
| Gravel | Sand | Silt | Clay | |||
| Perennial saline lake | 3 | 5.3 | 51.1 | 19.6 | 24 | Slightly gravelly muddy sand |
| 3 | 10.87 | 89 | 0.13 | 0.01 | Gravelly sand | |
| 3 | 21.2 | 77.42 | 1.36 | 0.01 | Gravelly sand | |
| Ephemeral saline pools | 3 | 6.75 | 82.71 | 2.00 | 8.54 | Gravelly muddy sand |
| 3 | 19.49 | 80.43 | 0.06 | 0.01 | Gravelly sand | |
| 3 | 10 | 34.85 | 33.4 | 21.75 | Gravelly sandy mud | |
| Dry and capillary mud flat | 3 | 0.86 | 89.00 | 2.13 | 8.01 | Muddy sand |
| 3 | 5.95 | 37.5 | 33.55 | 23.00 | Gravelly sandy mud | |
| 3 | 4.90 | 36.5 | 30.22 | 28.38 | Sandy mud | |
| Salt crust | 3 | 21.49 | 78.43 | 0.06 | 0.02 | Gravelly sand |
| 3 | – | 99.9 | 0.09 | 0.01 | Sand | |
| 3 | – | 95.9 | 2.3 | 1.8 | Sand | |
Depositional subenvironments in Ras Gemsa sabkha
Geomorphological and Sedimentological features enable identification of four well-defined zones located at different topographical levels (1) perennial saline pools; (2) ephemeral saline pools; (3) dry and capillary mud flat; and (4) supratidal flat of efflorescent halite crusts.
Perennial saline pools
Perennial saline pools extend along the deepest part of the sabkha area Fig. 2a, with a depth that varies between 20 and 50 cm. The maximum surface area of the pools occurs during winter season when several water bodies form one single pool. Ground and surface waters flow into the pools during flooding. Since the pools are closed and evaporation is high, water levels and salinity fluctuate seasonally, and the pools are much diminished in summer, to be subdivided into a group of completely smaller remnants. Windblown sand barriers, are common in the area, and influence to a considerable extent the subdivision of the pools. Such barriers are colonized by vegetation, which is in distinct association with a tendency for salt tolerance, along the periphery of the pools. The peripheral parts of the pools are gently elevated and are surrounded by concentric channels which are partially water-filled. The minerals in the pools are dominated by evaporites. Halite is the essential mineral with a subordinate amount of gypsum. A very thin film of halite precipitates on the brine surface, which tightly connected, mostly flattened euhedral crystals, of translucent halite develop later at the air–water interface as floating rafts Fig. 2b. They are held by surface tension until they are large enough to sink to the bottom of the brine forming a crust. Many crusts form as overgrowths on cumulated layers. Small biscuits may result from crystal growth diagenesis at local nucleation centers.
Fig. 2.
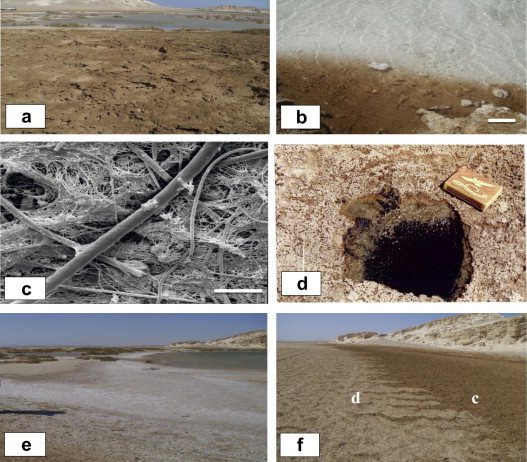
Depositional subenvironments in Ras Gemsa sabkha: (a) Perennial saline pools with halite floating crusts that grow at the periphery of the pools, (b) floating crusts of euhedral halite crystals. Scale is 15 cm, (c) SEM photomicrograph of the upper layer of microbial mat formed of filamentous cyanobacteria and algae with their EPS. Scale is 5 μm, (d) patches of oxidized reddish brown organic material at the surface followed in depth by black color of the anoxic part of the mat. Length of the match box is 5 cm, (e) ephemeral saline pools fringing the perennial ones and formed of halite raft texture, (f) dry sand flat with thin efflorescent salt crust, a few cm in thickness and the capillary mud flat is wet with the lack of interstitial saline minerals (marked as d and c).
Cohesive microbial mats of gelatinous appearance grow to a notable thickness at the margin of the perennial saline pools. Samples taken from the upper surface of the sediments show that the growth of microbial mats reaches up to 1.5 cm in overall thickness. This photosynthetic layer consists of a green zone that is dominated by filamentous cyanobacteria and unidentified algae with their EPS Fig. 2c. At depths greater than 2 cm, the microbial mat becomes increasingly black in color.
In some parts around the saline pools, irregular patches of oxidized organic material of reddish brown color are observed Fig. 2d. Sediments with black iron sulfide are a distinctive feature in the coastal microbial mats, where sulfate reducing bacteria are active. The sediments contain high amounts of iron as evidenced by the black color of the anoxic part of the mature mats and the brown oxidized layer of the oxic part of the freshly colonized sediment. The black color of iron reduction is recorded for a few mm below the surface and could reach the surface. This is probably due to the disturbance of the surface cyanobacteria layer by current action which removes the surface layer, leaving the iron-rich layer. According to Stal [30], some species of filamentous cyanobacteria are capable of binding iron to the polysaccharide sheaths where the iron is reduced concomitant with its binding. Krumbein et al. [31] explained that iron oxides may function as a redox buffer between anaerobic and aerobic organisms in the sediment, which helps in maintaining low sulfide concentrations, and may contribute to sediment stabilization.
Ephemeral saline pools
Ephemeral saline pools are mainly restricted to the southwestern part of the area. They fringe also the perennial saline pools Fig. 2e. Field observations during the years 2011 and 2012 indicate that most of the pools in the western area are of the ephemeral type, since they dry up once a year during the hot summer. However, water bodies may persist without drying up for a few months, as was observed in the late 2011. They have relatively flat, vegetation-free surfaces, and lack surface outflows. The pools area, range from 2 to 4 km2 during winter, being reduced to 1–3 km2 during summer. The pools normally reach a maximum water depth of 10 cm. During winter months, the sediments are submersed due to the slightly higher water table of the pools. In summer, the water level decreases and leaves white halite crusts. The pools have a characteristic asymmetrical morphology most probably due to their migration following the dominant wind direction (NW). When the brine reaches the halite saturation point, crystallization starts at the brine-air interphase as rafted textures. With time, crystals sink to the floor where syntaxial overgrowth may take place resulting in chevrons and cornet halite. Whenever the pools have completely dried up, a salt layer of variable thickness forms and cover the whole area of the pools. The salt layer typically reaches a maximum thickness of 5 cm; it is composed almost exclusively of cubic halite with minor quantities of gypsum. The duration of the evaporative concentration period is mostly governed by the hydrological balance, where crystallization is very high in summer and where dilution occurs in winter.
Dry sand and capillary mud flats
The shallow hypersaline pool surface water is widely surrounded by air-exposed saline flats with a general scarcity of water bodies Fig. 2f. The groundwater table stands closer to the surface (approximately 0.8 m), allowing the development of thinner efflorescent salt crusts (up to 1 cm thick). The efflorescence is composed almost exclusively of halite (over 90%) but is limited, almost entirely, to the surface. This high abundance of halite indicates the total evaporation of upward moving marine water at the sediment–air interface. The sediments are mainly terrigenous which consist of poorly sorted medium gravelly-sized siliciclastics sand. Halite crusts rarely exceed a few cm in thickness, which generally dissolve during winter. In the capillary mudflat, sediment surfaces are permanently wetted by capillary movement of the groundwater and lack any interstitial saline minerals Fig. 2f. Both dry and capillary mud flats are defined by the total absence of vegetation.
Supratidal flat of efflorescent halite crusts
The supratidal area is the marginal elevated zone that borders the saline pools; it is located inland from the pools border to a distance of about 200 m, so that it is not affected by tidal currents or even strong storms. There is no significant presence of microbial material in this zone. The sediments that may reach 60 cm in thickness are mainly clastics represented by sand, silt, and minor clay with halite and disseminated gypsum. The clastics can be regarded as a matrix for the host evaporites. The supratidal area acquires variable morphological shapes which pass abruptly from one to another over distances of a few meters. They are not exclusive to particular areas, but tend to have a patchy distribution around most of the pools. Polygonal crusts are thick, with small surface relief, mostly <10 cm high and develop into a distinctive pattern of ridges that are polygonal in plane form Fig. 3a. Due to scarcity of rainfall, the crusts form with clearly defined uplifted polygon margins. The diameter of the polygons usually ranges from 15 to 20 cm and varies with the thickness of the salt crust, which affects the mechanical strength of the crust [32]. White, efflorescent halite commonly forms along the margins of some of the polygons, also inside them, caused by evaporation of the groundwater brine that is drawn up to the surface by capillary pressure. The continuous growth and development of polygonal crusts without interruption for a long time produces a high-relief blocky surface, mostly >50 cm high Fig. 3b. Imitative salt crusts were also recognized and may be completely filled with sandy muddy sediments of the same grain size as the sediments underlying the crust Fig. 3c. They form when the surface was previously affected by wind. Smoot and Lowenstein [33] recorded similar crusts and mentioned that imitative efflorescent crusts rarely form on completely flat sediment surface, and that the crystallization of efflorescent salt partially preserves the preexisting surface morphology by leaving a cast of its original form. Other well recognized crusts often exhibit an irregular network of puffy or dome-like blisters Fig. 3d. They are rounded to oval in shape; the diameter varies between 2 and 3 cm. They include a higher proportion of underlying sediment and adhering eolian dust. Goodall et al. [32] have documented a similar structure in modern salt flats in SE Arabia and revealed that in blisters to form, the halite must be precipitated in an irregular manner and at different rates, which causes the salt crust surface to be characterized by a random network of rounded pressure ridges rather than the more organized polygonal pattern. They also added that the close proximity of the pressure ridges to each other interrupts growth and causes the polygonal network to lose its integrity and become more dome-like.
Fig. 3.

Supratidal flat of efflorescent halite crusts: (a) thick polygonal crusts with small surface relief. Halite is commonly growing along the margin and inside the polygons, (b) high-relief blocky crusts. N.B. Growth of halite along the fractures, (c) imitative salt crusts completely filled with sandy muddy sediments, (d) an irregular network of dome-like blisters.
Microbially induced surface sedimentary structures
Because the studied site is relatively isolated anthropogenic activity is low, and in the absence of metazoans grazing, microbial mats can grow freely without being disturbed. Microbial films and mats occur mainly along the margin of the saline pools. Field observations in such areas revealed a variety of structures induced by microbial mats and biofilm including frozen multidirected ripple marks, crinkle structures, jelly roll and petee structures.
Frozen multidirected ripple marks in the sense of Gerdes et al. [34] are patterns of consolidated ripple marks of various directions Fig. 4a. They are characteristic features of the upper intertidal to lower supratidal zones [5]. This ripple pattern arises from a set of subsequent storms each of which forms a generation of ripple marks. During the periods in between the storm events, the newly formed ripple marks are overgrown and biostabilized by microbial mats and thus cannot be reworked by the later events [10]. Field observations revealed that frozen multidirected ripple marks form during the spring growth season where a homogenous microbial mat veneer was observed over the sediment surface. SEM study of samples taken from these mats revealed that EPS of the common Microcoleus chthonoplastes was seen to bind sediment grains in the samples collected from the upper 2 mm of the surface layer. The processes of trapping, where particles are glued to the mat surface and binding where the mat gradually incorporates grains as it grows upwards are discussed by Gerdes et al. [34]. Sand grains of the studied samples that had been transported by wind are seen to be incorporated into the biofilm by the trapping and binding process Fig. 4b. Filamentous cyanobacteria, particularly M. chthonoplastes, have great effect in modifying the sediment surface as they form thick sheaths consisting of EPS which is very tough and resistant to degradation [21]. In addition, Gerdes and Krumbein [19] in their study of the tidal flats of the North Sea have revealed that the biomass production is increased in places characterized by the predominance of M. chthonoplastes. Accumulation of sedimentary particles by binding and trapping is a typical feature associated with this species [24]. Besides this, the binding meshwork of thick EPS effectively interweaves and stabilizes the surface sediments [35,26].
Fig. 4.

Frozen multidirected ripple marks: (a) two different generations of ripple marks (I and II, scale is 1 m), (b) thick biofilm binding and trapping sand grains leading to stabilization of the sediment surface, arrow points to M. chthonoplastes. Scale is 10 μm.
Salt-encrusted crinkle mats are confined to the periphery of the perennial saline pools. They appear as gently meandering uplifted microbial ridges with crests up to 2–5 cm. When viewed from up, such crests show more or less planar surfaces Fig. 5a. Crinkle structures result from the burial of microbial mats by freshly deposited sand [9]. High evaporation rates on the highest intertidal portion have resulted in the precipitation of gypsum and halite crystals within and beneath the microbial mat. The crystallization of halite and gypsum results in load pressure over the sediments which squeezes water out of the organic layers and probably enhance lithification processes. SEM study of representative samples taken from such structures showed that biofilms colonize first the deepest parts of the sediment surfaces as they provide greater moisture and protect the organisms against erosion [6]. They are found to fill the voids in and between the particles Fig. 5b and c. In advanced stages, the whole surface is covered by spider web-like structure of biofilm leading to a planar surface morphology Fig. 5d. Noffke et al. [35] documented similar features during their study in the tidal flats of the North Sea, where biomass production is high and named such microbial activity leveling. They revealed that filamentous mat-builders shelter their substrate against erosion by entangling sand and silt grains along the sediment surface. The microbes also secrete extracellular polymers that further increase the cohesion of surficial sediments [36]. In the fossil record, crinkle structures are documented in Archean sandstone [7], in Proterozoic [37], in the lower and middle Cambrian as well as the Silurian of Sweden [11].
Fig. 5.

Salt-encrusted crinkle mats: (a) meandering uplifted microbial ridges with salt-encrusted crinkles. Length of Marker pen is 15 cm, (b) thin section of microbial mats of filamentous cyanobacteria coated the sand and gypsum crystals. Scale is 500 μm, (c) biofilm colonizing the deepest parts of the sediment surfaces and fills the voids in and between the particles. Scale is 10 μm, (d) spider web structure of biofilm coating the whole particles of sand and gypsum crystals and leads to planar surface morphology. Scale is 2 μm.
Another feature of particular interest, which is confined to the very shallow parts of the pools where the thickness of the mat is about 0.5–0.7 cm, is the jelly roll structures Fig. 6a. They indicate the high production of biofilm and reflect the cohesive behavior of soft and jelly-like surfaces of microbial origin [38]. They are formed of 0.5–1 cm diameter separated and rounded burst open bubbles reflecting the flexible and cohesive behavior of the soft mat. Cohesive behavior during erosion, transport, and deposition is observed in both sandstones and mudstones and can be a very useful indicator of microbial mat colonization [38]. Additionally, experimental flume studies by Hagadorn and McDowell [25] revealed that microbial communities characterized by a thicker surface film provided greater erosional resistance and can inhibit the growth of ripples entirely, so that the bed shear stresses result in roll-up structures. They found that at flow velocities that produce ripples, no grain movement occurred, but at higher flow velocities (over ca 35 cm s−1), the entire surface and subsurface part of the mat was folded and then curled up on itself to form a roll-up structure. Cyanobacteria, even when they are not abundant, significantly affect the critical shear stress required for initiation of grain motion in medium sand [25].
Fig. 6.

(a) Jelly roll structure formed of burst open bubbles reflecting the flexible and cohesive behavior of the soft mat. Scale is 5 cm, (b) polygonal overthrust petee structures in halite-encrusted siliciclastic sediments. Scale is 50 cm.
During late summer, when the mat dries, it tends to break apart into overthrust, saucer-like petee structures Fig. 6b. The petees are thin crusts with small, mostly <10 cm high surface relief and develop into a distinctive pattern of polygonal rounded ridges in plane view. During wet seasons, microbial mats were found to colonize the outer petee surfaces, while during the dry season halite precipitates and leads to expansion and overthrust of the surface crust. The diameter of the polygons usually ranges from 25 to 50 cm and the relief is directly related to the groundwater level; the deeper the water table, the higher the relief of the petee structure is.
Preservation potential of microbes with evaporites
It is highly accepted that evaporites could be preserved over geological times where surface hydrological cycles are absent. They would be able of preserving traces of life independent of their producers. Evaporites, particularly halite, mineral precipitation permits the passage of photosynthetically active radiation and acts as UV-light scatterers so they can provide protection from cosmic radiation and allow certain life forms to survive in salt fluid inclusions for more than 100 million years [39,40]. Moreover, the interior of halite crusts seems to have unique microhabitats whose microenvironmental conditions cannot be found in soils or other lithic substrates [41]. This particular microhabitat is determined essentially by its hygroscopic nature which enhances the moisture conditions. A study by Wierzchos et al. [42] has shown that cyanobacteria which, grow within the pore spaces of rocks or below translucent rocks can retain more moisture than ambient conditions. In some cases however, they are endoevaporitic and found only within the halite rocks other than colonizing the surface of quartz grains. The fine halite and its intercrystalline spaces are occupied by air and/or high salt solution, a habitat that will not only aid in the retention of moisture because of capillary effects [43], but also might play an important role in conditioning the distribution and survival of microbial colonies [44]. Also cyanobacteria can carry out their metabolic activities under the stressed conditions of high salt and even low water [45]. EPS could act as a shield, slowing down desiccation and ameliorating the extreme external conditions [46]. Rothschild et al. [47] demonstrated that cyanobacteria inhabiting wet evaporite crusts of halite and gypsum were metabolically active for a long time after the dryness of the rock. The survival of archaea halophiles in dry salt over geological time scales has been reported by McGenity et al. [48].
Modern coastal sabkhas are widely colonized by microbial mats and biofilms. The abundance of a biofilm of EPS reflects the behavior developed by microbial mats living in such hypersaline systems. EPS layers allow cyanobacteria to increase their fossilization potential through early diagenesis of crystallization and cementation by gypsum and halite of these soft shaped layers. Preservation of EPS and biofilms depends on their rapid lithification before degradation [19]. Once lithified, these gel layers retain their biologic related morphology, which can be recognized in the fossil record. Furthermore, in carbonate environment the formation and persistence of ancient stromatolites depend on the binding and stabilizing of sediments by microbial mats and biofilms [31]. The initial biogenic stabilization of depositional systems may be an essential requirement to allow or enhance future lithification of the sediments [49].
The preservation potential of microbial mats with ancient evaporites has been documented by many authors. Barbieri et al. [50] studied the Upper Pleistocene evaporite deposits of a wide continental sabkha in southern Tunisia and found that biosignatures, with an intimate association with mucilaginous slime, are mostly contained in gypsum lithofacies precipitated from high salt-concentrated waters. These biosignatures include gypsified microfibers formed after the partial degradation of bacterial mucilaginous secretions. Recently, Noffke et al. [51] studied the Archean stromatolites in the Pilbara area of Western Australia which include a mixed carbonate–evaporite–siliciclastic coastal sabkha. The microbial mats generate a plethora of well-preserved MISS arising from interaction with sedimentary processes such as erosion or evaporite crystal growth. Upon comparison of fossil and modern biogenic structures and their facies-related distribution in sabkha settings, they strongly addressed the presence of microbial communities in the Paleoarchean of such hypersaline and extreme ecosystem.
Conclusions
Sabkhas, with its easier recognition and sampling is a good model not only for evaporites deposition in shallow marine environments, but also for preserving potential of traces of life within chemical precipitates. The climate of Ras Gemsa is hot and dry and solar radiation is intensive. These conditions favor the deposition of halite, which serves as a good conduit for light, reduces the effect of intensive harmful solar radiation, and provides protection from high cosmic radiation, which allows microbial mats to survive and flourish. The area is characterized by a low sedimentation rate, little wave action, lack of bioturbation, and is protected by vegetated patches of sea grasses, which results in an optimal development of microbial mats and biofilms. The microbial mats of Ras Gemsa sabkha produce distinctive sedimentary structures such as frozen multidirected ripples, salt-encrusted crinkle mats, jelly roll structures and petee structures. Most of these structures were found to be encrusted with halite. Thus within the halite rich rocks there will continually exist conditions suitable for the survival of microbes, particularly cyanobacteria. If the right balance is met between absence of surface hydrological cycles and rapid sealing provided by precipitating evaporite minerals, an ultimate lithification process with good preservation of microbially induced sedimentary structures can form. Intensive researches on microbial processes occurring in modern coastal sabkhas, which are extensively inhabited by microbial mats and biofilms, are needed to open the path of fully and better understanding of ancient microbial biota.
Conflict of interest
The author has declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
The author wishes to express her thanks to the anonymous referees for their constructive criticisms of an earlier version of the manuscript. Dr. G. Phillip, Cairo University, is highly acknowledged for his critical reading of the manuscript. I am much indebted to General Petroleum Company (GPC) for its virtuous hospitality during the trip. Dr. A. Abdel-Motelib and Dr. A. Wagdi, Cairo University, are highly acknowledged for their help in the field work.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Gerdes G., Dunaftschik-Piewak K., Riege H., Taher A.G., Krumbein W.E., Reineck H.E. Structural diversity of biogenic carbonate particles in microbial mats. Sedimentology. 1994;41:1273–1294. [Google Scholar]
- 2.Olveri E., Neri R., Bellanca A., Riding R. Carbonate stromatolites from a Messinian hypersaline setting in the Caltanissetta Basin, Sicily: petrographic evidence of microbial activity and related stable isotope and rare earth element signatures. Sedimentology. 2010;57:142–161. [Google Scholar]
- 3.Spadafora A., Perri E., Mckenzie J.A., Vasconcelos C.G. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology. 2010;57:27–40. [Google Scholar]
- 4.Schieber J. Microbial mats in terrigenous clastics: the challenge of identification in the rock record. In: Hagadorn JW, Pflueger F, Bottjer DJ, editors. Unexplored microbial worlds. Palaios 1999; 14: 3-13.
- 5.Noffke N., Gerdes G., Klenke Th., Krumbein W.E. Microbially induced sedimentary structures—Examples from modern sediments of siliciclastic tidal flats. ZBL für Geologie und Paläontologie. 1996;1(2):307–316. [Google Scholar]
- 6.Noffke N., Gerdes G., Klenke T., Krumbein W. Microbially induced sedimentary structures—a new category within the classification of primary sedimentary structures. J Sediment Res. 2001;71:649–656. [Google Scholar]
- 7.Noffke N. Microbially induced sedimentary structures in Archean sandstones: a new window into early life. Gondwana Res. 2007;11:336–342. [Google Scholar]
- 8.Schieber J., Bose P.K., Eriksson P.G., Banerjee S., Sarkar S., Altermann W., Catuneanu O. Elsevier; Amsterdam: 2007. Atlas of microbial mat features preserved within the siliciclastic rock record. [Google Scholar]
- 9.Noffke N. Springer; Berlin: 2010. Geobiology – microbial mats in sandy deposits from the Archean Era to today. [Google Scholar]
- 10.Cuadrado D.G., Carmona N.B., Bournod C. Biostabilization of sediments by microbial mats in a temperate siliciclastic tidal flat, Bahia Blanca estuary. Sediment Geol. 2011;237:95–101. [Google Scholar]
- 11.Calner M., Eriksson M.E. Microbial mats in siliciclastic depositional systems through time. In: Noffke N., Chafetz H., editors. The record of microbially induced sedimentary structures (MISS) in the Swedish paleozoic. SEPM Spec, Publication; 2012. pp. 29–36. [Google Scholar]
- 12.Gamper A., Heubeck C., Demskec D. Microbial mats in siliciclastic depositional systems through time. In: Noffke N., Chafetz H., editors. SEPM Spec, Publication; 2012. pp. 65–74. (Composition and microfacies of Archean microbial mats (Moodies Group, ca. 3.22 Ga, South Africa)). [Google Scholar]
- 13.Wehrmann A., Gerdes G., Höfling R. Microbial mats in siliciclastic depositional systems through time. In: Noffke N., Chafetz H., editors. SEPM Spec, Publication; 2012. (Microbial mats in a lower Triassic siliciclastic playa environment (Middle Buntsandstein North Sea)). [Google Scholar]
- 14.Khedr E.S. Recent coastal sabkhas from the Red Sea: a model of sabkhaization. Egypt J Geol. 1991;33(2):87–120. [Google Scholar]
- 15.Schreiber B.C., Lugli S., Babel M. Evaporites through Space and Time. Geol Soc London. 2007;285:470. Special Publication. [Google Scholar]
- 16.Renfro A.R. Genesis of evaporite associated-stratiform metaliferous deposits – a sabkha process. Econ Geol. 1974;69:33–45. [Google Scholar]
- 17.Mossman D.J. Stratiform barite in sabkha sediments, Walton-Cheverie, Nova Scotia. Econ Geol. 1986;81(8):2016–2021. [Google Scholar]
- 18.Leeder MR. Sedimentology and sedimentary basins from turbulence to tectonics.2nd ed. Wiley-Blackwell 2011.
- 19.Gerdes G., Krumbein W.E. Biolaminated deposits. Springer; Berlin: 1987. (Lect Notes Earth Sci). 9. [Google Scholar]
- 20.Noffke N. Turbulent lifestyle: microbial mats on Earth’s sandy beaches-Today and 3 billion years ago. GSA Today. 2009;18:1–4. [Google Scholar]
- 21.Decho A.W. Microbial exopolymer secretions in ocean environments: their roles in food webs and marine processes. Oceanogr Mar Biol Annual Rev. 1990;28:73–154. [Google Scholar]
- 22.Krumbein W.E. Paracelsus und die mucilaginischen Substanzen-500 Jahre EPS-Forschung. DGM-Mitt Jg. 1993:8–14. [Google Scholar]
- 23.Taher AG. Contribution of microbial mats and biofilms to biogenic stabilization of sediments. In: 3rd Int. Conf. Geology of the Arab World, Cairo University, Egypt; 1996: 235–54.
- 24.Noffke N., Krumbein W.E. A quantitative approach to sedimentary surface structures controlled by the interplay of microbial colonization and physical dynamics. Sedimentology. 1999;46:417–426. [Google Scholar]
- 25.Hagadorn J.W., McDowell C. Microbial influence on erosion, grain transport and bedform genesis in sandy substrates under unidirectional flow. Sedimentology. 2012;59:795–808. [Google Scholar]
- 26.Taher A.G. Abdel Motelib A. Microbial stabilization of sediments in a recent Salina, Lake Aghormi, Siwa Oasis, Egypt. Facies. 2014;60(1):45–52. [Google Scholar]
- 27.Sneh A., Friedman G.M. Hypersaline Ecosystems. In: Friedman G.M., Krumbein W.E., editors. Hypersaline Sea-marginal flats of the Gulfs of Elat and Suez. Springer; Berlin: 1985. pp. 104–124. [Google Scholar]
- 28.Aref MA. Petrological and sedimentological studies on some sulfur-bearing evaporite deposits on the western side of the Gulf of Suez, Egyp. Ph.D Thesis, Fac. Sci. Cairo Univ. Egypt.
- 29.Folk L. Hemplill; Austin, Texas: 1974. Petrology of sedimentary rocks. p. 182 [1992 P. 7] [Google Scholar]
- 30.Stal L.J. Biostabilization of sediments. In: Krumbein W.E., Stal L., Paterson D., editors. Ecophysiological interactions related to biogenic sediment stabilization. Germany; Oldenburg: 1994. pp. 41–53. [Google Scholar]
- 31.Krumbein W.E., Paterson D.M., Stal L. Oldenburg; Germany: 1994. Biostabilization of sediments. p. 525. [Google Scholar]
- 32.Goodall M., North C.P., Glennie K.W. Surface and subsurface sedimentary structures produced by salt crusts. Sedimentology. 2000;47:99–118. [Google Scholar]
- 33.Smoot J.P., Lowenstein T.K. Evaporites, petroleum and mineral resources. In: Melvin J.L., editor. Depositional environments of non-marine evaporates. Elsevier; Amsterdam: 1991. [Google Scholar]
- 34.Gerdes G., Krumbein W.E., Noffke N. Microbial sediments. In: Riding R.E., Awramik S.M., editors. Evaporite microbial sediments. Springer; Berlin: 2000. [Google Scholar]
- 35.Noffke N., Gerdes G., Klenke T. Benthic cyanobacteria and their influence on the sedimentary dynamics of peritidal depositional systems (siliciclastic, evaporitic salty and evaporitic carbonatic) Earth Sci Rev. 2003;62:163–176. [Google Scholar]
- 36.Decho A.W. Microbial sediments. In: Riding R., Awramik S., editors. Exopolymer microdomains as a structuring agent for heterogeneity within microbial biofilms. Springer; Berlin: 2000. pp. 9–15. [Google Scholar]
- 37.Hagadorn J.W., Bottjer D.J. Restriction of a late Neoproterozoic biotope; Suspect-microbial and trace fossils at the Vendian–Cambrian transition. Palaios. 1999;14:73–85. [Google Scholar]
- 38.Gerdes G. Structures left by modern microbial mats in their host sediment. In: Schieber J., Bose P., Eriksson P.G., Banerjee S., Altermann W., Catuneanu O., editors. Atlas of microbial mat features preserved within the siliciclastic rock record. Elsevier; 2007. pp. 5–38. [Google Scholar]
- 39.Kminek G., Bada J.L., Pogliano K., Ward J. Radiation dependent limit for the viability of bacterial spores in halite fluid inclusions and on Mars. Radiat Res. 2003;159:722–729. doi: 10.1667/0033-7587(2003)159[0722:rlftvo]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cockell S.A., Raven J.A. Zones of photosynthetic potential on Mars and the early Earth. Icarus. 2004;169:300–310. [Google Scholar]
- 41.De Los Rios A., Wierzchos J., Sancho L.G., Ascaso C. Acid microenvironments in microbial biofilms of Antarctic endolithic microecosystems. Environ Microbiol. 2003;5:231–237. doi: 10.1046/j.1462-2920.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- 42.Wierzchos J., Ascaso C., Mckay C.P. Endolithic Cyanobacteria in halite rocks from the hyperarid core of the Atacama Desert. Astrobiology. 2006;6:1–8. doi: 10.1089/ast.2006.6.415. [DOI] [PubMed] [Google Scholar]
- 43.McKay C.P., Friedmann E.I., Gómez-Silva B., Cáceres-Villanueva L., Andersen D.T., Landheim R. Temperature and moisture conditions in the extreme arid regions of the Atacama Desert: four years of observations including the El Niño of 1997–1998. Astrobiology. 2003;3:393–406. doi: 10.1089/153110703769016460. [DOI] [PubMed] [Google Scholar]
- 44.De Los Rios A., Valea S., Ascaso C., Davila A., Kastovsky J., McKay C.P., Gomez-Silva B., Wierzchos J. Comparative analysis of the microbial communities inhabiting halite evaporites of the Atacama Desert. Int Microbiol. 2010;13:79–89. doi: 10.2436/20.1501.01.113. [DOI] [PubMed] [Google Scholar]
- 45.Rothschild L.J. Earth analogs for Martian life: microbes in evaporites, a new model system for life on Mars. Icarus. 1990;88:246–260. doi: 10.1016/0019-1035(90)90188-f. [DOI] [PubMed] [Google Scholar]
- 46.Grilli C.M., Ocampo-Friedmann R., Friedmann E.I. Cytology of long-term desiccation in the desert cyanobacteria Chroococcidiopsis (Chroococcales) Phycologia. 1993;32:315–322. doi: 10.2216/i0031-8884-32-5-315.1. [DOI] [PubMed] [Google Scholar]
- 47.Rothschild L.J., Giver L.J., White M.R., Mancinelli R.L. Metabolic activity of microorganisms in evaporites. J Phycol. 1994;30:431–438. doi: 10.1111/j.0022-3646.1994.00431.x. [DOI] [PubMed] [Google Scholar]
- 48.McGenity T.J., Gemmell R.T., Grant W.D., Stan-Lot-ter H. Origins of halophilic microorganisms in ancient salt deposits: mini review. Environ Microbiol. 2000;2:243–250. doi: 10.1046/j.1462-2920.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- 49.Paterson D.M., Aspden R.J., Visscher P.T., Consalvey M., Andres M.S., Decho A.W., Stolz J. Reid RP Light-dependent biostabilization of sediments by stromatolite assemblages. PLoS ONE. 2008;3:1–10. doi: 10.1371/journal.pone.0003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbieri R., Stivalettaa N., Marinangelib L. Gabriele Orib G. Microbial signatures in sabkha evaporite deposits of Chott el Gharsa (Tunisia) and their astrobiological implications. Planet Space Sci. 2006;54:726–736. [Google Scholar]
- 51.Noffke N, Christian D, Wacey D, Hazen RM. A microbial ecosystem in an ancient sabkha of the 3.49 GA Pilbara, Western Australia and comparison with Mesoarchean, Neoproterozoic and Phanerozoic examples. GSA, annual meeting and exposition; 2013. No. 190–8.



