Abstract
Human trophoblast invasion and differentiation are essential for successful pregnancy outcome. The molecular mechanisms, however, are poorly understood. Interleukin (IL)-11, a cytokine, regulates endometrial epithelial cell adhesion. Leukemia inhibitory factor (LIF) is one of the key cytokines in the embryo implantation regulation. The present study aimed to assess the levels of LIF, IL-11, and IL-11 α receptor gene expression in the endometrium of women undergoing IVF and correlate their levels with the IVF pregnancy outcome. Also, the study aimed to detect any mutation in these three genes among IVF pregnant and non-pregnant women versus control menstrual blood of fertile women. Endometrial tissue biopsies were taken from 15 women undergoing IVF on the day of oocyte retrieval. The quantitative expression of IL-11, IL-11Rα, and LIF genes was assessed by real-time PCR and PCR products were sequenced. Menstrual blood from 10 fertile women was used as control to compare the DNA sequence versus DNA sequence of the studied genes in endometrial biopsies. LH, FSH, and E2 were assessed for enrolled patients by ELISA. Endometrial thickness was also assessed by pelvic ultrasonography. No significant difference was detected between quantitative expression of the three studied genes and pregnancy IVF outcome. Although DNA sequence changes were found in IL-11 and LIF genes of women with negative pregnancy IVF outcome compared to women with positive pregnancy IVF outcome, no DNA sequence changes were detected for IL-11Rα. Other studied parameters (e.g., age, LH, FSH, E2, and endometrial thickness) showed no significant differences or correlation of quantitative expression of the three studied involved genes. Data suggested that there were no significant differences between quantitative expression of IL-11, IL-11Rα, and LIF genes and the IVF pregnancy outcome. The present study may reveal that changes in IL-11 and LIF genes sequence may contribute in pregnancy IVF outcome.
Abbreviations: IL-11, interleukin 11; IL-11Rα, interleukin receptor α; LIF, leukemia inhibitory factor; IVF, in vitro fertilization; FSH, follicular stimulating hormone; LH, Luteinizing hormone; E2, Estradiol 2
Keywords: Interleukin-11 (IL-11), Interleukin-11 receptor α (IL-11Rα), Leukemia inhibitory factor (LIF), IVF, DNA sequence
Introduction
Embryo implantation is a complex process requiring synchronized endometrial receptivity and blastocyst competence [1]. The initial apposition, attachment, and adhesion of the blastocyst to an adequately prepared or receptive maternal endometrium occur via a coordinated dialog of locally produced molecules, including cytokines, adhesion, and extracellular matrix (ECM) molecules [2].
A class of cytokines, which play an important role in embryonic implantation, is the interleukin-(IL) 6 superfamily. That family consists of numerous cytokines, including leukemia inhibitory factor (LIF), IL-6, interleukin-11 (IL-11), neurotrophic factor, oncostatin-M, and cardiotrophin-1. An important characteristic of that class of cytokines is their sharing of intracellular signaling through gp130 [3]. IL-11 and LIF signal via a hetero-dimeric receptor complex comprising either the specific IL-11 receptor α chain or the low-affinity LIF receptor, associated with the common signaling component gp130. Binding of IL-11 or LIF to their receptors forms a complex that signals via activation of Janus kinases (JAKs) that subsequently phosphorylate tyrosine residues in the cytoplasmic domain of the gp130 subunit. This in turn triggers signaling cascades involving mitogen activated protein kinases (MAPKs) and signal transducer and activator of transcription (STAT) family, in particular STAT3 and STAT1 proteins, resulting in the activation of transcription of specific genes [4,5].
During the secretory phase of the menstrual cycle, human endometrial stromal cells spontaneously differentiate into decidualized stromal cells which are morphologically and biochemically distinct. If pregnancy ensues, decidualization proceeds further and provides the maternally derived component of the placenta. The molecular interactions that regulate the formation, maintenance, and remodeling of decidua are poorly understood although many factors are known to be involved [6]. IL-11 is absolutely required for decidualization of endometrial stromal cells and blastocyst implantation in mice [7]. In humans, IL-11 mRNA and protein are expressed in the endometrium throughout the menstrual cycle, while its expression in the stroma was reported to be restricted to the predecidualized stromal cells in the late secretory phase to help the blastocyst implantation. The expression of IL-11 and its receptor (IL-11Rα) was found to be maximal during decidualization, suggesting that their interactions in the decidua are important in that process [3,6].
Leukemia inhibitory factor (LIF) derived its name from its ability to induce the terminal differentiation of myeloid leukemia cells, thus preventing their continued growth. One of the main properties attributed to LIF is the regulation of embryo implantation. LIF had been shown to facilitate implantation in the mouse model and possibly in humans [8]. LIF is expressed in the luminal epithelium during the mid-late secretory phase (days 18–28) of the menstrual cycle, supporting a role in implantation [9]. It has been suggested that recombinant human LIF might help to improve the implantation rate in women with unexplained infertility [10]. Many in vitro fertilization (IVF) studies using gene-matrix technology had revealed some differences in the expression of many molecules, cytokines, and other factors in endometrium of infertile women compared with fertile women [11,12].
The aim of the present study was to assess the levels of LIF, IL-11, and IL-11 α receptor gene expression in the endometrium of women undergoing IVF and correlate their levels with the IVF pregnancy outcome. Also, the study aimed to detect any sequence mutation in these three genes among IVF pregnant and non-pregnant women versus control menstrual blood of fertile women.
Methodology
Patients and tissues
Fifteen women were enrolled in the current study; they were under IVF long protocol in The IVF Centre, Kasr El Aini Hospital, Cairo University, Egypt. Patients fulfilled the inclusion criteria that included the following: age between 23 and 35 years, FSH < 10 mIU/ml, no endometriosis, no previous uterine operations, no history of poor response in previous IVF cycles, no diabetes mellitus, and no antral follicle count (AFC) > 5. All patients gave their written informed consent to participate in the study.
Endometrial tissue samples were taken on the day of oocyte retrieval using soft suction plastic catheter. The original plane of this study was to take the endometrial biopsy twice on day of pick up and on day of transfer (day 5 post-LH surge), but we observed the occurrence of endometrial bleeding, so we stopped the procedure and assess results by chemical pregnancy rate. The pregnancy rate was 50% among the done cases, but the IVF board reconsiders the biopsy at day of pick up only. Standard long protocol was used. Down regulation started on day 21 of the previous cycle using decapeptyl 0.1 mg sc daily till withdrawal occurs, serum E 2 done on day 2 of cycle when less than 50 and endometrial thickness less than 5, stimulation with 150–300 IU of HMG was started. Folliculometry started 7 days then continued every other day till more than 4 follicles of 18 mm size are seen, and HCG 5000–10,000 iu GIVEN IM 36 hs before ovum pick up all embryos were day 3 6–8 cell embryos. The menstrual blood of 10 women with regular menstrual cycles and with no apparent endometrial dysfunction was taken as control samples. The study protocol and informed consents were approved by the Human Ethics Committee of Cairo University.
Total RNA isolation
Endometrial biopsies and menstrual control blood were lysed by RLT buffer (QIAGEN, Germantown, MD). The lysates were further prepared for total RNA extraction using the RNeasy mini kit (QIAGEN, Germantown, MD) according to the manufacturer’s instructions. DNase was applied to avoid DNA contamination. The RNA extract was stored at −80 °C until future use. RNA purity, yield, and concentration were determined through dual spectrophotometry (Beckman, USA), and 1 μg of RNA was run on a 1% agarose gel (Roche, Castle Hill, Australia) to ensure integrity of the RNA.
Quantitative RT-PCR (qRT-PCR)
Reverse transcriptase (RT) reaction mixture using High Capacity Reverse Transcriptase kit (Applied Biosystems, USA) containing 1 μg total RNA from each sample for cDNA synthesis, 0.5 μg random primer, 5 × RT buffer, 2.5 mmol/l dNTP, 20 U RNase inhibitor, and 200 U MMLV reverse transcriptase in a total volume of 25 μl was incubated at 37 °C for 60 min then heated to 95 °C for 5 min to inactivate MMLV. Minus RT for each sample was applied as negative control. RT was followed by qPCR, 50 ng of cDNA was added to 5× Fast-Start SYBR green master mixes with Rox (Roche Diagnostics, Indianapolis, IN) and 200 ng of primer mix (Sigma). The reaction was carried out in micro-optical plates (Applied Biosystems) and analyzed using StepOne real-time PCR system (Applied Biosystems). The PCR running method was as follows: 10 min at 95 °C for enzyme activation followed by 40 cycles of 15 s at 95 °C, 20 s at 55 °C, and 30 s at 72 °C for the amplification step. The primers used in the qRT-PCR evaluation were specific for target genes (Table 1). Relative mRNA expression was calculated by the comparative cycle threshold method (ΔΔCt) as outlined in the manufacturer’s user manual with GAPDH housekeeping gene. The fluorescence was plotted versus PCR cycle number for reaction, and each sample was indicated.
Table 1.
The oligonucleotide primers sequence of the studied genes.
| Gene | Primer sequence | Gene accession number |
|---|---|---|
| IL-11 | Forward: 5′-TCTCTCCTGGCGGACACG-3′ | NT_011109.16 |
| Reverse: 5′-AATCCAGGTTGTGGTCCCC-3′ | ||
| IL-11Rα | Forward: 5′-CACACCCTCGGCTACTTGAT-3′ | NT_008413.18 |
| Reverse: 5′-AAGAAAGGATTCCCAAAGACG-3′ | ||
| LIF | Forward: 5′-TGGTTCTGCACTGGAAACATG-3′ | NT_011520.12 |
| Reverse: 5′-GTAATAGAGAATAAAGAGGGCATTGG-3′ | ||
| GAPDH | Forward: 5′-CCTCTACTGGCGCTGCCAAGGCT-3′ | NT_009759.16 |
| Reverse: 5′-GTCCACCACTGACACGTTGG-3′ | ||
Serum hormonal levels assay
FSH, LH, and E2 were estimated by ELISA according to instructions of manufacturers.
DNA purification and sequencing analysis
IL-11 and LIF genes were analyzed by direct sequencing of the PCR products using SEQr kit (Applied Biosystems), according to manufacturer’s protocol. PCR products were purified using the QIAquick Gel Extraction Kit (QIAGEN). The relevant purified DNA samples of all the cases and controls were amplified and sequenced using automated sequencing with the aid of a Big Dye Terminator Sequencing Kit (PE/Applied Biosystems, Foster City, CA). The samples were run in an automated sequencer ABI Prism 310 Avant (PE/Applied Biosystems). All samples were sequenced twice to ensure the results.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (±SD), median, and range. Comparison between women who could achieve pregnancy and those who did not was done using Mann Whitney U test for independent samples. Correlation between various variables was done using Spearman rank correlation equation for non-normal variables. P values < 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
There was a statistical significant difference detected between control group and studied cases group as regards the studied cytokines genes expression levels but no significant difference as regards demographic data and hormonal levels (Table 2).
Table 2.
Demographic and Biochemical characteristics of control and study groups.
| Control group (n = 10) |
Study group (n = 15) |
p Value | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (range) | Mean ± SD | Median (range) | ||
| Age (years) | 29.4 ± 5.0 | 29.0 (23.0–38.0) | 29.0 ± 3.9 | 28.0 (23.0–35.0) | 0.968 |
| FSH (mIU/ml) | 5.7 ± 0.8 | 5.6 (4.6–7.0) | 5.6 ± 1.9 | 6.0 (3.4–9.4) | 0.778 |
| LH (mIU/ml) | 4.1 ± 0.6 | 4.0 (3.2–5.0) | 5.0 ± 3.7 | 4.2 (0.7–15.0) | 0.904 |
| IL-11 | 7.5 ± 0.3 | 7.5 (7.1–8.0) | 11.3 ± 2.1 | 9.3 (6.3–13.3) | <0.001 |
| IL-11Rα | 5.5 ± 0.4 | 5.5 (5.1–6.2) | 9.1 ± 2.1 | 9.3 (4.5–11.2) | 0.001 |
| LIF | 8.5 ± 0.2 | 8.5 (8.2–8.9) | 11.7 ± 2.5 | 12.6 (5.3–13.9) | 0.001 |
FSH: Follicular stimulating hormone, LH: Luteinizing hormone, and p value: significant if < 0.05.
There was no statistical significant difference detected between cases with IVF positive or negative pregnancy outcome for demographic data, hormonal levels, and the studied cytokines levels (Table 3).
Table 3.
Test for the difference between demographic and biochemical characteristics of patients with IVF negative and positive pregnancy outcome.
| Negative IVF pregnancy outcome (n = 10) |
Positive IVF pregnancy outcome (n = 5) |
p Value | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (range) | Mean ± SD | Median (range) | ||
| Age (years) | 28.6 ± 4.0 | 28.0 (23.0–35.0) | 30.0 ± 4.4 | 28.0 (27.0–35.0) | 0.538 |
| FSH (mIU/ml) | 5.5 ± 1.3 | 6.0 (3.8–7.0) | 5.8 ± 2.8 | 5.2 (3.4–9.4) | 0.705 |
| LH (mIU/ml) | 5.7 ± 4.5 | 4.9 (1.0–15.0) | 3.8 ± 1.5 | 3.5 (2.0–6.0) | 0.450 |
| E2 (pg/ml) | 52.1 ± 8.9 | 49 (44.0–67.0) | 46.7 ± 15.0 | 48.0 (31.0–61.0) | 0.569 |
| End. thickness (mm) | 12.4 ± 1.6 | 12.0 (10.0–15.0) | 11.0 ± 1.4 | 11.0 (9.0–13.0) | 0.117 |
| IL-11 | 12.5 ± 0.9 | 12.7 (11.4–13.9) | 12.7 ± 0.6 | 12.9 (11.8–13.3) | 0.667 |
| IL-11Rα | 9.7 ± 0.8 | 9.3 (8.7–11.2) | 9.9 ± 1.0 | 9.7 (8.6–11.1) | 0.539 |
| LIF | 12.3 ± 1.0 | 12.5 (10.6–13.3) | 11.5 ± 0.8 | 11.6 (10.5–12.4) | 0.140 |
FSH: Follicular stimulating hormone (mIU/ml), LH: Luteinizing hormone (mIU/ml), E2: Estradiol (pg/ml) and End. thickness: Endometrial thickness (mm). p Value: indicates significant value when < 0.05.
There was no statistical significant correlation between levels of IL11, IL-11Rα, and LIF gene expression and other data as age, FSH, LH, E2, and endometrial thickness (Table 4).
Table 4.
Correlation between IL11, IL-11Rα, and LIF gene expression and age, FSH, LH, E2, and endometrial thickness.
| IL-11 | IL-11 R | LIF | |
|---|---|---|---|
| Age | |||
| r | −0.307 | 0.174 | −0.147 |
| p | 0.358 | 0.608 | 0.665 |
| FSH | |||
| r | −0.127 | −0.528 | 0.132 |
| p | 0.709 | 0.095 | 0.698 |
| LH | |||
| r | −0.182 | −0.323 | 0.151 |
| p | 0.593 | 0.332 | 0.658 |
| E2 | |||
| r | 0.224 | 0.164 | 0.322 |
| p | 0.533 | 0.650 | 0.364 |
| Endometrial thickness | |||
| r | −0.260 | 0.086 | 0.409 |
| p | 0.349 | 0.760 | 0.130 |
r: Correlation coefficient.
DNA sequence analysis results
mRNA expression for the three studied genes was quantitated versus GAPDH as housekeeping gene. There were no statistical significance differences between the three studied genes expression quantitation. Blind DNA sequencing for PCR products of IL-11 and LIF was done in order to find possible DNA sequence changes that allow occurrence of pregnancy or not. There was DNA sequence transition from G (at IVF positive pregnancy cases) to T (at IVF negative pregnancy cases) at nucleotide 365 for IL-11 gene (Fig. 1) and transition from C (at IVF positive pregnancy cases) to T (at IVF negative pregnancy cases) at nucleotide 351 for LIF gene (Fig. 2). These sequence changes were compared to DNA sequence of studied genes in control menstrual blood of fertile women, and they were similar to DNA sequences of IVF positive pregnancy cases. No DNA sequence change was detected for IL-11Rα gene at both IVF positive and negative pregnancy cases.
Fig. 1.
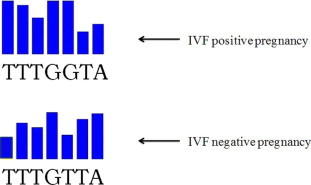
DNA sequence analysis for IL-11 gene showed G to T transition at nucleotide-365.
Fig. 2.
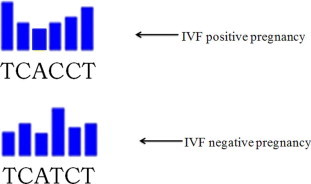
DNA sequence analysis for LIF gene showed C to T transition at nucleotide-351.
Discussion
Implantation is a complex process, in which the foreign blastocyst needs to be accepted by the maternal endometrium. Allowance for that, extensive preparation and bidirectional communication between the blastocyst and the endometrium are required [13]. Implantation process encompasses several distinct stages: apposition, adhesion, penetration, and trophoblast invasion. These steps can only take place during the window of implantation [14].
In human in vitro fertilization (IVF), embryo transfer can be accompanied by a low implantation rate even after a very successful IVF. The continuous tendency of improving implantation rates is of prime importance. The economic problems and psychological constraints linked to the necessity of undergoing multiple attempts before obtaining a successful pregnancy are a continuous worry for both medical practitioners and economists. Further specific and detailed knowledge about the molecule network that controls proper implantation is needed to be known in such a perspective.
Many molecules play important roles in the events of implantation such as adhesion molecules, chemokines, cytokines, growth factors, and invasive proteinases [15]. Both interleukin 11 (IL-11) and leukemia inhibitory factor (LIF) are two of few cytokines known to be absolutely required for blastocyst implantation, and both are obligatory for implantation in mice [7].
In the current study, we confirmed, by using quantitative RT-PCR, that there were no significant differences among gene expression levels of LIF, IL-11, and IL-11Rα between implantation versus non-implantation IVF.
In agreement with our results, Gazvani et al. [15] reported that there was no significant difference in IL-11 mRNA expression pattern in the peritoneal fluid and the endometrium of fertile and infertile women.
Additional confirmation to our results was added by Mikolajczyk et al. [12] who showed no statistically significant differences in uterine flushing in infertile women (with and without endometriosis) with regard to IL-11 and LIF levels when compared to fertile controls. Their results were also confirmed by the results of RT-PCR, where there were no differences between studied groups.
However, the present results disagree with those reported by Lédée-Bataille et al. [16] and Dimitriadis et al. [6,17] who concluded that LIF, IL-11, and IL-11Rα mRNA and protein are down-regulated in the endometrium of women with unexplained/idiopathic infertility or infertility and endometriosis compared with that of fertile controls. Also, reduced IL-11 and LIF secretion by endometrial epithelial cells may be responsible for the reduced implantation/pregnancy rates in excessive ovarian responders during IVF treatment [18,19].
This contradiction between results may be reasonably explained due to the different techniques used, the different timings for endometrial sampling, or the number of patients included in each study.
In the present study, changes in the sequences of IL-11 and LIF DNA were detected at failed IVF pregnancy outcome compared to DNA sequence for both genes of successful IVF pregnancy outcome. While in IL-11Rα, no DNA sequence changes were detected at both failed and successful IFV pregnancy outcomes.
Female mice with a null mutation of the IL-11 are infertile due to a defective post-implantation response to the implanting blastocyst, whereas female mice with no functional LIF gene are infertile due to an inability of normal embryos to implant [7,20].
Mutation of LIF gene results in reproductive failure in LIF −/− mice due to an inability to implant their blastocysts. This condition is reversed by infusion of LIF or by transfer of embryos to pseudo-pregnant recipient of wild-type mice [21]. Kralickova et al. [22] investigated the prevalence of the LIF gene mutations in the population of infertile women that consisted of nulligravid and secondary infertile patients. They revealed that the frequency of functionally relevant mutations of the LIF gene in infertile women is significantly enhanced in comparison with fertile controls.
Certain studies suggested that LIF gene mutations contribute to embryo implantation failure and thus to infertility and decreased pregnancy rates in Assisted Reproductive Technology (ART) [22,23]. These results coincided with our suggestion that the LIF gene mutations affect fertility.
The current work is just a pilot study, and a larger number of infertile patients to be compared with fertile women are needed for further investigations. Also, complete and detailed understanding of the complex regulatory mechanisms may provide new therapeutic targets for female infertility. Future work should include stem cells from a patient with a gene defect can be corrected, and scientists believe that when reintroduced into the patient, they could treat the effects of the mutation causing the disease.
Conclusions
Non significant correlation was found between quantitative mRNA of LIF, IL-11, and IL-11Rα gene expressions and IVF pregnancy outcomes. IL-11 and LIF genes mutation may contribute to IVF failure pregnancy outcomes. Our data may suspect diagnosis of endometrial associated implantation failure.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgement
This study was funded from Cairo University, Faculty of Medicine, Egypt.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Salamonsen L.A., Nie G., Hannan N.J., Dimitriadis E. Preparing fertile soil the importance of endometrial receptivity. Reprod Fertil Dev. 2009;21(7):923–934. doi: 10.1071/RD09145. [DOI] [PubMed] [Google Scholar]
- 2.Sharkey A.M., Smith S.K. The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol. 2003;17(2):289–307. doi: 10.1016/s1521-6934(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 3.Cork B.A., Tuckerman E.M., Li T.C., Laird S.M. Expression of interleukin (IL)-11 receptor by the human endometrium in vivo and effects of IL-11, IL-6 and LIF on the production of MMP and cytokines by human endometrial cells in vitro. Mol Hum Reprod. 2002;8(9):841–848. doi: 10.1093/molehr/8.9.841. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J.G., Chen J.R., Hernandez L., Alvord W.G., Stewart C.L. Dual control of LIF expression and LIF receptor function regulate STAT3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci. 2001;98(15):8680–8685. doi: 10.1073/pnas.151180898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitriadis E., White C.A., Jones R.L., Salamonsen L.A. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod. 2005;11(6):613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 6.Dimitriadis E., Stoikos C., Stafford-Bell M., Clark I., Paiva P., Kovacs G. Interleukin-11, IL-11 receptor alpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006;69(1):53–64. doi: 10.1016/j.jri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Robb L., Li R., Hartley L., Nandurkar H.H., Koentgen F., Begley C.G. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4(3):303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 8.Hu W., Feng Z., Teresky A.K., Levine A.J. P53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 9.Van Mourik M.S., Macklon N.S., Heijnen C.J. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukocyte Biol. 2009;85(1):4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 10.Aghajanova L. Leukemia inhibitory factor and human embryo implantation. Ann NY Acad Sci. 2004;1034:176–183. doi: 10.1196/annals.1335.020. [DOI] [PubMed] [Google Scholar]
- 11.Giudice L.C., Telles T.L., Lobo S., Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann NY Acad Sci. 2002;955:252–264. doi: 10.1111/j.1749-6632.2002.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 12.Mikolajczyk M., Wirstlein P., Skrzypczak J. Leukemia inhibitory factor and interleukin 11 levels in uterine flushing of infertile patients with endometriosis. Hum Reprod. 2006;21(12):3054–3058. doi: 10.1093/humrep/del225. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour H.N., Kelly R.W., Fraser H.M., Critchley H.Q. Endocrine regulation of menstruation. Endocrine Rev. 2009;27(1):17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 14.Karpovich N., Chobotova K., Carver J., Heath J.K., Barlow D.H., Mardon H.J. Expression and function of interleukin-11 and its receptor α in the human endometrium. Mol Hum Reprod. 2003;9(2):75–80. doi: 10.1093/molehr/gag012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazvani M.R., Bates M., Vince G., Christmas S., Lewis-Jones D.I., Kingsland C. Peritoneal fluid concentrations of interleukin-11 in women with endometriosis. Fertil Steril. 2000;74(6):1182–1186. doi: 10.1016/s0015-0282(00)01613-7. [DOI] [PubMed] [Google Scholar]
- 16.Lédée-Bataille N., Laprée-Delage G., Taupin J.L., Dubanchet S., Frydman R., Chaouat G. Concentration of leukemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod. 2002;17(1):213–218. doi: 10.1093/humrep/17.1.213. [DOI] [PubMed] [Google Scholar]
- 17.Dimitriadis E., Sharkey A.M., Tan Y.L., Salamonsen L.A., Sherwin J.R. Immuno-localization of phosphorylated STAT3, interleukin 11 and leukemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod Biol Endocrinol. 2007;5:44–52. doi: 10.1186/1477-7827-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menkhorst E., Salamonsen L., Robb L., Dimitriadis E. IL-11 antagonist inhibits uterine stromal differentiation, causing pregnancy failure in mice. Biol Reprod. 2009;80(5):920–927. doi: 10.1095/biolreprod.108.073601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap J., Foo C.F., Lee M.Y., Stanton P.G., Dimitriadis E. Proteomic analysis identifies interleukin 11 regulated plasma membrane proteins in human endometrial epithelial cells in vitro. Reprod Biol Endocrinol. 2011;9:73–87. doi: 10.1186/1477-7827-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marwood M., Visser K., Salamonsen L.A., Dimitriadis E. Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology. 2009;150(6):2915–2923. doi: 10.1210/en.2008-1538. [DOI] [PubMed] [Google Scholar]
- 21.Hirzel D.J., Wang J., Das S.K., Dey S.K., Mead R.A. Changes in uterine expression of leukemia inhibitory factor during pregnancy in the western spotted skunk. Biol Reprod. 1999;60(2):484–492. doi: 10.1095/biolreprod60.2.484. [DOI] [PubMed] [Google Scholar]
- 22.Kralickova M., Sima R., Vanecek T., Sima P., Rokyta Z., Ulcova-Gallova Z. Leukemia inhibitory factor gene mutations in the population of infertile women are not restricted to nulligravid patients. Eur J Obstet Gynecol Reprod Biol. 2006;127(2):231–235. doi: 10.1016/j.ejogrb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Steck T., Giess R., Suetterlin M.W., Bolland M., Wiest S., Poehls U.G., Dietl J. Leukemia inhibitory factor (LIF) gene mutations in women with unexplained infertility and recurrent failure of implantation after IVF and embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2004;112(1):69–73. doi: 10.1016/s0301-2115(03)00315-4. [DOI] [PubMed] [Google Scholar]



